Abstract
Intravenous tissue plasminogen activator is the only proven therapy for acute ischemic stroke. Not enough patients are eligible for treatment and additional new therapies are needed. Recently, laser technology has been applied to acute ischemic stroke. This noninvasive technique uses near-infrared wavelengths applied to the scalp within 24 h of symptom onset. The mechanism is incompletely understood but may involve increased mitochondrial adenosine triphosphate production. Animal models demonstrated safety and efficacy warranting randomized controlled trials in humans. NEST-1 (phase 2) and NEST-2 (phase 3) confirmed the safety of transcranial laser therapy, although efficacy was not found in NEST-2. Pooled analysis of NEST-1 and NEST-2 revealed a significantly improved success rate in patients treated with laser therapy. Further phase 3 testing is planned and may create a new paradigm for the treatment of acute ischemic stroke.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stroke affects nearly three quarters of a million people each year in the United States, of which more than three quarters are ischemic [1]. It is the leading cause of adult disability and the third most common cause of death in industrialized nations [2]. Despite its prevalence, the only proven treatment for acute ischemic stroke is intravenous tissue plasminogen activator (tPA) [3], and although approved in 1995, frustratingly few patients receive therapy [4, 5]. There are several reasons for this. Initially, neurologists and emergency medicine physicians were tentative to administer tPA because of the perceived risk of causing symptomatic intracranial hemorrhage (sICH). Data revealing an extremely low incidence of sICH addressed many of these concerns [6], although skepticism still exists in the emergency medicine community [7]. Nonetheless, the single largest factor currently limiting tPA use is that, per the NINDS study protocol, it must be administered within 3 h of symptom onset [3, 8]. The vast majority of stroke patients are not evaluated in this limited time window and although recent literature supports a modest benefit at 4.5 h from symptom onset [9, 10], under 5% of stroke patients currently receive tPA [4, 5]. Thus, there is a need for new therapies that can extend the treatment window.
History
Interactions between light and biology have been recognized since the concept of photosynthesis originated in the 17th century. Priestley, among others, elucidated the influence of sunlight on plants [11], and by the 1770s the basic reaction incorporating water and carbon dioxide uptake was outlined. Radiation remained poorly understood until the 1900s when Albert Einstein published his groundbreaking paper on photoelectric effects and concepts of absorption, spontaneous emission, and stimulated emission of electromagnetic radiation [12]. Five decades later the term “laser” (light amplification by stimulated emission of radiation) was coined and shortly thereafter the first human-made laser was created in 1960 [13].
We now understand that photobiological reactions such as photosynthesis entail the absorption of a specific wavelength of light by a specific molecule. These molecules, or photoreceptors, become electronically excited by absorbing light and subsequently participate in a metabolic reaction unrelated to the original light response. Thus, a laser is simply a tool that uses a light source to excite these molecules; however, the biological effects of any laser are wavelength specific when not simply used for tissue destruction.
Since inception, laser technology has been applied to a broad spectrum of industries and professions, but some of the most meaningful applications have been in medicine. The process began in the 1960s but lasers have infiltrated many fields of medicine. Dermatology, ophthalmology, dentistry, otolaryngology, cardiology, and neurology are only a few of the fields that have benefitted, and in some cases lasers have revolutionized the way we treat patients [14–16]. However, until now lasers’ medical applications have been confined to their ability to selectively ablate tissue.
Mechanism
Perhaps the most obvious question with transcranial laser therapy (TLT) is regarding the mechanism of action. It is known how lasers can cut or cauterize, but how a laser applied to the scalp during an ischemic stroke can improve outcomes is more difficult to comprehend.
Several postulated mechanisms seem unlikely. Heat production, although closely associated with lasers, did not appreciably elevate brain temperature in preclinical studies, suggesting that photothermal effects do not play a role [17•]. Hemodynamic effects (ie, recanalization and augmented collateral blood flow) are similarly unlikely because the interval from symptom onset to treatment extends to 24 h. This is much longer than with intravenous tPA in which recanalization does not improve outcomes when administered even 6 h after symptom onset [18–20]. Furthermore, final infarct volumes were not different between treated and untreated rat models [21].
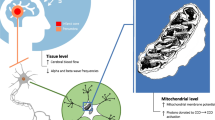
The most understood mechanism of action involves the relationship between lasers and cytochrome c oxidase. The cytochrome c oxidase enzyme complex contains two copper centers, the first of which contains a broad wavelength absorption peak when oxidized. Cytochrome c oxidase is located in the inner mitochondrial membrane and plays a central role in eukaryotic cells. As a terminal enzyme in the respiratory chain, it delivers protons across the inner mitochondrial membrane and enables the formation of adenosine triphosphate (ATP) by oxidative phosphorylation [22].
For several years, enhanced mitochondrial function with subsequent preservation of the ischemic penumbra and improved outcomes has been the hypothesized mechanism. Only recently has this been demonstrated using an in vivo model. Lapchak and De Taboada [23] were the first to demonstrate that TLT substantially increased cortical ATP production in an embolic stroke model. This same model was used previously to establish that TLT can improve clinical rating scores in rabbits and was similar to the model used to provide preliminary data for the NEST-1 and NEST-2 trials. After establishing decreased cortical ATP after embolization, consistent with the hypothesis of energy depletion after an ischemic event, laser therapy was found to attenuate embolization-induced decreases in cortical ATP content (P < 0.05). TLT did not completely normalize cortical ATP content but it increased overall ATP content by 41%. The authors hypothesized that this amount should be sufficient to improve or maintain mitochondrial function, improve neuronal survival, and produce behavioral improvement [23].
Neurogenesis or central nervous system (CNS) plasticity may be an additional mechanism of action contributing toward improved outcomes. Scientific dogma since the time of Cajal has held that neurogenesis does not occur in humans, but new research is challenging this concept [24]. Noting that improvement in neurologic outcome may not be evident for 2–4 weeks in the post-stroke rat model, it has been postulated that such delayed benefits may be due, in part, from induction of neurogenesis and migration of neurons [25]. This is supported by the absence of a reduction in lesion volume in TLT-treated rats and a significantly elevated number of newly formed neuronal cells in the ipsilateral subventricular zone. The presence of a large number of cells that positively react to doublecortin (a microtubule protein expressed in migrating neuroblasts) further suggests that increased numbers of cells migrating to the infarcted area contribute to the improved functional performance of the laser-treated rats. CNS insults trigger an increase in progenitor cell proliferation and migration to the damaged areas; infrared laser energy appears to further augment this process [21].
Mechanisms such as apoptosis have also been implicated. Carnevalli et al. [26] investigated the effects of laser therapy on the mitochondria, nucleus, and cytoskeleton by using specific fluorescent probes. Treated cells showed increased levels of cellular division, which was assessed by analyzing the cytoskeleton and the chromosomes. Moreover, cells maintained with nutritional deficiencies demonstrated membrane and genetic material that was more preserved compared with the controls. Therefore, TLT may prevent apoptosis and improve outcomes by exerting a neuroprotective effect, although these exact mechanisms are poorly understood [26].
Various ischemic models including traumatic brain injury, cardiac muscle ischemia, and skeletal muscle ischemia have revealed profound protective effects of laser therapy. Additional mechanisms thought to factor into benefits of laser therapy are increases in total antioxidants, angiogenesis, heat shock proteins, inhibition of nitric oxide synthase, and changes in cerebral microcirculation [27]. The precise mechanisms and cascades are not well defined, but it seems likely that many factors are intertwined and contribute toward the overall beneficial effects.
Animal Models
Applying laser technology to acute ischemic strokes began with animal models. In 2004, Lapchak et al. [28] used the rabbit small clot embolic stroke model and applied a gallium-arsenic diode near-infrared laser within 6 h of symptom onset. Significant and sustained improvement was demonstrated in behavioral function measured 24 h after treatment and again at 21 days from stroke onset [29]. Similar studies confirmed this beneficial effect despite using a rodent and a different model of inducing strokes (rat middle cerebral artery occlusion) [21]. There were differences in the treatment times for which TLT was effective, but both models resulted in statistically significant behavioral improvement that warranted human trials.
Clinical Trials
The NEST-1 was a prospective, multicenter, international, double-blind, randomized, placebo-controlled trial. The principal goals were to examine the safety and efficacy of TLT administered within 24 h of symptom onset. The NeuroThera Laser System (Photothera, Carlsbad, CA) is a noninvasive tool using the near-infrared portion of the electromagnetic spectrum with a laser wavelength that is invisible to the naked eye (808 nm). These low-energy (10-mW/cm²) lasers were applied at 20 predetermined locations on the scalp with 2 min of irradiation at each site. The treatment protocol lasted over 1 h and was applied consistently and independent of the vascular occlusion site. The NIHSS score, collapsed into a binary outcome, was prospectively identified as the primary end point; success occurred as a 90-day NIHSS score 0 to 1 or as a decrease in NIHSS score of ≥9 points from baseline to 90 days. Secondary outcome measures included the modified Rankin Scale (mRS), Barthel Index, and Glasgow Outcome Scale.
A total of 120 patients were randomized in a 2:1 ratio. Seventy-nine patients received active therapy at a median of 16 h from stroke onset, resulting in 70% of TLT-treated patients achieving a positive binary NIHSS outcome compared with 51% of the control group (P = 0.035). These results were further confirmed after controlling for age, sex, time to treatment, baseline severity, and previous stroke. Among TLT-treated patients, 38% achieved a final NIHSS score of 0 to 1 and improved by ≥9 points compared with 29% of placebo-treated patients. Secondary outcome measures also revealed that patients who received active treatment had improved outcomes [30•].
The encouraging phase 2 findings served as a basis for NEST-2, a phase 3 trial. This was a prospective, double-blind, randomized, placebo-controlled study that enrolled 660 patients. Inclusion criteria specified patients between 40 and 90 years old, prestroke mRS less than 3, baseline NIHSS score between 7 and 22, clinical diagnosis of ischemic stroke within 24 h but not treated with tPA, and no evidence of intracranial hemorrhage. Patients were randomized in a 1:1 ratio to receive TLT or placebo, and all patients underwent the identical procedure used in NEST-1. Unlike in NEST-1, the primary efficacy outcome measure was the 90-day dichotomous mRS, with success defined as a score of 0 to 2 or failure from 3 to 6.
Of the 660 patients, 331 received TLT and 327 received placebo therapy. In the TLT group, 120 (36.3%) achieved successful outcomes versus 101 (30.9%) in the control group (P = 0.094). Outcomes showed a trend toward better outcomes with TLT, but ultimately these failed to reach statistical significance (P < 0.05). Furthermore, subset analysis did not indicate that there was a time-to-treatment effect within 24 h after symptom onset.
Although we do not have prestroke mRS data from the two trials to compare the degree of disability upon entrance to the study, the initial NIHSS scores suggest that patients with more severe deficits were enrolled in the NEST-2 trial. There was a significant difference in the initial NIHSS scores between NEST-1 (mean NIHSS, 12.2; SD, 3.9) and NEST-2 (mean NIHSS, 13.1; SD, 4.6).
On post hoc analysis, after those with the most severe strokes (NIHSS, 16–22) were excluded, a statistically significant benefit was found. For these remaining 434 patients, the dichotomous mRS success rate of TLT showed an absolute improvement rate of 9.7% (51.6% TLT vs 41.9% sham) [17•]. Post hoc analysis must be viewed with skepticism, but TLT may require identification of the optimal patient group to statistically prove its benefits. When explaining why NEST-2 was unsuccessful in reaching statistical significance, the authors likened it to the obstacles encountered when developing tPA.
Several large randomized studies with intravenous tPA failed to achieve statistical significance despite highly suggestive trends toward efficacy [18, 19, 31]. Only after a specific treatment population and treatment time window were identified was the benefit of tPA clearly established. The fine-tuning process for tPA lasted many years, but the strong trend of good clinical outcomes combined with an excellent safety profile has fostered optimism the wait will be far shorter for TLT.
Pooled Analysis
To further analyze the two trials, we performed a retrospective analysis of prospectively collected data. Just as in NEST-2, binary mRS 90-day end point with success (mRS, 0–2) and failure (mRS, 3–6) was the primary efficacy outcome measure used. The null hypothesis that improved outcomes were not different between TLT and sham was tested by using logistic regression with two prespecified covariates (baseline stroke severity and time from stroke onset to randomization). Combining patients from the two studies provided 778 patients (120 from NEST-1, 658 from NEST-2) and the success rate in the TLT group was strongly significant compared with the sham group (P = 0.003; OR, 1.67; 95% CI, 1.19–2.35).
Safety
Although NEST-2 did not reach significance in its primary efficacy outcome measure, it did suggest that TLT was extremely safe. NEST-2 included a broad range of stroke patients regarding baseline severity, prestroke disability, and time to treatment and demonstrated that mortality rates, serious adverse event (SAE) rates, and adverse events were virtually identical. The TLT group had 58 (17.5%) deaths and 125 (37.8%) SAEs compared with 57 (17.4%) deaths and 137 (41.8%) SAEs in the placebo group. No SAEs were directly attributable to TLT. Hemorrhagic transformation at day 5 occurred in 49 (14.8%) in the TLT group and 56 (17.1%) in the placebo group [17•]. No differences in safety outcomes were found between the groups confirming the safety analysis of previous studies [30•].
Future Studies
The future of TLT requires a third randomized double-blind prospective study (NEST-3). Based on experience from NEST-1 and NEST-2, the protocol for NEST-3 will likely alter inclusion and exclusion criteria (baseline mRS, baseline NIHSS) to identify those who will benefit the most. Simultaneously, the combination of TLT and thrombolytics is being tested in a separate study. A recently completed trial using rabbits and an embolic model tested TLT applied after administration of intravenous tPA. The results showed that this combination did not adversely affect hemorrhage rate, volume, or survival compared with tPA alone [32•]. It was only intended to test safety, but theoretically the different mechanisms of action of TLT and tPA could synergistically treat strokes. Based on this study a clinical trial testing TLT plus tPA was recommended and is on the horizon.
Conclusions
The application of TLT in acute ischemic stroke is in its early stages. Compelling evidence that it is safe and effective has led to the tantalizing possibility of significantly expanding the treatment window and changing the paradigm for stroke treatment. Confirmatory studies are needed, and to this end, protocols are currently being developed that identify the optimal laser settings, patient population, and time to treatment. Projecting further, it will be fascinating to see if TLT and tPA can be used in combination to more effectively treat strokes.
Abbreviations
- NEST-1:
-
NeuroThera Effectiveness and Safety Trial-1
- NEST-2:
-
NeuroThera Effectiveness and Safety Trial-2
- NEST-3:
-
NeuroThera Effectiveness and Safety Trial-3
- NIHSS:
-
National Institutes of Health Stroke Scale
- NINDS:
-
National Institute of Neurological Disorders and Stroke
References
Papers of particular interest, published recently, have been highlighted as follows: • Of importance
Lloyd-Jones D, Adams R, Carnethon M, et al.: Heart disease and stroke statistics—2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2009, 119:480–486.
Thom T, Haase N, Rosamond W, et al.: Heart disease and stroke statistics—2006 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2006, 113:e85–e151.
Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group [no authors listed]. N Engl J Med 1995, 333:1581–1587.
Kidwell CS, Shephard T, Tonn S, et al.: Establishment of primary stroke centers: a survey of physician attitudes and hospital resources. Neurology 2003, 60:1452–1456.
Kleindorfer D, Lindsell CJ, Brass L, et al.: National US estimates of recombinant tissue plasminogen activator use: ICD-9 codes substantially underestimate. Stroke 2008, 39:924–928.
Saver JL: Hemorrhage after thrombolytic therapy for stroke: the clinically relevant number needed to harm. Stroke 2007, 38:2279–2283.
Hoffman JR, Schriger DL: A graphic reanalysis of the NINDS trial. Ann Emerg Med 2009, 54:329–336, 336.e1–336.e35.
Alberts MJ: tPA in acute ischemic stroke: United States experience and issues for the future. Neurology 1998, 51:S53–S55.
Hacke W, Kaste M, Bluhmki E, et al.: Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 200, 359:1317–1329.
Hacke W, Donnan G, Fieschi C, et al.: Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet 2004, 363:768–774.
Priestley J: Experiments and Observations on Different Kinds of Air. Edited by Bowyer W, Nichols J. London: Oxford University; 1774.
Einstein A: Zur quantentheorie der strahlung [on the quantum theory of radiation]. Phys 1917, 18:121–128.
Maiman T: Simulated optical radiation in ruby. Nature 1960:493.
Solomon KD, Fernandez de Castro LE, Sandoval HP, et al.: Lasik world literature review: quality of life and patient satisfaction. Ophthalmology 2009, 116:691–701.
Spencer JM, Hadi SM: The excimer lasers. J Drugs Dermatol 2004, 3:522–525.
Mavrogiannis M, Thomason JM, Seymour RA: Lasers in periodontology. Dent Update 2004, 31:535–538, 541–542, 545–547.
• Zivin JA, Albers GW, Bornstein N, et al.: Effectiveness and safety of transcranial laser therapy for acute ischemic stroke. Stroke 2009, 40:1359–1364. This is the first phase 3 study of TLT; safety was demonstrated but efficacy did not meet formal statistical significance.
Hacke W, Kaste M, Fieschi C, et al.: Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke: the European Cooperative Stroke Study (ECASS). JAMA 1995, 274:1017–1025.
Hacke W, Kaste M, Fieschi C, et al.: Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian Acute Stroke Study Investigators. Lancet 1998, 352:1245–1251.
Clark WM, Albers GW: The ATLANTIS rt-PA (Alteplase) Acute Stroke Trial: final results. Stroke 1999, 30:234.
Oron A, Oron U, Chen J, et al.: Low-level laser therapy applied transcranially to rats after induction of stroke significantly reduces long-term neurological deficits. Stroke 2006, 37:2620–2624.
Streeter J, De Taboada L, Oron U: Mechanisms of action of light therapy for stroke and acute myocardial infarction. Mitochondrion 2004, 4:569–576.
Lapchak PA, De Taboada L: Transcranial near infrared laser treatment (NILT) increases cortical adenosine-5'-triphosphate (ATP) content following embolic strokes in rabbits. Brain Res 2009 Oct 23 (Epub ahead of print).
Shen J, Xie L, Mao X, et al.: Neurogenesis after primary intracerebral hemorrhage in adult human brain. J Cereb Blood Flow Metab 2008, 28:1460–1468.
Detaboada L, Ilic S, Leichliter-Martha S, et al.: Transcranial application of low-energy laser irradiation improves neurological deficits in rats following acute stroke. Lasers Surg Med 2006, 38:70–73.
Carnevalli CM, Soares CP, Zangaro RA, et al.: Laser light prevents apoptosis in Cho K-1 cell line. J Clin Laser Med Surg 2003, 21:193–196.
Oron A, Oron U, Streeter J, et al.: Low-level laser therapy applied transcranially to mice following traumatic brain injury significantly reduces long-term neurological deficits. J Neurotrauma 2007, 24:651–656.
Lapchak PA, Wei J, Zivin JA: Transcranial infrared laser therapy improves clinical rating scores after embolic strokes in rabbits. Stroke 2004, 35:1985–1988.
Lapchak PA, Salgado KF, Chao CH, Zivin JA: Transcranial near-infrared light therapy improves motor function following embolic strokes in rabbits: an extended therapeutic window study using continuous and pulse frequency delivery modes. Neuroscience 2007, 148:907–914.
• Lampl Y, Zivin JA, Fisher M, et al.: Infrared laser therapy for ischemic stroke: a new treatment strategy: results of the NeuroThera Effectiveness and Safety Trial-1 (NEST-1). Stroke 2007, 38:1843–1849. This is the first phase 2 study with TLT that demonstrated safety and effectiveness.
Clark WM, Wissman S, Albers GW, et al.: Recombinant tissue-type plasminogen activator (Alteplase) for ischemic stroke 3 to 5 hours after symptom onset. The ATLANTIS Study: a randomized controlled trial. Alteplase Thrombolysis for Acute Noninterventional Therapy in Ischemic Stroke. JAMA 1999, 282:2019–2026.
• Lapchak PA, Han MK, Salgado KF, et al.: Safety profile of transcranial near-infrared laser therapy administered in combination with thrombolytic therapy to embolized rabbits. Stroke 2008, 39:3073–3078. This is the first study to demonstrate that TLT could be administered safely with tPA in rabbits.
Disclosure
Dr. Justin Zivin is the Principal Investigator of NEST-2, is a consultant for Photothera Inc., and is on the Scientific Advisory Board of Photothera Inc. No other potential conflicts of interest relevant to this article were reported.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Stemer, A.B., Huisa, B.N. & Zivin, J.A. The Evolution of Transcranial Laser Therapy for Acute Ischemic Stroke, Including a Pooled Analysis of NEST-1 and NEST-2. Curr Cardiol Rep 12, 29–33 (2010). https://doi.org/10.1007/s11886-009-0071-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11886-009-0071-3