Abstract
Purpose of Review
Clonal hematopoiesis of indeterminate potential (CHIP) has been identified as a novel cardiovascular risk factor. Here we review the relationship of lifestyle and environmental risk factors predisposing to somatic mutations and CHIP and provide an overview on age-related cardiovascular outcomes.
Recent Findings
CHIP has been associated with accelerated atherosclerosis and cardiovascular disease in both epidemiological and experimental studies. The most commonly mutated candidate driver genes are DNMT3A, TET2, JAK2, and ASXL1. The underlying mechanisms appear predominantly related to inflammatory pathways. Although age is the dominant risk factor for developing CHIP, emerging evidence suggests that other factors such as smoking, obesity/type 2 diabetes, or an unhealthy diet play a role in the occurrence of somatic mutations.
Summary
Evidence suggests a strong link between vascular risk factors, somatic hematopoietic mutations, and age-related cardiovascular disease. Further studies on CHIP biology are required to identify targeted interventions for risk reduction in patients with CHIP and inform the utility of screening strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The human immune system relies upon hematopoietic stem cells (HSCs), which are precursors to erythroid, lymphoid, and myeloid cells and platelets that regulate immunity and inflammation.
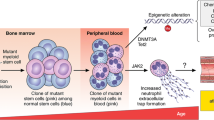
Due to a combination of genetic predisposition, environmental exposures, and random chance, some HSCs acquire specific somatic mutations with leukemogenic potential, which result in cellular survival advantages and clonal expansion of cells in that lineage. This phenomenon, the clonal expansion of HSCs harboring leukemogenic mutations in the absence of other criteria for hematologic neoplasia, dysplasia, or cytopenia, is termed clonal hematopoiesis of indeterminate potential (CHIP) [1, 2].
Human aging is associated with an increased frequency of somatic mutations in HSCs over the lifetime. The prevalence of CHIP in peripheral blood is low (< 0.5%) from birth until 50 years of age after which it begins to rise, affecting 10% of persons aged 70 to 80 years [3]. Most patients with CHIP have somatic mutations in regulator or DNA repair genes such as DNMT3A, TET2, or ASXL1, which increase in frequency with age [4••, 5]. Although such somatic mutations greatly increase the risk of acquiring additional driver mutations resulting in a 10- to 100-fold increased relative risk of hematologic malignancy, the main cause of death in individuals with CHIP is atherosclerotic cardiovascular disease (CVD). The absolute risk for acquiring a hematologic malignancy remains modest (0.5 to 1% per year) [6].
In this review, we first address the relationship of selected cardiovascular risk factors with CHIP and describe the most commonly found somatic mutations. Second, we provide an overview on age-related cardiovascular outcomes related to the presence of CHIP. Finally, we provide our perspective on the potential clinical utility of screening for CHIP for CVD prevention.
Cardiovascular Risk Factors and CHIP
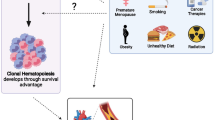
Age is the dominant risk factor for CHIP, which parallels other chronic diseases of aging [6]. Emerging evidence suggests that certain environmental factors and lifestyle exposures may play a role in the induction of somatic mutations and the development of CHIP [6–8] (Fig. 1). Adherence to a healthy lifestyle is a major approach to controlling CVD, associated with more favorable CVD risk factor profiles and with lower CVD incidence and mortality [9–12]. Because many CVD risk factors are influenced by lifestyle, modifiable behavioral factors may also be associated with a lower presence of CHIP. Although research is still limited on which CVD risk factors are related to CHIP, we briefly summarize the available evidence below.
Smoking and Chronic Obstructive Lung Disease
Smoking has been positively associated with presence of CHIP, although findings have not been uniform. [5, 13–16]. Prior inconsistencies are most likely explained by active as compared to former smoking status. Using data from the UK Biobank, a smoking history was significantly associated with CHIP [17]. However, this association was largely driven by those who were current smokers rather than former smokers. Interestingly, among specific CHIP mutations, ASXL1 mutations seem to be particularly enriched with current and past smokers [17]. In line with these findings, individuals with CHIP were recently shown to be at significantly higher risk of compared to non-carriers [18]. Moreover, smoking exposure was found to be associated with a small but significantly increased risk of having CHIP. Detailed analysis further showed that inactivation of TET2 was associated with the development of emphysema and inflammation in models using cigarette smoke exposure [18]. Most recent evidence stems from two-sample Mendelian randomization analyses [16], showing that smoking is strongly associated with mosaic chromosomal alterations but not with CHIP. These recent findings support a causal association between smoking and mosaic chromosomal alterations and suggest that smoking may variably shape the fitness of clones bearing somatic mutations [16].
Diet
The relationship between diet quality and presence of CHIP has yielded conflicting results [19, 20]. While some authors did not find any association, other results suggest that an unhealthy diet may be associated with a higher prevalence of CHIP [19, 20]. In an analysis using data from a large cohort of postmenopausal women, Haring et al. did not find a relationship between adherence to a healthy diet (assessed by the Alternative Healthy Eating Index-2010) and prevention of major chronic diseases and presence of CHIP [19]. On the other hand, recent evidence stemming from the UK Biobank suggests that an unhealthy diet quality as defined by intake of fruits and vegetables, red meat, processed food, and added salt is associated with a higher prevalence of CHIP and higher rates of adverse CVD events and death independent of CHIP status [20]. Differences may be explained by study population characteristics, measurement errors, or other factors and warrant further clarification.
Obesity and Type 2 Diabetes
Adipose tissue can synthesize cytokines such as TNF-α and IL-6 and has been shown to promote inflammation and atherogenesis independent of effects on insulin resistance or lipoproteins [21]. Having a normal body mass index compared to being obese is associated with lower frequency of CHIP in postmenopausal women [19]. Jaiswal et al. reported a 1.3-fold increased odds of CHIP in diabetes patients [22]. Obesity, diabetes, and CHIP may be related to one another through increased activation of pro-inflammatory pathways [21]. In fact, DNMT3A and TET2 may mediate atherosclerotic cardiovascular risk through regulating lipid and glucose metabolism [15, 23].
DNMT3A is significantly increased in adipose tissue–derived macrophages in mice fed with high-fat diet [24]. Similarly, a recent report by Fuster et al. indicates that clonal expansion in TET2 deficient cells can aggravate insulin resistance, obesity, and aging in mice [25]. Thus, a combination of aging, adipose tissue accumulation, and CHIP-associated mutations might activate the production of inflammatory cytokines. In agreement with this hypothesis, TET2-deficient HSCs have been shown to produce increased levels of monocytes and inflammatory cytokines such as IL-1β and IL-6 [26, 27]. In fact, serum levels of IL-6 are generally high in people with CHIP, paralleling the observation that obesity or aging also induce an inflammatory response in the bone marrow by promoting accumulation of adipocytes [4••, 28, 29].
A positive feedback loop between self-renewal of HSCs and progression of inflammation in TET2-mutated CHIP has been proposed [25, 27]. TET2-mutated HSCs preferentially produce myeloid progenitors. As a result, monocytes and macrophages are increased. In macrophages with TET2 mutations, the NLRP3 inflammasome pathway is activated and IL-1β is increasingly secreted. This eventually leads to an overproduction of IL-1β in the adipose tissue explaining a relationship between CHIP and type 2 diabetes mellitus as the IL-1β-mediated autoinflammatory process is regarded as a major factor in the loss of beta-cell mass in type 2 diabetes [25, 30]. In turn, IL-1β promotes the self-renewal of HSCs with TET2 mutations. This positive feedback loop would be responsible for the pathogenesis of diabetes and atherosclerosis in TET2-mutated CHIP [27].
Hyperlipidemia
Chronic elevation of blood lipid levels in conjunction with immune cell recruitment and inflammation accelerates the development of atherosclerotic plaques. Interestingly, however, CHIP mutations have not been not associated with lipid levels apart from the association with JAK2 CHIP, which is correlated with a decrease in total cholesterol and LDL despite an increased risk for coronary heart disease [4••, 8]. The lack of a clear relationship between most CHIP mutations and hyperlipidemia has been a surprising finding. Insights were recently provided by Heyde et al. who demonstrated that mild hypercholesterolemia in non-atherosclerotic wild-type mice did not induce clonal expansion, suggesting that in the absence of inflammation, elevated cholesterol alone is not sufficient to drive clonal hematopoiesis [31••].
Premature Menopause
A history of premature menopause was found to be independently associated with increased odds of CHIP in two large cohorts of postmenopausal women [32]. Moreover, in gene-specific analyses, only DNMT3A was significantly associated with premature menopause. Interestingly, the risks of developing CHIP appeared to differ in women with natural versus surgical premature menopause, implying that postmenopausal reductions in estrogen and other sex steroid hormones alone may not explain the relationship between premature menopause and CHIP. Independent of established CHIP risk factors, premature menopause was associated with 1.4-fold odds—and natural premature menopause with 1.7-fold odds—of CHIP [32].
HIV and Chronic Infection
HIV infection is associated with greater risk for hematologic malignancy and coronary artery disease is a major cause of morbidity. Bick et al. showed that in individuals living with HIV, the prevalence of CHIP is increased twofold compared to matched controls. Interestingly, ASXL1 was the most commonly implicated mutated CHIP gene [33].
Cancer Treatment and Radiation
Bolton et al. examined the effects of different cancer therapies on CHIP [34]. Those most associated with CHIP prevalence were external beam radiation therapy, cytotoxic chemotherapy, and radionuclide therapy. Within cytotoxic chemotherapy, topoisomerase II inhibitors (e.g., doxorubicin) had the strongest association along with platinum agent carboplatin. CHIP has also been associated with environmental radiation. Recently presented analyses from WHI suggest ambient exposure to radon was associated with CHIP prevalence [35]. Furthering the work on ambient radiation and CHIP, Mencia-Trinchant et al. conducted a unique study on a pair of twin astronauts using data from the NASA Twins Study [36•]. These astronauts exhibited CHIP almost two decades prior to the mean age at which it is typically detected and showed larger shifts in clone size than age-matched controls or radiotherapy patients.
Somatic Mutations and CHIP
The most commonly mutated candidate driver genes in CHIP are DNMT3A, TET2, and ASXL1 (Table 1) [8, 15]. These three somatic mutations account for 75% of all CHIP cases [4••]. Additional mutations are seen in JAK2, which is particularly associated with increased rates of thrombosis, as well as the DNA damage response pathway genes PPM1D and TP53, and mRNA splicing factors SRSF2 and SF3B1. Early analysis found mutations in DNMT3A, TET2, and ASXL1 to be associated with a 1.7-fold to 2.0-fold increased risk of incident coronary heart disease, while the JAK2 V617F mutation was associated with a 12-fold increased risk [15]. The vast majority of individuals (approximately 90%) with CHIP driver mutations have only one identified mutation. Across age groups, JAK2 CHIP carriers are the youngest. Relative to JAK2, ASXL1, and TET2 carriers are 3.3 and 3.9 years older, while PPM1D, SF3B1, and SRSF2 carriers are 5.0, 6.9, and 7.7 years older, respectively [4••].
DNMT3A
With a frequency of approximately 50% in all CHIP cases, DNA methyltransferase 3a (DNMT3A) is considered to be the most commonly mutated gene in CHIP [4••]. DNMT3A represents an epigenetic regulator of gene expression and encodes a methyltransferase enzyme that catalyzes DNA methylation. Pathogenic mutations of DNMT3A promote HSC self-renewal and the expression of multipotency genes while suppressing differentiation factor expression. This enables DNMT3A mutations to affect all hematopoietic lineages, inducing pro-inflammatory T-cell polarization and activating the inflammasome complex.
Using CRISPR gene technology, it was possible to show in mouse models that DNMT3A CHIP can both cause inflammation and be promoted by inflammation itself [37]. Murine macrophages carrying DNMT3A mutations showed increased expression of several cytokines. On the other hand, interferon-gamma is sufficient to drive clonal expansion of DNMT3A mutant HSCs, in which via increased resistance to stress-induced apoptosis and differentiation, defects can easily outcompete wild-type HSCs in peripheral blood.
TET 2
With a presence of approximately 20% in all CHIP cases, TET2 is the second most frequently mutated gene in CHIP. TET2 acts in antagonistic fashion to DNMT3A by catalyzing the oxidation of the DNA-methyl group (demethylation) and also affects transcription by recruiting histone modifiers. TET2 loss-of-function mutations cause epigenetic dysregulation, promote HSC self-renewal, and preferentially lead towards myeloid lineage differentiation [38].
Analyses of macrophages from mice that received bone marrow with TET2 deficient cells showed elevated expression of several chemokine and cytokine genes that contribute to a pro-inflammatory state and accelerated atherosclerosis [15]. TET2 deficient carriers showed an increased level of circulating IL-1ß due to NLRP3-inflammasome induction and an accelerated cardiac fibrosis. The atheroprotective effect of NLRP3 inflammasome inhibitors, as well as their protection against the development of heart failure in TET2-deficient mice, supports these findings [26, 39].
ASXL1
ASXL1 (additional sex combs-like 1) is the third most mutated gene in CHIP (approximately 5 to 10%), leading to altered histone modification [4••]. ASXL1 deletion facilitates aberrant gene expression and results in myeloid transformation, but the mechanisms by which ASXL1 mutations lead to increased inflammation are not entirely clear [27, 40]. Observational studies point to a link between ASXL1 mutations in blood cells with smoking and among patients with HIV. Dawoud et al. utilized whole-exome sequencing data of the UK Biobank and found a significantly higher risk of having CHIP for those who were current or former smokers [17]. The majority of participants (69%) with an ASXL1 CHIP were current or former smokers confirming that mutations in ASXL1 and genes coding for spliceosomes are strongly associated with exposure to DNA-damaging agents such as smoking [17].
JAK2
JAK2 (activated janus kinase 2) is a non-receptor tyrosine kinase that transmits intracellular signals downstream of cytokine receptors and accounts for a small percentage of all CHIP, which do not only appear in older age groups. JAK2 tyrosine phosphorylates and activates TET2 in response to cytokines, linking extracellular signals with epigenetic changes in hematopoiesis. JAK2 mutations in CHIP tend to occur at a younger age and carry the strongest risk of premature cardiac disease among CHIP variants [4••, 15]. The presence of JAK2 CHIP carrier status is associated with higher levels of IL-18, and downstream increases in IL-6 production. Inflammation and atherosclerotic disease have been shown to occur in JAK2 CHIP carriers even in the presence of reduced LDL cholesterol [4••, 8].
The JAK2V617F mutation is commonly linked to myeloproliferative neoplasms, and in these diseases, it is associated with thromboembolic complications, increased blood viscosity, and platelet adhesion, as well as reduced venous blood return [41, 42]. Indeed, the JAK2-gain-of-function mutation has been shown to promote the risk of venous and coronary thrombosis and pulmonary embolus, due to its enhanced formation of neutrophil extracellular traps, components of innate immunity [43] Importantly, however, the JAK2V617F mutation was also found to be associated with thrombosis in patients without the presence of myeloproliferative neoplasms or other hematologic disorders [43]. Moreover, Wang et al. showed that the JAK2V617F mutation expression promotes neutrophil infiltration and early atherosclerotic lesion formation and plaque instability in a mouse model of hypercholesterolemia [44]. Edelmann et al. further evaluated the role of JAK2V617F mutation in thrombus formation and found that the mutation upregulates β1 and β2 integrin expression, which are both essential regulators for attachment of leukocytes to endothelial cells [45]. Collectively, current evidence suggests that JAK2 mutations in CHIP can promote CVD by altering hematopoiesis-boosting innate immunity responses, and promoting thrombotic diseases [46].
TP53, PPM1D, SF3B1, SRSF2
Mutations in DNA damage repair genes such as TP53 or PPM1D are less frequent than other CHIP mutations [4••]. In case of a detected DNA damage, the activated tumor suppressor p53 induces the expression of PPM1D protein phosphatase (Mn2+/Mg2+-dependent 1D) which leads to dephosphorylation of p53 and ultimately to apoptosis. Hematopoietic cell lines with PPM1D loss-of-function mutations outcompete normal cells by increased resistance to apoptosis and are strongly associated with CHIP after prior exposure to cytotoxic chemotherapies such as cisplatin, etoposide, and doxorubicin [47]. Thus, especially in the case of chemotherapy treatment of solid tumors, hematopoietic mutations in TP53 and PPM1D appear to promote the outgrowth of clones that can lead to subsequent malignancy and risk for leukemic transformation [47, 48].
Mutations of the mRNA spliceosome complex components SF3B1 and SRSF2 are not well studied yet, but seem to be the most common genetic alterations in patients with myelodysplastic syndrome [49, 50]. Mutations in the splicing factors SF3B1 and SRSF2 have been reported to share convergent effects on aberrant splicing of mRNAs that promote nuclear factor κB signaling [50].
CHIP Without Driver Mutations
CHIP is commonly defined as somatic mutation with variant allele frequency > 2% in peripheral blood of individuals with no evidence of hematologic disease.47, 48 Thus, CHIP without known candidate driver mutations is technically excluded from this classification [51, 52]. Nonetheless, clonal hematopoiesis without driver mutations carries increased risk of hematologic cancers and all-cause mortality, although its links to CVD are poorly understood [5, 53]. In fact, in a significant proportion of cases of clonal hematopoiesis, no clear candidate driver mutation is identified [4••, 5]. Mosaic chromosomal alteration represents one presentation of CHIP without driver mutations. It includes larger structural somatic alterations such as deletions, duplications, or copy number neutral loss of heterozygosity [54, 55]. Similar to candidate driver mutations, mosaic chromosomal laterations are common at very old age and have been related to lymphoid malignancies like CLL [56]. Interestingly, cardiovascular risk is not altered in cases of mosaic chromosomal alteration, even when associated with DNMT3A or TET2 loss (with the notable exception of JAK2) [8, 54, 55].
Genotypic Associations with CHIP
While CHIP driver mutations are acquired somatic mutations, certain germline variation may predispose to the development of CHIP during life course. Using whole-genome sequencing data from a large cohort unselected for candidate driver mutation, Bick et al. could identify three germline risk loci associated with a predilection to TET2 CHIP [4••, 8] One set of loci involved genes that maintain genome integrity (e.g., TERT and CHEK2) and which have been implicated in stem cell maintenance/self-renewal and the risk of neoplasm in multiple organ systems; other germline loci are associated with increased hematopoietic stem cell self-renewal (e.g., TET2) and only associated with hematologic malignancies; finally, a third set of germline loci are associated with the acquisition of CHIP mutations in specific driver genes. Specifically, variations at the TCL1A promoter were associated with increased risk of DNMT3A CHIP, but not other CHIP subsets [4••, 8].
CHIP and Age-Related Cardiovascular Risk
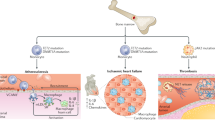
In a seminal paper on “Clonal Hematopoiesis and Risk of Atherosclerotic Cardiovascular Disease,” Jaiswal et al. showed that presence of CHIP in peripheral blood cells was associated with accelerated atherosclerosis and coronary heart disease [15, 22]. Subsequent studies have expanded our understanding on CHIP carrier status and CVD (Table 2). Reports on associations of CHIP with multiple other age-related cardiovascular conditions such as heart failure, stroke, or aortic valve stenosis have been published to this point [13, 57, 58] (Fig. 1).
Coronary Heart Disease
There is now consistent evidence for an epidemiological association between presence of CHIP and myocardial infarction or coronary revascularization procedures [15]. In experimental analysis, it could be further shown that clonal hematopoiesis associated with TET2 deficiency leads to accelerated atherosclerosis mainly driven by interactions between clonal monocytes-macrophages and the endothelium and an increased expression of pro-inflammatory genes [15, 39, 59, 60]. Moreover, RNA sequencing of cells with loss-of-function mutations in TET2 showed augmented expression of pro-inflammatory mediators implicated in the pathogenesis of atherosclerosis including IL-1β and IL-6 [15].
Heart Failure
In addition to the relationship between CHIP and coronary heart disease, CHIP has been recently associated with ischemic heart failure and reduced left ventricular ejection fraction [13, 61]. However, even outside the context of ischemic events, CHIP was found to be associated with deteriorating cardiac function. In fact, the presence of CHIP mutations in patients with chronic heart failure has been identified as an independent predictor of mortality [62]. Interestingly, current evidence suggests that heart failure patients carrying more than one CHIP mutation have a worse prognosis; higher cumulative clone size is also an adverse prognostic factor, supporting a dose–response relationship [63, 64].
The underlying mechanisms by which CHIP and its mutations in ASXL1, DNMT3A, TET2, or JAK2 are related to heart failure development and progression are not well understood. It appears that different CHIP mutations may exert this effect through different signaling pathways and inflammatory profiles. ASXL1, TET2, and JAK2 sequence variations have been each associated with an increased risk of heart failure, whereas the association of DNMT3A with heart failure shows inconsistent results across studies.
Additional information on the pathways by which the DNMT3A or TET2 mutations alter cardiac function came from Sano et al. who investigated the potential mechanisms in a murine model using a CRISPR/Cas9 system [37]. The researchers used a lentiviral vector to deliver Cas9 and guide RNA, introducing inactivating mutations in TET2 and DNMT3A in bone marrow cells using a model of hypertensive heart failure. Interestingly, only mice with inactivating mutations in TET2 had expanded mutant hematopoietic cells and increased expression of IL-1B and IL-6, whereas mice with inactivating mutations in DNMT3A did not demonstrate expansion of hematopoietic cells. Other data showed that circulating monocytes of patients with heart failure carrying DNMT3A mutations demonstrated a pro-inflammatory transcriptome with significantly increased expression of inflammatory genes compared with monocytes derived from patients with heart failure without DNMT3A mutations, especially inflammatory interleukin IL-1β, IL6, IL8, the inflammasome NLRP3, and the macrophage inflammatory proteins CCL3, CCL4, and resistin, of which the latter mediates monocyte-endothelial adhesion and may all together contribute to an aggravation of chronic heart failure [65].
Aortic Valve Stenosis
Mas-Peiro et al. examined the incidence of CHIP in patients with severe degenerative aortic valve stenosis [57]. It appeared that CHIP prevalence was enriched among patients with severe aortic stenosis, as somatic DNMT3A or TET2 driver mutations were detected in a third of patients. CHIP carriers had an increased mortality rate following TAVI procedure, were found to have increased inflammatory activation of T-cells and demonstrated higher circulating levels of non-classical monocytes that secrete pro-inflammatory cytokines, including TNF-α, IL-1β, and IL-8.
Stroke
CHIP was recently found to be associated with a 14% increased odds of incident stroke when analyzed across eight cohorts [58]. Interestingly and unexpectedly, this relationship was primarily driven by 24% increased odds of hemorrhagic stroke. Unselected subtypes of ischemic stroke were not associated with CHIP. However, in further analyses of ischemic stroke subtypes, CHIP was strongly associated with small vessel stroke, with stronger relationships with mutations in TET2. The mechanisms linking CHIP to hemorrhagic stroke are not clear, but again inflammatory signaling pathways linked to aneurysm formation, accelerated arteriosclerosis, blood vessel fragility, and cerebral amyloid angiopathy are potentially involved [58, 66–68].
CHIP Implications: a New Target for Cardiovascular Disease Prevention
CHIP has been identified as a major, non-lipid/non-traditional mediator of cardiovascular risk. It has been linked to multiple cardiovascular outcomes including coronary heart disease, heart failure, stroke, and aortic valve disease. The underlying pathophysiological mechanisms are most likely related to inflammatory pathways. These exciting findings raise several questions:
Should cardiovascular risk assessment and management include screening for CHIP in light of recent CHIP findings? Diagnosing CHIP requires deep sequencing of peripheral blood and it is not yet a routine clinical test. However, as methods for assaying CHIP become more accessible and cost-efficient and as additional treatments targeting CHIP become available, screening for CHIP status may become a routine part of clinical care. Assessing CHIP status offers promise for advancing individualized precision medicine, with the ultimate aim of preventing the accumulation of acquired somatic mutations, atherosclerotic lesion development, and progression. Research on CHIP represents an extension of the study of cardiovascular genetics and atherosclerosis biology beyond inherited germline mutations [6, 69]. Clinical management of individuals with CHIP remains limited, with few treatment strategies beyond traditional risk factor modification. As new data emerge, international recommendations for diagnosis, management algorithms, and treatment options for CHIP can be promulgated.
How may clinicians be able to leverage CHIP data in the future and what treatment strategies may be indicated ? Screening for CHIP and its major driver mutations can help clinicians to identify patients at high-cardiovascular risk warranting more intensified lifestyle-related or even pharmacologic interventions. One potential intervention in patients with CHIP could be to specifically target certain inflammatory pathways. Modulation of IL-1β in the CANTOS trial and IL-1 and Il-8 with colchicine in COLCOT may therefore provide potential tools and stimulate the development of future targeted interventions [40, 70, 71]. Genetically reduced IL-6 signaling in DNMT3A and TET2 CHIP carriers has been shown to substantially reduce CVD risk [72]. Additionally, most recent data from the CANTOS trial raise the possibility that in individuals with established CVD and elevated high-sensitivity C-reactive protein level above 2.0 mg/L, those with TET2 variants may respond better to Canakinumab, an anti-IL-1β antibody, with respect to CVD event reduction than those without CHIP [73•]. Other suggested therapeutic approaches involve inhibition of JAK2 or downstream integrins, which may reduce thrombotic CVD complications in CHIP patients carrying JAK2 mutations, or tight control of glucose levels to mitigate CVD risk in patients with TET2-driven CHIP [43, 46, 74, 75].
In conclusion, the recognition of a strong link between somatic mutations, clonal hematopoiesis, and age-related cardiovascular risk provides new insights into the pathophysiology of atherothrombotic cardiovascular conditions and novel approaches to prevention and treatment. This exciting field may have future implications for clinical practice and population health.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Jaiswal S, Ebert BL. Clonal hematopoiesis in human aging and disease. Science. 2019;366(6465).
Ebert BL, Libby P. Clonal hematopoiesis confers predisposition to both cardiovascular disease and cancer: a newly recognized link between two major killers. Ann Intern Med. 2018;169(2):116–7.
Laurie CC, Laurie CA, Rice K, Doheny KF, Zelnick LR, McHugh CP, et al. Detectable clonal mosaicism from birth to old age and its relationship to cancer. Nat Genet. 2012;44(6):642–50.
•• Bick AG, Weinstock JS, Nandakumar SK, Fulco CP, Bao EL, Zekavat SM, et al. Inherited causes of clonal haematopoiesis in 97,691 whole genomes. Nature. 2020;586(7831):763–8. Findings from the study suggest that germline genetic variation shapes haematopoietic stem cell function, leading to CHIP through mechanisms that are specific to clonal haematopoiesis as well as shared mechanisms that lead to somatic mutations across tissues.
Zink F, Stacey SN, Norddahl GL, Frigge ML, Magnusson OT, Jonsdottir I, et al. Clonal hematopoiesis, with and without candidate driver mutations, is common in the elderly. Blood. 2017;130(6):742–52.
Libby P, Sidlow R, Lin AE, Gupta D, Jones LW, Moslehi J, et al. Clonal hematopoiesis: crossroads of aging, cardiovascular disease, and cancer: JACC review topic of the week. J Am Coll Cardiol. 2019;74(4):567–77.
Khetarpal SA, Qamar A, Bick AG, Fuster JJ, Kathiresan S, Jaiswal S, et al. Clonal hematopoiesis of indeterminate potential reshapes age-related CVD: JACC review topic of the week. J Am Coll Cardiol. 2019;74(4):578–86.
Marnell CS, Bick A, Natarajan P. Clonal hematopoiesis of indeterminate potential (CHIP): linking somatic mutations, hematopoiesis, chronic inflammation and cardiovascular disease. J Mol Cell Cardiol. 2021;161:98–105.
Corlin L, Short MI, Vasan RS, Xanthakis V. Association of the duration of ideal cardiovascular health through adulthood with cardiometabolic outcomes and mortality in the Framingham Offspring Study. JAMA Cardiol. 2020.
Ford ES, Bergmann MM, Boeing H, Li C, Capewell S. Healthy lifestyle behaviors and all-cause mortality among adults in the United States. Prev Med. 2012;55(1):23–7.
Mozaffarian D, Afshin A, Benowitz NL, Bittner V, Daniels SR, Franch HA, et al. Population approaches to improve diet, physical activity, and smoking habits: a scientific statement from the American Heart Association. Circulation. 2012;126(12):1514–63.
Li Y, Schoufour J, Wang DD, Dhana K, Pan A, Liu X, et al. Healthy lifestyle and life expectancy free of cancer, cardiovascular disease, and type 2 diabetes: prospective cohort study. BMJ. 2020;368: l6669.
Dorsheimer L, Assmus B, Rasper T, Ortmann CA, Ecke A, Abou-El-Ardat K, et al. Association of mutations contributing to clonal hematopoiesis with prognosis in chronic ischemic heart failure. JAMA Cardiol. 2019;4(1):25–33.
Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371(26):2488–98.
Jaiswal S, Natarajan P, Silver AJ, Gibson CJ, Bick AG, Shvartz E, et al. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N Engl J Med. 2017;377(2):111–21.
Levin MG, Nakao T, Zekavat SM, Koyama S, Bick AG, Niroula A, et al. Genetics of smoking and risk of clonal hematopoiesis. Sci Rep. 2022;12(1):7248.
Dawoud AAZ, Tapper WJ, Cross NCP. Clonal myelopoiesis in the UK Biobank cohort: ASXL1 mutations are strongly associated with smoking. Leukemia. 2020;34(10):2660–72.
Miller PG, Qiao D, Rojas-Quintero J, Honigberg MC, Sperling AS, Gibson CJ, et al. Association of clonal hematopoiesis with chronic obstructive pulmonary disease. Blood. 2022;139(3):357–68.
Haring B, Reiner AP, Liu J, Tobias DK, Whitsel E, Berger JS, et al. Healthy lifestyle and clonal hematopoiesis of indeterminate potential: results from the women’s health initiative. J Am Heart Assoc. 2021;10(5): e018789.
Bhattacharya R, Zekavat SM, Uddin MM, Pirruccello J, Niroula A, Gibson C, et al. Association of diet quality with prevalence of clonal hematopoiesis and adverse cardiovascular events. JAMA Cardiol. 2021;6(9):1069–77.
Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105(9):1135–43.
Jaiswal S, Natarajan P, Ebert BL. Clonal hematopoiesis and atherosclerosis. N Engl J Med. 2017;377(14):1401–2.
Bonnefond A, Skrobek B, Lobbens S, Eury E, Thuillier D, Cauchi S, et al. Association between large detectable clonal mosaicism and type 2 diabetes with vascular complications. Nat Genet. 2013;45(9):1040–3.
You D, Nilsson E, Tenen DE, Lyubetskaya A, Lo JC, Jiang R, et al. Dnmt3a is an epigenetic mediator of adipose insulin resistance. Elife. 2017;6.
Fuster JJ, Zuriaga MA, Zorita V, MacLauchlan S, Polackal MN, Viana-Huete V, et al. TET2-loss-of-function-driven clonal hematopoiesis exacerbates experimental insulin resistance in aging and obesity. Cell Rep. 2020;33(4): 108326.
Sano S, Oshima K, Wang Y, MacLauchlan S, Katanasaka Y, Sano M, et al. Tet2-mediated clonal hematopoiesis accelerates heart failure through a mechanism involving the IL-1β/NLRP3 inflammasome. J Am Coll Cardiol. 2018;71(8):875–86.
Asada S, Kitamura T. Clonal hematopoiesis and associated diseases: a review of recent findings. Cancer Sci. 2021;112(10):3962–71.
Ambrosi TH, Scialdone A, Graja A, Gohlke S, Jank AM, Bocian C, et al. Adipocyte accumulation in the bone marrow during obesity and aging impairs stem cell-based hematopoietic and bone regeneration. Cell Stem Cell. 2017;20(6):771–84 e6.
Masamoto Y, Arai S, Sato T, Yoshimi A, Kubota N, Takamoto I, et al. Adiponectin enhances antibacterial activity of hematopoietic cells by suppressing bone marrow inflammation. Immunity. 2016;44(6):1422–33.
Dinarello CA, Donath MY, Mandrup-Poulsen T. Role of IL-1beta in type 2 diabetes. Curr Opin Endocrinol Diabetes Obes. 2010;17(4):314–21.
•• Heyde A, Rohde D, McAlpine CS, Zhang S, Hoyer FF, Gerold JM, et al. Increased stem cell proliferation in atherosclerosis accelerates clonal hematopoiesis. Cell. 2021;184(5):1348–61 e22. This study could show that hematopoietic stem cell division rates are increased in mice and humans with atherosclerosis. Mathematical analysis demonstrates that increased stem cell proliferation expedites somatic evolution and expansion of clones with driver mutations.
Honigberg MC, Zekavat SM, Niroula A, Griffin GK, Bick AG, Pirruccello JP, et al. Premature menopause, clonal hematopoiesis, and coronary artery disease in postmenopausal women. Circulation. 2021;143(5):410–23.
Bick AG, Popadin K, Thorball CW, Uddin MM, Zanni M, Yu B, et al. Increased CHIP prevalence amongst people living with HIV. medRxiv. 2020.
Bolton KL, Ptashkin RN, Gao T, Braunstein L, Devlin SM, Kelly D, et al. Cancer therapy shapes the fitness landscape of clonal hematopoiesis. Nat Genet. 2020;52(11):1219–26.
Anthony KM MJ JS, Natarajan P, Desai P, Hayden KM, Bick AG, Carty CL, et al. Radon is associated with clonal hematopoiesis of indeterminate potential in the women’s health initiative. AHA Sci Sessions. 2020. Circulation.
• Mencia-Trinchant N, MacKay MJ, Chin C, Afshinnekoo E, Foox J, Meydan C, et al. Clonal hematopoiesis before, during, and after human spaceflight. Cell Rep. 2020;33(10): 108458. The results of this study suggest that longitudinal monitoring of CHIP may serve as an important metric for overall cancer and cardiovascular risk.
Sano S, Oshima K, Wang Y, Katanasaka Y, Sano M, Walsh K. CRISPR-mediated gene editing to assess the roles of Tet2 and Dnmt3a in clonal hematopoiesis and cardiovascular disease. Circ Res. 2018;123(3):335–41.
Buscarlet M, Provost S, Zada YF, Bourgoin V, Mollica L, Dubé MP, et al. Lineage restriction analyses in CHIP indicate myeloid bias for TET2 and multipotent stem cell origin for DNMT3A. Blood. 2018;132(3):277–80.
Fuster JJ, MacLauchlan S, Zuriaga MA, Polackal MN, Ostriker AC, Chakraborty R, et al. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science. 2017;355(6327):842–7.
Bhattacharya R, Bick AG. Clonal hematopoiesis of indeterminate potential: an expanding genetic cause of cardiovascular disease. Curr Atheroscler Rep. 2021;23(11):66.
Neubauer H, Cumano A, Müller M, Wu H, Huffstadt U, Pfeffer K. Jak2 deficiency defines an essential developmental checkpoint in definitive hematopoiesis. Cell. 1998;93(3):397–409.
Misawa K, Yasuda H, Araki M, Ochiai T, Morishita S, Shirane S, et al. Mutational subtypes of JAK2 and CALR correlate with different clinical features in Japanese patients with myeloproliferative neoplasms. Int J Hematol. 2018;107(6):673–80.
Wolach O, Sellar RS, Martinod K, Cherpokova D, McConkey M, Chappell RJ, et al. Increased neutrophil extracellular trap formation promotes thrombosis in myeloproliferative neoplasms. Sci Transl Med. 2018;10(436).
Wang W, Liu W, Fidler T, Wang Y, Tang Y, Woods B, et al. Macrophage inflammation, erythrophagocytosis, and accelerated atherosclerosis in Jak2 (V617F) mice. Circ Res. 2018;123(11):e35–47.
Edelmann B, Gupta N, Schnoeder TM, Oelschlegel AM, Shahzad K, Goldschmidt J, et al. JAK2-V617F promotes venous thrombosis through β1/β2 integrin activation. J Clin Invest. 2018;128(10):4359–71.
Papa V, Marracino L, Fortini F, Rizzo P, Campo G, Vaccarezza M, et al. Translating evidence from clonal hematopoiesis to cardiovascular disease: a systematic review. J Clin Med. 2020;9(8).
Hsu JI, Dayaram T, Tovy A, De Braekeleer E, Jeong M, Wang F, et al. PPM1D mutations drive clonal hematopoiesis in response to cytotoxic chemotherapy. Cell Stem Cell. 2018;23(5):700–13 e6.
Silver AJ, Jaiswal S. Clonal hematopoiesis: pre-cancer PLUS. Adv Cancer Res. 2019;141:85–128.
Yimpak P, Tantiworawit A, Rattanathammethee T, Angsuchawan S, Laowatthanapong S, Tasuya W, et al. Alteration of SF3B1 and SRSF2 genes in myelodysplastic syndromes patients in upper northern Thailand. Asian Pac J Cancer Prev. 2019;20(4):1215–21.
Lee SC, North K, Kim E, Jang E, Obeng E, Lu SX, et al. Synthetic lethal and convergent biological effects of cancer-associated spliceosomal gene mutations. Cancer Cell. 2018;34(2):225–41 e8.
Calvillo-Argüelles O, Jaiswal S, Shlush LI, Moslehi JJ, Schimmer A, Barac A, et al. Connections between clonal hematopoiesis, cardiovascular disease, and cancer: a review. JAMA Cardiol. 2019;4(4):380–7.
Shlush LI. Age-related clonal hematopoiesis. Blood. 2018;131(5):496–504.
Genovese G, Kähler AK, Handsaker RE, Lindberg J, Rose SA, Bakhoum SF, et al. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N Engl J Med. 2014;371(26):2477–87.
Loh PR, Genovese G, McCarroll SA. Monogenic and polygenic inheritance become instruments for clonal selection. Nature. 2020;584(7819):136–41.
Loh PR, Genovese G, Handsaker RE, Finucane HK, Reshef YA, Palamara PF, et al. Insights into clonal haematopoiesis from 8,342 mosaic chromosomal alterations. Nature. 2018;559(7714):350–5.
Terao C, Suzuki A, Momozawa Y, Akiyama M, Ishigaki K, Yamamoto K, et al. Chromosomal alterations among age-related haematopoietic clones in Japan. Nature. 2020;584(7819):130–5.
Mas-Peiro S, Hoffmann J, Fichtlscherer S, Dorsheimer L, Rieger MA, Dimmeler S, et al. Clonal haematopoiesis in patients with degenerative aortic valve stenosis undergoing transcatheter aortic valve implantation. Eur Heart J. 2020;41(8):933–9.
Bhattacharya R, Zekavat SM, Haessler J, Fornage M, Raffield L, Uddin MM, et al. Clonal hematopoiesis is associated with higher risk of stroke. Stroke. 2021. STROKEAHA121037388.
Libby P, Sidlow R, Lin AE, Gupta D, Jones LW, Moslehi J, et al. Clonal hematopoiesis: crossroads of aging, cardiovascular disease, and cancer: JACC review topic of the week. J Am Coll Cardiol. 2019;74(4):567–77.
Steensma DP. Clinical consequences of clonal hematopoiesis of indeterminate potential. Blood Adv. 2018;2(22):3404–10.
Yu B, Roberts MB, Raffield LM, Zekavat SM, Nguyen NQH, Biggs ML, et al. Supplemental association of clonal hematopoiesis with incident heart failure. J Am Coll Cardiol. 2021;78(1):42–52.
Pascual-Figal DA, Bayes-Genis A, Díez-Díez M, Hernández-Vicente Á, Vázquez-Andrés D, de la Barrera J, et al. Clonal hematopoiesis and risk of progression of heart failure with reduced left ventricular ejection fraction. J Am Coll Cardiol. 2021;77(14):1747–59.
Assmus B, Cremer S, Kirschbaum K, Culmann D, Kiefer K, Dorsheimer L, et al. Clonal haematopoiesis in chronic ischaemic heart failure: prognostic role of clone size for DNMT3A- and TET2-driver gene mutations. Eur Heart J. 2021;42(3):257–65.
Dorsheimer L, Assmus B, Rasper T, Ortmann CA, Abou-El-Ardat K, Kiefer KC, et al. Hematopoietic alterations in chronic heart failure patients by somatic mutations leading to clonal hematopoiesis. Haematologica. 2020;105(7):e328–32.
Abplanalp WT, Mas-Peiro S, Cremer S, John D, Dimmeler S, Zeiher AM. Association of clonal hematopoiesis of indeterminate potential with inflammatory gene expression in patients with severe degenerative aortic valve stenosis or chronic postischemic heart failure. JAMA Cardiol. 2020;5(10):1170–5.
Carrillo-Jimenez A, Deniz Ö, Niklison-Chirou MV, Ruiz R, Bezerra-Salomão K, Stratoulias V, et al. TET2 regulates the neuroinflammatory response in microglia. Cell Rep. 2019;29(3):697–713 e8.
Yang SJ, Shao GF, Chen JL, Gong J. The NLRP3 inflammasome: an important driver of neuroinflammation in hemorrhagic stroke. Cell Mol Neurobiol. 2018;38(3):595–603.
Young AM, Karri SK, You W, Ogilvy CS. Specific TNF-alpha inhibition in cerebral aneurysm formation and subarachnoid hemorrhage. Curr Drug Saf. 2012;7(3):190–6.
Sidlow R, Lin AE, Gupta D, Bolton KL, Steensma DP, Levine RL, et al. The clinical challenge of clonal hematopoiesis, a newly recognized cardiovascular risk factor. JAMA Cardiol. 2020;5(8):958–61.
Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377(12):1119–31.
Tardif JC, Kouz S, Waters DD, Bertrand OF, Diaz R, Maggioni AP, et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med. 2019;381(26):2497–505.
Bick AG, Pirruccello JP, Griffin GK, Gupta N, Gabriel S, Saleheen D, et al. Genetic interleukin 6 signaling deficiency attenuates cardiovascular risk in clonal hematopoiesis. Circulation. 2020;141(2):124–31.
• Svensson EC, Madar A, Campbell CD, He Y, Sultan M, Healey ML, et al. TET2-driven clonal hematopoiesis and response to canakinumab: an exploratory analysis of the CANTOS randomized clinical trial. JAMA Cardiol. 2022. The results of this study suggest that in patients with a history of myocardial infarction and elevated high-sensitivity C-reactive protein, the presence of CHIP variant TET2 clones may predispose patients to improved outcomes with targeted anti-inflammatory therapy.
Wu D, Hu D, Chen H, Shi G, Fetahu IS, Wu F, et al. Glucose-regulated phosphorylation of TET2 by AMPK reveals a pathway linking diabetes to cancer. Nature. 2018;559(7715):637–41.
Cimmino L, Dolgalev I, Wang Y, Yoshimi A, Martin GH, Wang J, et al. Restoration of TET2 function blocks aberrant self-renewal and leukemia progression. Cell. 2017;170(6):1079–95 e20.
Morgan PK, Fang L, Lancaster GI, Murphy AJ. Hematopoiesis is regulated by cholesterol efflux pathways and lipid rafts: connections with cardiovascular diseases. J Lipid Res. 2020;61(5):667–75.
Dharan NJ, Yeh P, Bloch M, Yeung MM, Baker D, Guinto J, et al. HIV is associated with an increased risk of age-related clonal hematopoiesis among older adults. Nat Med. 2021;27(6):1006–11.
Cremer S, Kirschbaum K, Berkowitsch A, John D, Kiefer K, Dorsheimer L, et al. Multiple somatic mutations for clonal hematopoiesis are associated with increased mortality in patients with chronic heart failure. Circ Genom Precis Med. 2020;13(4): e003003.
Acknowledgements
We thank Armin Schweitzer, Saarland University Hospital, for his help in preparing the figure of this manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Cardiovascular Genomics
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Haring, B., Wissel, S. & Manson, J.E. Somatic Mutations and Clonal Hematopoiesis as Drivers of Age-Related Cardiovascular Risk. Curr Cardiol Rep 24, 1049–1058 (2022). https://doi.org/10.1007/s11886-022-01724-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11886-022-01724-2