Abstract
Purpose of Review
This review provides an overview of genetic and non-genetic causes of premature coronary artery disease (pCAD).
Recent Findings
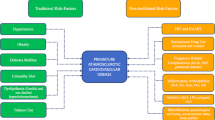
pCAD refers to coronary artery disease (CAD) occurring before the age of 65 years in women and 55 years in men. Both genetic and non-genetic risk factors may contribute to the onset of pCAD. Recent advances in the genetic epidemiology of pCAD have revealed the importance of both monogenic and polygenic contributions to pCAD. Familial hypercholesterolemia (FH) is the most common monogenic disorder associated with atherosclerotic pCAD. However, clinical overreliance on monogenic genes can result in overlooked genetic causes of pCAD, especially polygenic contributions. Non-genetic factors, notably smoking and drug use, are also important contributors to pCAD. Cigarette smoking has been observed in 25.5% of pCAD patients relative to 12.2% of non-pCAD patients. Finally, myocardial infarction (MI) associated with spontaneous coronary artery dissection (SCAD) may result in similar clinical presentations as atherosclerotic pCAD.
Summary
Recognizing the genetic and non-genetic causes underlying pCAD is important for appropriate prevention and treatment. Despite recent progress, pCAD remains incompletely understood, highlighting the need for both awareness and research.

Similar content being viewed by others
Data Availability
No datasets were generated or analyzed during the current study.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015; 385(9963):117–171
Khera AV, et al. Genetic risk, adherence to a healthy lifestyle, and coronary disease. N Engl J Med. 2016;375(24):2349–58.
Malakar AK, Choudhury D, Halder B, Paul P, Uddin A, Chakraborty S. A review on coronary artery disease, its risk factors, and therapeutics. J Cell Physiol. 2019;234(10):16812–23.
Thygesen K, et al. Fourth universal definition of myocardial infarction (2018). Circulation. 2018;138(20):e618–51.
Arnett DK et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019 https://doi.org/10.1161/CIR.0000000000000678
Wilmot KA, O’Flaherty M, Capewell S, Ford ES, Vaccarino V. Coronary heart disease mortality declines in the United States from 1979 through 2011: evidence for stagnation in young adults, especially women. Circulation. 2015;132(11):997–1002.
Zheng ZJ, Croft JB, Giles WH, Mensah GA. Sudden cardiac death in the United States, 1989 to 1998. Circulation. 2001;104(18):2158–63.
Pejic RN. Familial hypercholesterolemia. Ochsner J. 2014;14(4)669-672
Ison HE, Clarke SL, and Knowles JW. Familial hypercholesterolemia. In: Adam MP, Mirzaa GM, Pagon RA, Wallace SE, Bean LJH, Gripp KW, and Amemiya A, Editors. GeneReviews, Seattle (WA): University of Washington, Seattle, 2014
Vallejo-Vaz AJ, et al. Global perspective of familial hypercholesterolaemia: a cross-sectional study from the EAS Familial Hypercholesterolaemia Studies Collaboration (FHSC). Lancet. 2021;398(10312):1713–25.
Albiero R, Seresini G. Atherosclerotic spontaneous coronary artery dissection (A-SCAD) in a patient with COVID-19: case report and possible mechanisms. Eur Heart J Case Rep. 2020;4(FI1):1–6.
Klarin D, Natarajan P. Clinical utility of polygenic risk scores for coronary artery disease. Nat Rev Cardiol. 2021;19(5):291–301.
Lali R, Cui E, Ansarikaleibari A, Pigeyre M, Paré G. Genetics of early-onset coronary artery disease: from discovery to clinical translation. Curr Opin Cardiol. 2019;34(6):706–13.
Zdravkovic S, Wienke A, Pedersen NL, Marenberg ME, Yashin AI, De Faire U. Heritability of death from coronary heart disease: a 36-year follow-up of 20 966 Swedish twins. J Intern Med. 2002;252(3):247–54.
Di Scipio M, et al. A versatile, fast and unbiased method for estimation of gene-by-environment interaction effects on biobank-scale datasets. Nat Commun. 2023;14(1):5196.
Khera AV, Kathiresan S. Genetics of coronary artery disease: discovery, biology and clinical translation. Nat Rev Genet. 2017;18(6):331–44.
Sayols-Baixeras S, Lluís-Ganella C, Lucas G, Elosua R. Pathogenesis of coronary artery disease: focus on genetic risk factors and identification of genetic variants. Appl Clin Genet. 2014;7:15–32.
Marenberg ME, Risch N, Berkman LF, Floderus B, de Faire U. Genetic susceptibility to death from coronary heart disease in a study of twins. N Engl J Med. 1994;330(15):1041–6.
Scheuner MT, Whitworth WC, McGruder H, Yoon PW, Khoury MJ. Familial risk assessment for early-onset coronary heart disease. Genet Med. 2006;8(8):525–31.
Scheuner MT, Whitworth WC, McGruder H, Yoon PW, Khoury MJ. Expanding the definition of a positive family history for early-onset coronary heart disease. Genet Med. 2006;8(8):491–501.
Genetic Alliance and New York-Mid-Atlantic Consortium for Genetic and Newborn Screening Services, Understanding genetics: a New York, mid-Atlantic guide for patients and health professionals. Lulu.com, 2009
Hu P et al. Prevalence of familial hypercholesterolemia among the general population and patients with atherosclerotic cardiovascular disease Circulation. 2020. https://doi.org/10.1161/CIRCULATIONAHA.119.044795. This work reassessed the global prevalence of familial hypercholesterolemia through systematic review and meta-analysis, after observing previous estimates likely underreported the disease. The current overall global prevalence is reported to be 1:311.
Shah AS and Wilson DP. Genetic disorders causing hypertriglyceridemia in children and adolescents. MDText.com, Inc., 2023
Goyal A, Cusick AS, and Reilly E. Familial hypertriglyceridemia. StatPearls Publishing, 2023
de Beer F, et al. Expression of type III hyperlipoproteinemia in apolipoprotein E2 (Arg158 –> Cys) homozygotes is associated with hyperinsulinemia. Arterioscler Thromb Vasc Biol. 2002;22(2):294–9.
Myrie SB, Steiner RD, Mymin D. Sitosterolemia. Seattle: University of Washington; 2020.
Alshaikhli A and Vaqar S. Tangier disease. StatPearls Publishing, 2023
Burnett JR, Hooper AJ, McCormick SPA, Hegele RA. Tangier Disease. Seattle: University of Washington; 2019.
Faeh D, Chiolero A, Paccaud F. Homocysteine as a risk factor for cardiovascular disease: should we (still) worry about? Swiss Med Wkly. 2006;136(47–48):745–56.
Alrefaei AF, Abu-Elmagd M. LRP6 receptor plays essential functions in development and human diseases. Genes. 2022;13(1):120.
Singh R, et al. Rare nonconservative LRP6 mutations are associated with metabolic syndrome. Hum Mutat. 2013;34(9):1221–5.
Mani A, et al. LRP6 mutation in a family with early coronary disease and metabolic risk factors. Science. 2007;315(5816):1278–82.
Xu Y, et al. Functional analysis LRP6 novel mutations in patients with coronary artery disease. PLoS ONE. 2014;9(1):e84345.
Srivastava R, Zhang J, Go G-W, Narayanan A, Nottoli TP, Mani A. Impaired LRP6-TCF7L2 activity enhances smooth muscle cell plasticity and causes coronary artery disease. Cell Rep. 2015;13(4):746–59.
Morris CA. Williams syndrome. In: Adam MP, Mirzaa GM, Pagon RA, Wallace SE, Bean LJH, Gripp KW, and Amemiya A, Editors., GeneReviews®. Seattle (WA): University of Washington, Seattle; 1999.
Dadlani GH, et al. Cardiovascular screening in Williams syndrome. Prog Pediatr Cardiol. 2020;58:101267.
Sickles CK and Gross GP. Progeria. StatPearls Publishing; 2022
Gordon LB, Ted Brown W, and Collins FS. Hutchinson-Gilford progeria syndrome. University of Washington, Seattle; 2019
Olive M, et al. Cardiovascular pathology in Hutchinson-Gilford progeria: correlation with the vascular pathology of aging. Arterioscler Thromb Vasc Biol. 2010;30(11):2301–9.
Prakash A, et al. Cardiac abnormalities in patients with Hutchinson-Gilford progeria syndrome. JAMA Cardiol. 2018;3(4):326–34.
Germain DP. Pseudoxanthoma elasticum. Orphanet J Rare Dis. 2017;12(1):1–13.
G. Lefthériotis et al. The vascular phenotype in Pseudoxanthoma elasticum and related disorders: contribution of a genetic disease to the understanding of vascular calcification. Front Genet. 2013;4. https://doi.org/10.3389/fgene.2013.00004.
Vikulova DN, Trinder M, Mancini GBJ, Pimstone SN, Brunham LR. Familial hypercholesterolemia, familial combined hyperlipidemia, and elevated lipoprotein(a) in patients with premature coronary artery disease. Can J Cardiol. 2021;37(11):1733–42.
Gratton J, Humphries SE, Futema M. Prevalence of FH-causing variants and impact on LDL-C concentration in European, South Asian, and African Ancestry groups of the UK Biobank-brief report. Arterioscler Thromb Vasc Biol. 2023;43(9):1737–42.
Toft-Nielsen F, Emanuelsson F, Benn M. Familial hypercholesterolemia prevalence among ethnicities-systematic review and meta-analysis. Front Genet. 2022;13:840797.
Alnouri F et al. Xanthomas can be misdiagnosed and mistreated in homozygous familial hypercholesterolemia patients: a call for increased awareness among dermatologists and health care practitioners. Glob Heart. 2020;15(1). https://doi.org/10.5334/gh.759
Civeira F, et al. Tendon xanthomas in familial hypercholesterolemia are associated with cardiovascular risk independently of the low-density lipoprotein receptor gene mutation. Arterioscler Thromb Vasc Biol. 2005. https://doi.org/10.1161/01.ATV.0000177811.14176.2b.
Bell A and Shreenath AP. Xanthoma. In: StatPearls, StatPearls Publishing, 2022
Brunham LR, et al. Canadian cardiovascular society position statement on familial hypercholesterolemia: Update 2018. Can J Cardiol. 2018;34(12):1553–63.
Abul-Husn NS et al. Genetic identification of familial hypercholesterolemia within a single U.S. health care system. Science. 2016. https://doi.org/10.1126/science.aaf7000
Sharifi M, Futema M, Nair D, Humphries SE. Genetic architecture of familial hypercholesterolaemia. Curr Cardiol Rep. 2017;19(5):44.
Abifadel M, et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet. 2003;34(2):154–6.
Garcia CK, et al. Autosomal recessive hypercholesterolemia caused by mutations in a putative LDL receptor adaptor protein. Science. 2001;292(5520):1394–8.
Berge KE, et al. Accumulation of dietary cholesterol in sitosterolemia caused by mutations in adjacent ABC transporters. Science. 2000;290(5497):1771–5.
Khera AV, et al. Diagnostic yield and clinical utility of sequencing familial hypercholesterolemia genes in patients with severe hypercholesterolemia. J Am Coll Cardiol. 2016;67(22):2578–89.
Reiner Ž. Hypertriglyceridaemia and risk of coronary artery disease. Nat Rev Cardiol. 2017;14(7):401–11.
Berglund L, et al. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice Guideline. J Clin Endocrinol Metab. 2012;97(9):2969–89.
Santamarina-Fojo S. The familial hyperchylomicronemia syndrome. JAMA. 1991;265(7):904.
Hulley SB, Rosenman RH, Bawol RD, Brand RJ. Epidemiology as a guide to clinical decisions. The association between triglyceride and coronary heart disease. N Engl J Med. 1980;302(25):1383–9.
Gotto AM Jr. Triglyceride as a risk factor for coronary artery disease. Am J Cardiol. 1998;82(9A):22Q-25Q.
Pradhan AD et al. Triglyceride lowering with pemafibrate to reduce cardiovascular risk. N Engl J Med. 2022.https://doi.org/10.1056/NEJMoa2210645
The AIM-HIGH Investigators. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. 2011. https://doi.org/10.1056/NEJMoa1107579
H. N. Ginsberg et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010; 362(17). https://doi.org/10.1056/NEJMoa1001282
The HPS2-THRIVE Collaborative Group. Effects of extended-release niacin with laropiprant in high-risk patients. 2014. https://doi.org/10.1056/NEJMoa1300955
Musunuru K. Treating coronary artery disease: beyond statins, ezetimibe, and PCSK9 inhibition. Annu Rev Med. 2021; 72. https://doi.org/10.1146/annurev-med-080819-044918
Burgess S and Thompson SG. Mendelian randomization: methods for using genetic variants in causal estimation. CRC Press, 2015
Thomsen M, Varbo A, Tybjærg-Hansen A, Nordestgaard BG. Low nonfasting triglycerides and reduced all-cause mortality: a Mendelian randomization study. Clin Chem. 2014;60(5):737–46.
Holmes MV, et al. Mendelian randomization of blood lipids for coronary heart disease. Eur Heart J. 2015;36(9):539–50.
Khera AV, et al. Association of rare and common variation in the lipoprotein lipase gene with coronary artery disease. JAMA. 2017;317(9):937–46.
Do R, et al. Exome sequencing identifies rare LDLR and APOA5 alleles conferring risk for myocardial infarction. Nature. 2015;518(7537):102–6.
Jørgensen AB, Frikke-Schmidt R, Nordestgaard BG, Tybjærg-Hansen A. Loss-of-function mutations in APOC3 and risk of ischemic vascular disease. N Engl J Med. 2014;371(1):32–41.
Dewey FE, et al. Inactivating variants in ANGPTL4 and risk of coronary artery disease. N Engl J Med. 2016;374(12):1123–33.
Burnett JR, Hooper AJ, Hegele RA. “Familial lipoprotein lipase deficiency”, in GeneReviews® [Internet]. Seattle: University of Washington; 2017.
Hegele RA. APOC3 interference for familial chylomicronaemia syndrome. touchREV Endocrinol. 2022;18(2)82–83
Gaudet D, et al. Antisense inhibition of apolipoprotein C-III in patients with hypertriglyceridemia. N Engl J Med. 2015;373(5):438–47.
Pechlaner R, et al. Very-low-density lipoprotein-associated apolipoproteins predict cardiovascular events and are lowered by inhibition of APOC-III. J Am Coll Cardiol. 2017;69(7):789–800.
Hopkins PN, Wu LL, Hunt SC, Brinton EA. Plasma triglycerides and type III hyperlipidemia are independently associated with premature familial coronary artery disease. J Am Coll Cardiol. 2005;45(7):1003–12.
Bennet AM, et al. Association of apolipoprotein E genotypes with lipid levels and coronary risk. JAMA. 2007;298(11):1300–11.
[49] Genetic polymorphism in human apolipoprotein E. In: Methods in Enzymology, Academic Press, 1986, pp. 823–851
Koopal C, David Marais A, Westerink J, and Visseren FLJ. Autosomal dominant familial dysbetalipoproteinemia: a pathophysiological framework and practical approach to diagnosis and therapy. J Clin Lipidol. 2017; 11(1)12–23.e1
Pathogenesis of type III hyperlipoproteinemia (dysbetalipoproteinemia): questions, quandaries, and paradoxes. J Lipid Res. 1999;40(11)1933–1949
Mahley RW. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science. 1988;240(4852):622–30.
Remnant lipoprotein metabolism: key pathways involving cell-surface heparan sulfate proteoglycans and apolipoprotein E. J Lipid Res. 1999;40(1)1–16
Roy N, Gaudet D, and Brisson D. Palmar striated xanthomas in clinical practice. J Endocr Soc. 2022; 6(8)bvac103
C. Koopal et al. Vascular risk factors, vascular disease, lipids and lipid targets in patients with familial dysbetalipoproteinemia: a European cross-sectional study. Atherosclerosis. 2015;240(1) https://doi.org/10.1016/j.atherosclerosis.2015.02.046
Villeneuve S, Brisson D, Marchant NL, Gaudet D. The potential applications of apolipoprotein E in personalized medicine. Front Aging Neurosci. 2014;6:154.
Koopal C, Marais AD, Visseren FLJ. Familial dysbetalipoproteinemia: an underdiagnosed lipid disorder. Curr Opin Endocrinol Diabetes Obes. 2017;24(2):133–9.
Tada H, et al. Diagnosis and management of sitosterolemia 2021. J Atheroscler Thromb. 2021;28(8):791–801.
Bastida JM, Girós ML, Benito R, Janusz K, Hernández-Rivas JM, González-Porras JR. Sitosterolemia: diagnosis, metabolic and hematological abnormalities, cardiovascular disease and management. Curr Med Chem. 2019;26(37):6766–75.
Pang X, et al. Homocysteine induces the expression of C-reactive protein via NMDAr-ROS-MAPK-NF-κB signal pathway in rat vascular smooth muscle cells. Atherosclerosis. 2014;236(1):73–81.
Shenoy V, Mehendale V, Prabhu K, Shetty R, Rao P. Correlation of serum homocysteine levels with the severity of coronary artery disease. Indian J Clin Biochem. 2014;29(3):339–44.
Ganguly P, Alam SF. Role of homocysteine in the development of cardiovascular disease. Nutr J. 2015;14:6.
Zhang S, Bai Y-Y, Luo L-M, Xiao W-K, Wu H-M, Ye P. Association between serum homocysteine and arterial stiffness in elderly: a community-based study. J Geriatr Cardiol. 2014;11(1):32–8.
Moll S, Varga EA. Homocysteine and MTHFR mutations. Circulation. 2015;132(1):e6-9.
Ebbing M, et al. Mortality and cardiovascular events in patients treated with homocysteine-lowering B vitamins after coronary angiography: a randomized controlled trial. JAMA. 2008;300(7):795–804.
Antoniades C, Antonopoulos AS, Tousoulis D, Marinou K, Stefanadis C. Homocysteine and coronary atherosclerosis: from folate fortification to the recent clinical trials. Eur Heart J. 2009;30(1):6–15.
Bønaa KH, et al. Homocysteine lowering and cardiovascular events after acute myocardial infarction. N Engl J Med. 2006;354(15):1578–88.
Lonn E, et al. Homocysteine lowering with folic acid and B vitamins in vascular disease. N Engl J Med. 2006;354(15):1567–77.
Toole JF, et al. Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death: the Vitamin Intervention for Stroke Prevention (VISP) randomized controlled trial. JAMA. 2004;291(5):565–75.
Thériault S, Lali R, Chong M, Velianou JL, Natarajan MK, Paré G. Polygenic contribution in individuals with early-onset coronary artery disease. Circ Genom Precis Med. 2018;11(1):e001849.
Tam V, Patel N, Turcotte M, Bossé Y, Paré G, Meyre D. Benefits and limitations of genome-wide association studies. Nat Rev Genet. 2019;20(8):467–84.
Zuk O, et al. Searching for missing heritability: designing rare variant association studies. Proc Natl Acad Sci USA. 2014;111(4):E455–64.
Aragam KG, et al. Discovery and systematic characterization of risk variants and genes for coronary artery disease in over a million participants. Nat Genet. 2022;54(12):1803–15.
Matsunaga H, et al. Transethnic meta-analysis of genome-wide association studies identifies three new loci and characterizes population-specific differences for coronary artery disease. Circ Genom Precis Med. 2020;13(3):e002670.
Samani NJ, et al. Genomewide association analysis of coronary artery disease. N Engl J Med. 2007;357(5):443–53.
Helgadottir A, et al. A common variant on chromosome 9p21 affects the risk of myocardial infarction. Science. 2007;316(5830):1491–3.
McPherson R, et al. A common allele on chromosome 9 associated with coronary heart disease. Science. 2007;316(5830):1488–91.
Wellcome Trust Case Control Consortium. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447(7145):661–78.
Almontashiri NAM. The 9p21.3 risk locus for coronary artery disease: a 10-year search for its mechanism. J Taibah Univ Med Sci. 2017;12(3):199–204.
Ross R. Atherosclerosis–an inflammatory disease. N Engl J Med. 1999;340(2):115–26.
Zhuang J, et al. Methylation of p15INK4b and expression of ANRIL on chromosome 9p21 are associated with coronary artery disease. PLoS ONE. 2012;7(10):e47193.
Musunuru K. Enduring mystery of the chromosome 9p21.3 locus. Circ Cardiovasc Genet. 2013;6(2):224–5.
Jarinova O et al. Functional analysis of the chromosome 9p21.3 coronary artery disease risk locus. Arterioscler Thromb Vasc Biol. 2009;29(10). https://doi.org/10.1161/ATVBAHA.109.189522
Motterle A et al. Functional analyses of coronary artery disease associated variation on chromosome 9p21 in vascular smooth muscle cells. Hum Mol Genet. 2012; 21(18). https://doi.org/10.1093/hmg/dds224
Privé F, Arbel J, Aschard H, and Vilhjálmsson B J. Identifying and correcting for misspecifications in GWAS summary statistics and polygenic scores. bioRxiv. 2022;2021.03.29.437510. https://doi.org/10.1101/2021.03.29.437510.
Privé F, Arbel J, Vilhjálmsson BJ. LDpred2: better, faster, stronger. Bioinformatics. 2020;36(22–23):5424–31.
Jiang X, Holmes C, McVean G. The impact of age on genetic risk for common diseases. PLoS Genet. 2021;17(8):e1009723.
Patel AP, et al. A multi-ancestry polygenic risk score improves risk prediction for coronary artery disease. Nat Med. 2023;29(7):1793–803.
Khera AV, et al. Genome-wide polygenic scores for common diseases identify individuals with risk equivalent to monogenic mutations. Nat Genet. 2018;50(9):1219–24.
Fahed AC, et al. Polygenic background modifies penetrance of monogenic variants for tier 1 genomic conditions. Nat Commun. 2020;11(1):3635.
Mars N, et al. Polygenic and clinical risk scores and their impact on age at onset and prediction of cardiometabolic diseases and common cancers. Nat Med. Apr.2020;26(4):549–57.
Genomic risk prediction of coronary artery disease in 480,000 adults: implications for primary prevention. J Am Coll Cardiol. 2018;72(16)883–1893
Natarajan P, et al. Polygenic risk score identifies subgroup with higher burden of atherosclerosis and greater relative benefit from statin therapy in the primary prevention setting. Circulation. 2017;135(22):2091–101.
Mega JL, et al. Genetic risk, coronary heart disease events, and the clinical benefit of statin therapy. Lancet. 2015;385(9984):2264.
Lee S, Abecasis GR, Boehnke M, Lin X. Rare-variant association analysis: study designs and statistical tests. Am J Hum Genet. 2014;95(1):5–23.
Lali R, et al. Calibrated rare variant genetic risk scores for complex disease prediction using large exome sequence repositories. Nat Commun. Oct.2021;12(1):1–15. This work was the first to create rare variant polygenic risk scores for prediction of coronary artery disease. Despite the much lower power of rare variants relative to common variants, the rare variant polygenic risk score could identify 1.5% of the population with a greater than two-fold risk of CAD.
Sudlow C, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12(3):e1001779.
Uddin MdM et al. Clonal hematopoiesis of indeterminate potential, DNA methylation, and risk for coronary artery disease. Nat Commun. 2022;13(1). https://doi.org/10.1038/s41467-022-33093-3
Jaiswal S, et al. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N Engl J Med. 2017;377(2):111–21.
Stein A et al. Clonal hematopoiesis and cardiovascular disease: deciphering interconnections. Basic Res Cardiol. 2022;117(1). https://doi.org/10.1007/s00395-022-00969-w
Challen G, Goodell MA. Clonal hematopoiesis: mechanisms driving dominance of stem cell clones. Blood2020. https://doi.org/10.1182/blood.2020006510.10.1182/blood.2020006510
Mayerhofer E, et al. Prevalence and therapeutic implications of clonal hematopoiesis of indeterminate potential in young patients with stroke. Stroke. 2023;54(4):938–46.
Cobo I, Tanaka T, Glass CK, Yeang C. Clonal hematopoiesis driven by DNMT3A and TET2 mutations: role in monocyte and macrophage biology and atherosclerotic cardiovascular disease. Curr Opin Hematol. 2022;29(1):1–7.
Marnell CS, Bick A, Natarajan P. Clonal hematopoiesis of indeterminate potential (CHIP): linking somatic mutations, hematopoiesis, chronic inflammation and cardiovascular disease. J Mol Cell Cardiol. 2021;161:98–105.
Gumuser ED, et al. Clonal hematopoiesis of indeterminate potential predicts adverse outcomes in patients with atherosclerotic cardiovascular disease. J Am Coll Cardiol. 2023;81(20):1996–2009.
Kar SP, et al. Genome-wide analyses of 200,453 individuals yield new insights into the causes and consequences of clonal hematopoiesis. Nat Genet. 2022;54(8):1155–66.
Nikpay M, et al. A comprehensive 1,000 genomes-based genome-wide association meta-analysis of coronary artery disease. Nat Genet. 2015;47(10):1121–30.
Cole JH, Miller JI 3rd, Sperling LS, Weintraub WS. Long-term follow-up of coronary artery disease presenting in young adults. J Am Coll Cardiol. 2003;41(4):521–8.
Navas-Nacher EL, Colangelo L, Beam C, Greenland P. Risk factors for coronary heart disease in men 18 to 39 years of age. Ann Intern Med. 2001;134(6):433–9.
Petrie JR, Guzik TJ, Touyz RM. Diabetes, hypertension, and cardiovascular disease: clinical insights and vascular mechanisms. Can J Cardiol. 2018;34(5):575–84.
Jorge-Galarza E, et al. Control of blood pressure levels in patients with premature coronary artery disease: results from the Genetics of Atherosclerotic Disease study. J Clin Hypertens. 2020;22(7):1253–62.
Poorzand H, et al. Risk factors of premature coronary artery disease in Iran: a systematic review and meta-analysis. Eur J Clin Invest. 2019;49(7):e13124.
Malmberg K, Båvenholm P, Hamsten A. Clinical and biochemical factors associated with prognosis after myocardial infarction at a young age. J Am Coll Cardiol. 1994;24(3):592–9.
Maahs DM, et al. Cardiovascular disease risk factors in youth with diabetes mellitus: a scientific statement from the American Heart Association. Circulation. 2014;130(17):1532–58.
D’Agostino RB Sr, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117(6):743–53.
Koz C, et al. Conventional and non-conventional coronary risk factors in male premature coronary artery disease patients already having a low Framingham risk score. Acta Cardiol. 2008;63(5):623–8.
O’Sullivan JW, Raghavan S, Marquez-Luna C, Luzum JA, Damrauer SM, Ashley EA, O’Donnell CJ, Willer CJ, Natarajan P et al. Polygenic risk scores for cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2022;146(8)e93–e118
Becker DH and Gardner LB. Prevention in clinical practice. Springer Science & Business Media; 2012
Ridker PM, Buring JE, Rifai N, Cook NR. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score. JAMA. 2007;297(6):611–9.
Yusuf S et al. Cardiovascular risk and events in 17 low-, middle-, and high-income countries. 2014. https://doi.org/10.1056/NEJMoa1311890
Woodward M, Brindle P, Tunstall-Pedoe H. Adding social deprivation and family history to cardiovascular risk assessment: the ASSIGN score from the Scottish Heart Health Extended Cohort (SHHEC). Heart. 2007;93(2):172.
Hippisley-Cox J, Coupland C, and Brindle P. Development and validation of QRISK3 risk prediction algorithms to estimate future risk of cardiovascular disease: prospective cohort study. BMJ. 2017;357. https://doi.org/10.1136/bmj.j2099
Assmann G, Cullen P, and Schulte H. Simple scoring scheme for calculating the risk of acute coronary events based on the 10-year follow-up of the prospective cardiovascular Münster (PROCAM) study. Circulation. 2002;105(3). https://doi.org/10.1161/hc0302.102575
Palmieri L, et al. CUORE project: implementation of the 10-year risk score. Eur J Cardiovasc Prev Rehabil. 2011;18(4):642–9.
Sofogianni A, Stalikas N, Antza C, and Tziomalos K. Cardiovascular risk prediction models and scores in the era of personalized medicine. J Pers Med. 2022;12(7) https://doi.org/10.3390/jpm12071180. https://doi.org/10.3390/jpm12071180
DeFilippis AP, et al. An analysis of calibration and discrimination among multiple cardiovascular risk scores in a modern multiethnic cohort. Ann Intern Med. 2015;162(4):266–75.
Zeitouni M, et al. Risk factor burden and long-term prognosis of patients with premature coronary artery disease. J Am Heart Assoc. 2020. https://doi.org/10.1161/JAHA.120.017712.
Sivapalaratnam S, et al. Family history of premature coronary heart disease and risk prediction in the EPIC-Norfolk prospective population study. Heart. 2010;96(24):1985.
Bastuji-Garin S, et al. The Framingham prediction rule is not valid in a European population of treated hypertensive patients. J Hypertens. 2002;20(10):1973–80.
Brindle P, et al. Predictive accuracy of the Framingham coronary risk score in British men: prospective cohort study. BMJ. 2003;327(7426):1267.
Khan RJ, Stewart CP, Davis SK, Harvey DJ, Leistikow BN. The risk and burden of smoking related heart disease mortality among young people in the United States. Tob Induc Dis. 2015;13(1):16.
Sadeghian S, Graili P, Salarifar M, Karimi AA, Darvish S, Abbasi SH. Opium consumption in men and diabetes mellitus in women are the most important risk factors of premature coronary artery disease in Iran. Int J Cardiol. 2010;141(1):116–8.
Caliri AW, Tommasi S, Besaratinia A. Relationships among smoking, oxidative stress, inflammation, macromolecular damage, and cancer. Mutat Res - Rev Mut Res. 2021;787:108365.
Incalza MA, D’Oria R, Natalicchio A, Perrini S, Laviola L, Giorgino F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vascul Pharmacol. 2018;100:1–19.
Patel KM, et al. Macrophage sortilin promotes LDL uptake, foam cell formation, and atherosclerosis. Circ Res. 2015;116(5):789–96.
Sun R, Mendez D, Warner KE. Trends in nicotine product use among US adolescents, 1999–2020. JAMA Netw Open. 2021;4(8):e2118788.
Dutra LM and Glantz SA. E-cigarettes and national adolescent cigarette use: 2004–2014. Pediatrics. 2017;139(2). https://doi.org/10.1542/peds.2016-2450
Harrell MB, et al. Flavored e-cigarette use: characterizing youth, young adult, and adult users. Prev Med Rep. 2017;5:33–40.
Wang TW, Neff LJ, Park-Lee E, Ren C, Cullen KA, King BA. E-cigarette use among middle and high school students — United States, 2020. MMWR Surveill Summ. 2020;69(37):1310.
Leventhal AM, et al. Association of electronic cigarette use with initiation of combustible tobacco product smoking in early adolescence. JAMA. 2015;314(7):700.
Martinelli T, et al. Exploring the gateway hypothesis of e-cigarettes and tobacco: a prospective replication study among adolescents in the Netherlands and Flanders. Tob Control. 2023;32(2):170–8.
Matsuzawa Y, Lerman A. Endothelial dysfunction and coronary artery disease: assessment, prognosis, and treatment. Coron Artery Dis. 2014;25(8):713–24.
Kuntic M, et al. Short-term e-cigarette vapour exposure causes vascular oxidative stress and dysfunction: evidence for a close connection to brain damage and a key role of the phagocytic NADPH oxidase (NOX-2). Eur Heart J. 2020;41(26):2472–83.
von Hundelshausen P, et al. RANTES deposition by platelets triggers monocyte arrest on inflamed and atherosclerotic endothelium. Circulation. 2001;103(13):1772–7.
Badimon L, Badimon JJ, Penny W, Webster MW, Chesebro JH, Fuster V. Endothelium and atherosclerosis. J Hypertens Suppl. 1992;10(2):S43-50.
Wold LE, et al. Cardiopulmonary consequences of vaping in adolescents: a scientific statement from the American Heart Association. Circ Res. 2022;131(3):e70–82.
Dydyk AM, Jain NK, and Gupta M. Opioid use disorder. In: StatPearls [Internet], StatPearls Publishing;2023
Chang HY, Kharrazi H, Bodycombe D, Weiner JP, and Alexander GC. Healthcare costs and utilization associated with high-risk prescription opioid use: a retrospective cohort study. BMC Med. 2018;16(1). https://doi.org/10.1186/s12916-018-1058-y
Doshi R, et al. Frequency of cardiovascular events and in-hospital mortality with opioid overdose hospitalizations. Am J Cardiol. 2019;124(10):1528–33.
Krantz MJ, Palmer RB, Haigney MCP. Cardiovascular complications of opioid use: JACC state-of-the-art review. J Am Coll Cardiol. 2021;77(2):205–23.
Nakhaee S, Ghasemi S, Karimzadeh K, Zamani N, Alinejad-Mofrad S, and Mehrpour O. The effects of opium on the cardiovascular system: a review of side effects, uses, and potential mechanisms. Subst Abuse Treat Prev Policy 2020;15(1). https://doi.org/10.1186/s13011-020-00272-8. https://doi.org/10.1186/s13011-020-00272-8
Asgary S, Sarrafzadegan N, Naderi G-A, Rozbehani R. Effect of opium addiction on new and traditional cardiovascular risk factors: do duration of addiction and route of administration matter? Lipids Health Dis. 2008;7(1):42.
Ziaee M, Hajizadeh R, Khorrami A, Sepehrvand N, Momtaz S, Ghaffari S. Cardiovascular complications of chronic opium consumption: a narrative review article. Iran J Public Health. 2019;48(12):2154–64.
Baldo BA. Toxicities of opioid analgesics: respiratory depression, histamine release, hemodynamic changes, hypersensitivity, serotonin toxicity. Arch Toxicol. 2021;95(8):2627–42.
Dick DM and Agrawal A. The genetics of alcohol and other drug dependence. Alcohol Res Health 2008;31(2)
Rosenbloom JM, Burns SM, Kim E, August DA, Ortiz VE, and Houle TT. Race/ethnicity and sex and opioid administration in the emergency room. Anesth Analg 2019;128(5) https://doi.org/10.1213/ANE.0000000000003517
Momtazi S, Rawson R. Substance abuse among Iranian high school students. Curr Opin Psychiatry. 2010;23(3):221–6.
Maino A, et al. Opium as a risk factor for early-onset coronary artery disease: results from the Milano-Iran (MIran) study. PLoS ONE. 2023;18(4):e0283707.
Marmor M, Penn A, Widmer K, Levin RI, Maslansky R. Coronary artery disease and opioid use. Am J Cardiol. 2004;93(10):1295–7.
Piano MR. Alcohol’s effects on the cardiovascular system. Alcohol Res. 2017;38(2):219–41.
Grucza RA, Norberg KE, Bierut LJ. Binge drinking among youths and young adults in the United States: 1979–2006. J Am Acad Child Adolesc Psychiatry. 2009;48(7):692–702.
Alterman AI, Bridges KR, Tarter RE. Drinking behavior of high risk college men: contradictory preliminary findings. Alcohol Clin Exp Res. M1986;10(3):305–10.
Quigley LA, Marlatt GA. Drinking among young adults: prevalence, patterns, and consequences. Alcohol Health Res World. 1996;20(3):185–91.
Kannel WB, Ellison RC. Alcohol and coronary heart disease: the evidence for a protective effect. Clin Chim Acta. 1996;246(1–2):59–76.
Langer RD, Criqui MH, Reed DM. Lipoproteins and blood pressure as biological pathways for effect of moderate alcohol consumption on coronary heart disease. Circulation. 1992;85(3):910–5.
Biddinger KJ, et al. Association of habitual alcohol intake with risk of cardiovascular disease. JAMA Netw Open. 2022;5(3):e223849. A study involving Mendelian randomization focused in the UK Biobank cohort which observed various levels of alcohol intake relative to cardiovascular risk. Risk differences were observed across levels of intake, where light alcohol consumption was noted to cause minimal increases in cardiovascular risk and heavier consumption caused exponential increases in cardiovascular risk. Additionally, adjustment for healthy lifestyle factors reduced the cardioprotective effects of modest alcohol intake.
Larsson SC, Burgess S, Mason AM, Michaëlsson K. Alcohol consumption and cardiovascular disease: a Mendelian randomization study. Circ Genom Precis Med. 2020;13(3):e002814.
Hu C, et al. Causal associations of alcohol consumption with cardiovascular diseases and all-cause mortality among Chinese males. Am J Clin Nutr. 2022;116(3):771–9.
Pletcher MJ, Varosy P, Kiefe CI, Lewis CE, Sidney S, Hulley SB. Alcohol consumption, binge drinking, and early coronary calcification: findings from the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Epidemiol. 2005;161(5):423–33.
Englund MM, Egeland B, Oliva EM, Collins WA. Childhood and adolescent predictors of heavy drinking and alcohol use disorders in early adulthood: a longitudinal developmental analysis. Addiction. 2008;103(s1):23–35.
Shoar NS, Marwaha R, and Molla M. Dextroamphetamine-amphetamine. In: StatPearls [Internet], StatPearls Publishing; 2023
Schrantee A, et al. Dopaminergic system dysfunction in recreational dexamphetamine users. Neuropsychopharmacology. 2015;40(5):1172–80.
Potula R, et al. Methamphetamine causes mitrochondrial oxidative damage in human T lymphocytes leading to functional impairment. J Immunol. 2010;185(5):2867–76.
Graham DG, Tiffany SM, Bell WR Jr, Gutknecht WF. Autoxidation versus covalent binding of quinones as the mechanism of toxicity of dopamine, 6-hydroxydopamine, and related compounds toward C1300 neuroblastoma cells in vitro. Mol Pharmacol. 1978;14(4):644–53.
Mahtta D, et al. Recreational substance use among patients with premature atherosclerotic cardiovascular disease. Heart. 2021;107(8):650–6.
Batra V, et al. Early onset cardiovascular disease related to methamphetamine use is most striking in individuals under 30: a retrospective chart review. Addict Behav Rep. 2022;15:100435.
Schneiderman N, Ironson G, Siegel SD. Stress and health: psychological, behavioral, and biological determinants. Annu Rev Clin Psychol. 2005;1(1):607–28.
Henein MY, Vancheri S, Longo G and Vancheri F. The impact of mental stress on cardiovascular health-part II. J Clin Med Res. 2022; 11(15). https://doi.org/10.3390/jcm11154405. https://doi.org/10.3390/jcm11154405
Sadeghi B, Mashalchi H, Eghbali S, Jamshidi M, Golmohammadi M, Mahvar T. The relationship between hostility and anger with coronary heart disease in patients. J Educ Health Promot. A2020;9:223.
Satyjeet F, et al. Psychological stress as a risk factor for cardiovascular disease: a case-control study. Cureus. 2020;12(10):e10757.
Yao B-C, Meng L-B, Hao M-L, Zhang Y-M, Gong T, Guo Z-G. Chronic stress: a critical risk factor for atherosclerosis. J Int Med Res. 2019;47(4):1429–40.
Di Carli MF, et al. Effects of cardiac sympathetic innervation on coronary blood flow. N Engl J Med. 1997;336(17):1208–16.
Liu M, et al. An evidence of brain-heart disorder: mental stress-induced myocardial ischemia regulated by inflammatory cytokines. Neurol Res. 2020;42(8):670–5.
Kershaw KN, Lane-Cordova AD, Carnethon MR, Tindle HA, Liu K. Chronic stress and endothelial dysfunction: the multi-ethnic study of atherosclerosis (MESA). Am J Hypertens. 2017;30(1):75–80.
Chang PP, Ford DE, Meoni LA, Wang N-Y, Klag MJ. Anger in young men and subsequent premature cardiovascular disease: the precursors study. Arch Intern Med. 2002;162(8):901–6.
Henning RJ. Obesity and obesity-induced inflammatory disease contribute to atherosclerosis: a review of the pathophysiology and treatment of obesity. Am J Cardiovasc Dis. 2021;11(4):504–29.
Piché M-E, Tchernof A, Després J-P. Obesity phenotypes, diabetes, and cardiovascular diseases. Circ Res. 2020;126(11):1477–500.
de Onis M, Blössner M, Borghi E. Global prevalence and trends of overweight and obesity among preschool children. Am J Clin Nutr. 2010;92(5):1257–64.
Grundy SM. Multifactorial causation of obesity: implications for prevention. Am J Clin Nutr. 1998;67(3 Suppl):563S-S572.
Din-Dzietham R, Liu Y, Bielo M-V, Shamsa F. High blood pressure trends in children and adolescents in national surveys, 1963 to 2002. Circulation. 2007;116(13):1488–96.
Cole CB, Nikpay M, Stewart AFR, McPherson R. Increased genetic risk for obesity in premature coronary artery disease. Eur J Hum Genet. 2016;24(4):587–91.
Raj M. Obesity and cardiovascular risk in children and adolescents. Indian J Endocrinol Metab. 2012;16(1):13–9.
Mihuta M-S, et al. “Subclinical atherosclerosis progression in obese children with relevant cardiometabolic risk factors can be assessed through carotid intima media thickness”, NATO Adv. Sci Inst Ser E Appl Sci. 2021;11(22):10721.
Freedman DS, Khan LK, Dietz WH, Srinivasan SR, Berenson GS. Relationship of childhood obesity to coronary heart disease risk factors in adulthood: the Bogalusa heart study. Pediatrics. 2001;108(3):712–8.
Beauloye V, Zech F, Tran HTM, Clapuyt P, Maes M, Brichard SM. Determinants of early atherosclerosis in obese children and adolescents. J Clin Endocrinol Metab. 2007;92(8):3025–32.
McGill HC Jr, et al. Obesity accelerates the progression of coronary atherosclerosis in young men. Circulation. 2002;105(23):2712–8.
Yip A, Saw J. Spontaneous coronary artery dissection-a review. Cardiovasc Diagn Ther. 2015;5(1):37–48.
Garcia-Guimarães M, et al. Spontaneous coronary artery dissection: mechanisms, diagnosis and management. Eur Cardiol. 2020;15:1–8.
Nakashima T, et al. Prognostic impact of spontaneous coronary artery dissection in young female patients with acute myocardial infarction: a report from the Angina Pectoris-Myocardial Infarction Multicenter Investigators in Japan. Int J Cardiol. 2016;207:341–8.
Saw J, Aymong E, Mancini GBJ, Sedlak T, Starovoytov A, Ricci D. Nonatherosclerotic coronary artery disease in young women. Can J Cardiol. 2014;30(7):814–9.
Nishiguchi T, et al. Prevalence of spontaneous coronary artery dissection in patients with acute coronary syndrome. Eur Heart J Acute Cardiovasc Care. 2016;5(3):263–70.
White RE. Estrogen and vascular function. Vascul Pharmacol. 2002;38(2):73–80.
Rensing BJ, Kofflard M, van den Brand MJBM, Foley DP. Spontaneous dissections of all three coronary arteries in a 33-week-pregnant woman. Catheter Cardiovasc Interv. 1999;48(2):207–10.
Mikkola TS, Clarkson TB. Estrogen replacement therapy, atherosclerosis, and vascular function. Cardiovasc Res. 2002;53(3):605–19.
Tikkanen MJ, Nikkila EA, Vartiainen E. Natural oestrogen as an effective treatment for type-II hyperlipoproteinaemia in postmenopausal women. Lancet. 1978;2(8088):490–1.
Grodstein F. A prospective, observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease. Ann Intern Med. 2000;133(12):933.
Hayes SN, et al. Spontaneous coronary artery dissection: JACC state-of-the-art review. J Am Coll Cardiol. 2020;76(8):961–84.
Marcoff L, Rahman E. Menstruation-associated spontaneous coronary artery dissection. J Invasive Cardiol. 2010;22(10):E183–5.
Aggarwal A, Srivastava S, Velmurugan M. Newer perspectives of coronary artery disease in young. World J Cardiol. 2016;8(12):728.
Klein LW. Acute coronary syndromes in young patients with angiographically normal coronary arteries. Am Heart J. 2006;152(4). https://doi.org/10.1016/j.ahj.2006.03.020
Tanaka M et al. Evaluation of characteristics and degree of remodeling in coronary atherosclerotic lesions by 64-detector multislice computed tomography (MSCT). Atherosclerosis. 2009;203(2). https://doi.org/10.1016/j.atherosclerosis.2008.07.013
Kullo IJ, Edwards WD, and Schwartz RS. Vulnerable plaque: pathobiology and clinical implications. Ann Intern Med. 1998;129(12). https://doi.org/10.7326/0003-4819-129-12-199812150-00010
Author information
Authors and Affiliations
Contributions
A. L. wrote the following sections: Introduction, Genetics of pCAD, Polygenic Causes of pCAD, Clinical Risk Factors and Clinical Risk Scores, and Conclusion as well as revised writing for all sections. H. P. wrote the following sections: Smoking, Alcohol, Amphetamines, Stress and Exercise, and Spontaneous Coronary Artery Dissection (SCAD). D. G. wrote the following sections: Opioid Usage and collaborated for research on sections written by H. P. M. D. prepared Fig. 1 and aided with research on clinical aspects. R. L. revised genetics section and provided expertise regarding overall topic. G. P. assigned topics related to pCAD and is the principal investigator of the project. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Le, A., Peng, H., Golinsky, D. et al. What Causes Premature Coronary Artery Disease?. Curr Atheroscler Rep (2024). https://doi.org/10.1007/s11883-024-01200-y
Accepted:
Published:
DOI: https://doi.org/10.1007/s11883-024-01200-y