Abstract
Purpose of Review
There is growing evidence that enolase is involved in allergy. This manuscript reviews the impact of enolase in allergic disease and describes several sources of this allergen including molds, plants, animals, and pollens, among others. IgE epitopes are carefully analyzed as they may account for cross-reactivity.
Recent Findings
Enolase has been previously associated to food allergy and contact dermatitis. However, other groups and we have identified recently novel enolases derived from diverse pollens in patients suffering asthma and allergic rhinitis. Exposure to outdoor enolases may cause respiratory disease.
Summary
Enolase has been identified across various species and its amino acid sequence is highly conserved among different sources of this allergen. The demonstration that enolase is involved in many allergic diseases including respiratory allergies, is of clinic relevance. Thus, the development of novel molecular-based diagnostic and therapeutic strategies may pave the way for improved diagnosis and therapeutics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Enolase, an enzyme widely distributed in most organisms, has been shown to be a relevant allergen. Here, the main recent findings are compiled and analyzed to highlight the importance of the sources of this allergen including food, fungus, and aeroallergens derived from pollens and fungal spores, which may increase the risk of developing respiratory allergies. In addition, its ability to trigger allergic reactions in contact sources is emphasized. In the past, enolase was considered to be an insignificant allergen, however its potential as a sensitizing agent and its ability to trigger cross-reactivity are now recognized.
The body of evidence presented here raises the possibility that enolase may play a broader role as a panallergen, not limited to fungi but also to plants and animals, possibly spanning different eukaryotic domains. Enolase, also known as 2-phosphopyruvate hydratase, is a key enzyme involved in glycolysis, an ancient metabolic pathway that is widespread in many biological species. It catalyzes the reversible conversion of 2-phosphoenolpyruvate to 3-phosphoglycerate, and the direction of the reaction depends on the substrate concentration in the medium and the presence of metal ions, as it has two metal-binding sites [1]. There are three tissue-specific isoforms: α-enolase, which is found in most body tissues, β-enolase in muscle tissue; and γ-enolase in neuronal tissue. All enolases are homodimers consisting of two identical, antiparallel-aligned monomers containing the active site of the TIM barrel towards the carboxyl end that have a molecular weight of 82,000 to 100,000 Da per dimer [2]. Although the canonical function of enolase is glycolysis, recent evidence suggests that this enzyme may also fulfill other functions in the cell, classifying it as a "moonlighting" protein [3].
The most remarkable aspect of enolase is its clinical relevance, which is related to its immunogenic capacity, although it is an enzyme that is highly conserved in organisms and ideally considered harmless. α-Enolase has been associated with various pathologies, including infections, different types of cancer, autoimmune and degenerative diseases [4]. Similarly, enolase is an allergenic protein that can be recognized by the immune system of atopic individuals, triggering mild to severe symptoms. It originates from sources that span multiple biological kingdoms of the eukaryotic domain [5]. Enolase has been recognized widely as an allergen in filamentous fungi and yeasts, among which it is considered a panallergen [6]. There are also several allergenic enolases in the animal kingdom, most of which are classified as food allergens in vertebrates, such as birds and fish, and invertebrates, including crustaceans and insects. In the plant kingdom, allergenic enolases are found in pollen and latex, contact allergens. Since enolases are ubiquitous in foods, animals, insects, fungal, and plants, coupled with their ability to elicit an allergic response, their relevance as an allergen stands out [5]. We are discussing below the role of enolases in allergic diseases.
Food Enolases
Several enolases have been identified in food sources. In total, there are seven allergenic enolases, of which six β-enolases (present in muscle) are cataloged as vertebrate food allergens such as fish and chicken. These six β-enolases are Gad m 2 from Gadus morhua (Atlantic cod), Gad m 2 from Gallus domesticus (chicken), Pan h 2 from Pangasianodon hypophthalmus (striped catfish), Sal s 2 from Salmo salar (Atlantic salmon), Thu a 2 from Thunnus albacares (yellowfin tuna) and the recently registered Cyp c 2 from Cyprinus carpio (common carp) (www.allergen.org).
In 2022, Sližienė et al., overexpressed and characterized the carp (Cyprinus carpio) β-enolase, using serum from fish allergy sufferers and was officially registered as the food allergen Cyp c 2. In addition, in the same study, monoclonal IgG antibodies directed against Cyp c 2 were produced by hybridomas and tested with allergenic fish extracts from two commercial companies and with homemade extracts from chicken and pig muscles. Two different hybridoma clones were obtained, one of which recognized a conformational epitope and the other only linear epitopes, so that the latter was used to assess cross-reactivity. This monoclonal IgG was able to recognize the β-enolases of commercial extracts of different fish (cod, carp, herring, and salmon). However, for one of the commercial extracts, it was not possible to detect the β-enolase of the herring extract, demonstrating the lack of standardization of commercial extracts in terms of the amounts of allergens they consist of, which could affect the outcome of diagnoses and treatments [7]. Cyp c 2 shares 83.1, 92.61%, and 93.76% amino acid sequence identity with the β-enolases Thu a 2, Pan h 2, and Sal s 2, respectively (Fig. 1). In addition, they report the recognition of these monoclonal antibodies against fungal enolase, Alt a 6, against baker's yeast extract, and against chicken and pig β-enolases, achieving positive immunodetection in all cases and reporting the first indication to date of the presence of allergenic β-enolase in pigs. Mapping of the reported linear epitope turned out to be a portion of 76 amino acids, which shares a percentage of sequence identity of 60 to 70% with other food allergens such as Atlantic salmon, striped catfish, and chicken, being the first report of a linear epitope in β-enolases [9]. These results seem to indicate that β-enolases are relevant allergens in vertebrates and could represent cross-reactivity with fungal sources, as it has 64.12% amino acid sequence identity with Alt a 6 and between 61.57% and 65.66% with the other fungal enolases reported in the WHO/UIUS (Fig. 1), indicating the first report of possible cross-reactivity between animal enolase and a fungal one, opening up to date unexplored areas of research. Considering that fish, chicken, and possibly pork are indispensable sources of protein and nutrients for the world population, the presence of allergenic enolases in these sources is significant and increasingly demonstrates the relevant role of enolase as a food allergen and the possible existence of cross-reactivity between them [7,8,9,10]since only among the vertebrate β-enolases registered so far, a percentage of amino acid identity between 79.17% and 93.76% is reported. In 2021, the allergenicity of Lepidorhombus whiffiagonis (whiff) enolase was reported after being recognized by the serum of patients allergic to whiff fish and immunodetected in the raw and boiled extract [11].
Identity matrix and dendogram of allergenic enolases amino acid sequences. A Enolase’s identity matrix. Enolases from different taxonomic groups are highlighted in different shadows of gray. Light gray from fungus and yeast, medium gray from animals, and dark gray for plants. B Enolases phylogenetic tree obtained with Clustal Omega [18]
On the other hand, it is important to highlight the inclusion of the first invertebrate enolase in the official list of allergens, Per a 14 from Periplaneta americana (American cockroach). Cockroaches have been associated with allergic diseases since 1964, suggesting that they are important sources of aeroallergens [12]. In 2010, enolase from Blattella germanica (German cockroach) was identified as a possible minor allergen by immunoproteomics and tested for its reactivity with the serum of allergic individuals [13]. However, its identity as an allergen was not recorded by the WHO/UIUS. In 2022, a study was published in which a total of eight new American cockroach allergens, including an enolase, were registered. Initially, an in-silico prediction and immunoproteomics approach to select potential allergens, which were subsequently overexpressed in E. coli and exposed to the serum of patients with a positive skin test to a commercial extract of P. americana. The ELISA test showed that 70% of patients in the study group (n = 20) exhibited reactivity to Per a 14 enolase, indicating an important role as an aeroallergen of Periplaneta americana [14]. The amino acid sequence of Per a 14 is 69.68% to 73.61% identical to previously reported animal enolases from fish and chicken (Fig. 1).
Allergenic enolases are also found in some crustaceans, mainly shrimps [15,16,17]. In a recent study, the allergenicity of enolase from Penaeus monodon (black tiger shrimp) was revealed using an immunoproteomics approach. One hundred percent of a total of eight sera from patients allergic to shrimp reacted to the enolase in the extract of Penaeus monodon, showing a higher reactivity than allergens considered more relevant, such as tropomyosin and hemocyanin, both of which showed reactivity in seven of the eight sera tested [15]. These findings suggest that enolase allergy may be underestimated. To date, no allergenic shrimp enolase has been officially registered in the WHO/UIUS database.
Fungal Enolases
Aeroallergens from fungi can cause severe allergic respiratory diseases, including rhinitis, asthma, and bronchopulmonary allergic mycosis. Fungi rank third after mites and pollen, with the worldwide prevalence of fungal allergies estimated at 3–10% [19, 20]. The concentration of fungal spores can vary depending on geographical location, environmental conditions or meteorological factors and can sometimes be higher, or lower than pollens [20, 21].
Enolases are considered panallergens among the fungal families, as they are ubiquitous in molds, and yeasts [22]. The prevalence of enolases that bind IgE is between 20 and 30% [23]. Studies on A. alternata asthma allergic patients’ sera have shown 22% enolase IgE recognition, while clinical studies on asthma patients sensitized to Penicillium showed a 30% recognition of IgE against Pen c 22 (enolase from P. citrinum) [24]. Indicating its clinical impact on allergic diseases. The Allergen Nomenclature Subcommittee (www.allergen.org) has documented five enolases in molds, and two in yeast, specifically Rhodotorula mucilaginosa[25]. There are also reports of allergenicity to enolase from Saccharomyces cerevisiae in bakers [26, 27]. S. cerevisiae is widely used in the food industry, where it goes through high hydrostatic pressure for processing and sterilization. In 2023, Jia et al. investigated the allergenic properties of S. cerevisiae-enolase under pressurization and found that high pressure (300–600 MPa) led to disruption of its ternary and secondary structures, thereby enhancing its allergenic potential [28]. Recently, two enolases from indoor fungi have been reported: Ulo c 6 (Ulocladium chartarum), which grows in buildings with high humidity, and Pae v 6 (Paecilomyces variotii), which is found in decomposing organic foods. The IgE-binding was 40% (out of 85 patients) for Ulo c 6, and 61% for Pae v 6 (out of 57 patients). In addition, there is evidence of direct allergenic activity of rPae v 6 based on CD63-based basophil activation models. Therefore, rPae v 6, with its high exposure in indoor environments and prevalence, shows its relevance as an aeroallergen [29, 30].
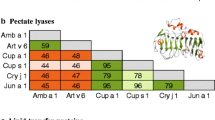
Fungal enolases show a high degree of amino acid sequence identity, ranging from 79.91% to 94.29%, and yeasts (72.02% to 76.38%) because they are phylogenetically related (Fig. 1B). According to Crameri et al., fungal enolases have similar three-dimensional structures with conserved epitopes, leading to IgE cross-reactivity between enolases from fungi, yeasts, and latex [19, 22]. The cross-reactivity among fungal enolases is shown in Fig. 2. For example, it has been demonstrated that C. albicans and S. cerevisiae enolases share some cross-reactive IgE epitopes. In 1995, Ito et al. showed that 20% of C. albicans enolase-allergic individuals (4 out of 20) recognized S. cerevisiae; both enolases share 75% amino acid sequence identity [31]. In addition, it has been reported that Asp f 22 (A. fumigatus) and Alt 6 (A. alternata) enolases can reduce each other’s IgE binding to approximately 30% and 39%[32]. Wagner et al., in 2000 found that Hev b 9 reduced 24% IgE-binding to rCla h 6 by, but not rAlt a 6 [33]. In 2022 Čelakovská et al. evaluated the sensitization of molds with food allergens in atopic dermatitis patients. Interestingly, the patients positive to Alt a 6, and Asp f 3 showed considerably allergic reactions to hazelnuts, and the patients sensitized to these two allergens and to Alt a 1 had tendency to react to walnuts. But no hypersensitivity reactions to other foods such as peach, kiwi, apples, spices, oranges, celery, carrot, soy, wheat, and cow milk [34]. Because so far only cross-reactivity of fungal enolases with yeast and latex has been reported and there is still limited information about cross-reaction with other plant and animal enolases, fungus enolases could be classified as "stenallergens" (Greek: "stenos": tight), a term suggested by Hauser et al. in 2010, because enolases are widely distributed in animal, fungal, and plant kingdoms, but they exhibit a restricted cross-reactivity pattern [35]. Moreover, fungal and plants enolases share amino acid sequence identity of 54.5% -62.59% whereas fungal and animal enolases share 59.16% -65.74% (Fig. 1A). Therefore, the identification of cross-reactive epitopes in enolases among the different kingdoms of life is crucial for the improvement in clinical diagnostics and allergy immunotherapy.
Cross-reactivity among allergenic enolases. The intersection of rows and columns represent the cross-reaction between allergenic enolases registered in WHO/IUIS. Experimental cross-reactivity is illustrated by white boxes with dotted border, predicted cross-reactivity by dark boxes with black border), and reactivity with unrecorded sources by light gray boxes with dark border. The light gray areas delimit the enolases from fungi and yeast, the medium gray area delimits those from animals, and the dark gray areas delimit those from plants
Diagnosis and treatment of fungal aeroallergens pose significant challenges due to difficulties in obtaining fungal enolases. Currently, diagnostic kits are ineffective because allergens lack stability or an optimal concentration to trigger allergic reactions. Fungal allergens are challenging to obtain due to the specific growth conditions required for each species [20, 36]. Molecular allergy diagnostics present a promising solution by expressing recombinant enolases that show IgE reactivity and allergenicity. This facilitates effective personalized management of fungal enolase allergies and improves diagnostics and immunotherapy.
Plant Enolases as Aeroallergens
In 2021, enolase was officially registered as an allergen in only three sources from the plant kingdom (Plantae), namely in two pollen aeroallergens and one contact allergen in the list of systematic allergen nomenclature approved by the Allergen Nomenclature Subcommittee of the World Health Organization and the International Union of Immunological Societies (WHO/IUIS) (www.allergen.org). The recent identification of enolase in the pollen derived from different plants has raised concerns regarding its ability to cause respiratory disease. Respiratory allergies are a growing public health problem worldwide and affect the quality of life of millions of people. It is triggered by aeroallergens present in the environment that cause a variety of respiratory symptoms, such as rhinitis, and asthma [37].
In 2004, Cyn d 22, an enolase from Cynodon dactylon (Bermuda grass), was registered as the first pollen-allergenic enolase, followed by Amb a 12 from Ambrosia artemisiifolia (ragweed) in 2017. Hev b 9, from the latex tree (Hevea brasiliensis), was officially registered in 2013 (www.allergen.org) as the only contact allergenic enolase. In our previous review in 2021, we highlighted the use of discovery proteomics to uncover novel allergenic enolases, particularly from pollen sources (tree of heaven, coconut palm, privet, red oak, poplar) and gave evidence of these allergens as sensitizers in allergic respiratory disease [5]. Other pollen sources in which enolase has recently been identified using an immunoproteomic approach include Ligustrum Lucidum, pecan (Carya illinoinensis) and mesquite (Prosopis velutina) [38, 39]. Since 2021, two enolase pollen allergens have been added: Art si 12 from Artemisia sieversiana (Sieversian wormwood) and Pla a 6 from Platanus acerifolia (London plane tree). In the case of Art si 12, no publications were found to support its allergenicity, and it appears in the WHO/IUIS database as confidential until publication. On the other hand, Jiao et al., in 2022, reported the purification and expression of enolase Pla a 6 and demonstrated allergenicity in in the serum of allergic patients using ELISA, western blot, inhibition ELISA, and basophil activation assay. Recombinant Pla a 6 is the first overexpressed pollen enolase that exhibits similar IgE binding to natural Pla a 6 and can inhibit IgE binding to the whole pollen extract by 45.77%, as well as giving a positive result in the basophil activation test, demonstrating its importance as an allergen [40].
Amb a 12, an enolase from Ambrosia artemisiifolia, was recently reported. Indeed, Grijincu et al., overexpressed this enolase using a prokaryotic (E. coli) and a eukaryotic vector (insect cells) and showed low specific IgE frequency and allergenicity with both proteins [41]. However, the immunogenic capacity of Amb a 12 was demonstrated by immunization of rabbits, where the IgG produced showed the ability to recognize enolases in other pollens such as mugwort (Artemisia), timothy grass (Phleum pratense), and birch pollen (Betula verrucosa) as well as in food allergens such as wheat flour, kiwi, and peach fruit pulp, suggesting common epitopes between these allergens and possible cross-reactivity. This finding supports the role of enolase as a panallergen [41]. There is currently limited information available on the potential cross-reactivity of enolases among plant species, and interest is recently emerging. However, the demonstration by Grijincu et al., on the potential cross-reactivity of Amb a 12 enolase to fresh foods, suggests its involvement in the Oral Allergy Syndrome [41]. Many other studies are required to delve into the cross-reactivity of enolases. Similarly, the cross-reaction among the contact allergen Hev b 9 (Hevea brassiliensis) and the fungal enolases Alt a 6 and Cla h 6 [33], can be explained by their high amino identity, 60.74% and 60.46%, respectively (Fig. 1). As can be seen in Fig. 1, Hev b 9 has a percentage identity of 89.64% with Art si 12 and 90.23% with Pla a 6 (highly similar). In general, plant enolases share a percentage of amino acid sequence identity in a range of 86.77% to 90.23% (Fig. 1).
Since there are only five reports of allergenic enolases belonging to the plant kingdom this draws attention to the relatively scarce reports of allergenic enolases in plants and pollen, considering the vast array of unexplored species and the better-characterized allergens in which enolase hasn't been documented. Likewise, to date, there are no reports of cross-reactivity among plant enolases, as can be seen in Fig. 2. Identification, overexpression, and cross-reactivity information between enolases is vital to improve diagnoses and treatments, mainly in respiratory allergies. Figure 1 shows that the amino acid sequence identities between plant and fungal enolases are between 50.54% and 62.59%.
Enolases IgE Epitopes
The IgE conformational epitopes of allergenic enolases have not been fully explored, except for fungal enolases. In 1995, Ito et al. identified IgE-binding epitopes for C. albicans enolase, suggesting the region from Gln361 to Ile-399 near the C-terminal residues [42]. Subsequently, Simon-Nobe et al. identified IgE antigenic determinants of rCla h 6 at Asp136-Pro143 and, through structural analysis, proposed theoretical epitopes that include Ala124-Leu130 and Arg163 as part of the main IgE epitope [43]. This IgE epitope is shaded in blue in Fig. 3A. The three-dimensional structure of allergic enolases is essential for IgE recognition epitopes for Cla h 6 lie from the residues in the C-terminal region of the third α-helix to the loop that connects to the β4-sheet; this epitope also includes the turn that lies between the third and fourth α-helices shown in Fig. 3C. The linear epitopes reported are exposed on the surface of the allergen, facilitating IgE recognition and cross-reactivity among fungal and latex enolases.
Enolase's conserved amino acid sequence and structure. A Amino acid sequence alignment of allergenic enolases: Hev b 9 – Hevea brasilensis, Cla h 6 - Cladosporium herbarum, Asp f 22 - Aspergillus fumigatus, Pen c 22 - Penicillium citrinum, Cur l 2 - Curvularia lunata, Alt a 6 - Alternaria alternata, Ulo c 6 - Ulocladium chartarum, Pae v 6 - Paecilomyces variotii, Rho m 1 - Rhodotorula mucilaginosa, and Sac c enolase - Saccharomyces cerevisiae. Residues shaded in blue correspond to the reported IgE linear epitopes for Cla h 6 [43]. Residues shaded in orange are the reported mAb IgG 6E4 epitope [7]. The alignment sequence was obtained with Clustal Omega [18]. An asterisk (*) indicates fully conserved residues, a colon (:) are similar properties, a period (.) indicates weakly similar properties, and a space () indicates different properties. B Three-dimensional models alignment of enolases including Hev b 9 (purple), Cla h 6 (gray), and Pen c 22 (pink) obtained with AlphaFold [49], and verified using the MolProbity [50], finally figures were obtained using PyMOL(http://www.pymol.org). The RMSD of the compared Cla h 6 with Hev b 9 is 0.535 Å, and for Pen c 22 is 0.337. C Three-dimensional model of Cla h 6 with IgE epitopes [43]
Figure 3A shows a sequence amino acid alignment of fungal, yeast, and latex enolases, in which Asp f 22 and Pae v 6 share 88.23%, Pen c 22, Cur l 2 and Ulo c 6 share 82.35%, Hev b 9 and Sac c enolase 64.7%, whereas Rho m 1 shares 47.05% identity in the epitope described by Simon-Nobe et al. 2008. This could suggest that this IgE epitope could be relevant to the cross-reactivity of fungal enolases. Moreover, there is a conserved identity amino acid sequence between fungal and latex enolases (55.53 - 62.59%) (Fig. 1) and with a similar three-dimensional structure, where fungal enolases Cla h 6 and Pen c 22 are more similar compared to Hev b 9 shown in Fig. 3B.
Sližienė et al. in 2022 obtained IgG monoclonal antibodies against Cyp c 2 (anti-MBP-Eno) through the hybridoma. One of these, mAb 6E4, recognized a linear epitope on Cyp c 2, which comprised Asn222-Pro299 (shaded in orange in Fig. 3A), lying close to the C-terminal region from the sixth α-helix to the eighth β-sheet [7]. Figure 3A shows the alignment of enolases from different sources, including plants (Hev b 9), fish (Cyp c 2), yeast (Rho m 1 and Sac c) and fungi (Cla h 6, Asp f 22, Pen c 22, Cur l 2, Alt a 6, Ulo c 6 and Pae v 6). The recognition site for mAb 6E4 shares an identity of 60–55% and the main IgE epitope lies proximal to the C-terminal of the enolases, exhibiting conserved residues within this domain.
Interestingly, enolase structures reported in the Protein Data Bank are found to be dimers; for example, shrimp enolase from Litopenaeus vannamei (PDB:8UEL) [17], Aspergillus fumigatus enolase (PDB:7RHV) [44], Saccharomyces cerevisiae (PDB: 1ONE) [45], Candida albicans (PDB: 7V67) [46], and Homo sapiens (yellow, PDB ID: 3B97) [47]. Dimerization of allergens can facilitate the cross-linking of IgE-FcεRI, thereby enhancing allergenicity [48]. Therefore, it is crucial to identify the conformational epitopes of enolases and to determine their oligomeric states with higher allergenic potential. In silico enolase epitope mapping predictions combined with experimental identification of their conformational antigenic determinant regions should be undertaken. This knowledge will facilitate the development of hypoallergenic enolases for improved diagnostics and therapeutic approaches.
Conclusions
It is now well established that allergic enolases play a significant role in allergic disease. They can sensitize individuals through ingestion, skin contact, or inhalation, particularly from airborne sources like pollen and mold. Enolases derived from various sources, such as fish, fungi, and latex, have been extensively studied and associated with allergic conditions. Recent research suggests that novel serum IgE-binding enolases may contribute to allergic respiratory diseases like asthma and allergic rhinitis. Currently, it is difficult to diagnose enolase allergy in the clinic. Diagnostic tests based on natural allergen extracts are the most common methods for allergy diagnosis. However, they are composed of mixtures of allergic and nonallergic material, which do not allow identifying the disease-eliciting allergen. Fish enolases are the ones that have been investigated the most using fish-allergen assays, and more recently, we have identified several pollen enolases using proteomics technology. Interestingly, Yong-Sing et al. found a novel IgE-binding enolase from Platanus acerifolia pollen using sera from patients sensitized to platanus in combination with chromatographic techniques. However, commercially available diagnostic tests to be used in clinics are limited. To date, 18 enolases have been officially recognized by the World Health Organization/International Union of Immunological Societies Allergen Nomenclature Subcommittee. However, there are many more that have been identified as allergenic in fungi, plants, and animals, highlighting the importance of enolase as an allergen. The development of specific diagnostic tests for proper identification of the individual or cross-reactive enolases will allow the delivery of personalized care and prevention.
Data Availability
No datasets were generated or analysed during the current study.
References
Pancholi V. Multifunctional a α-enolase: its role in diseases. Cell Mol Life Sci. 2001;58:902–20. https://doi.org/10.1007/pl00000910.
Gerlt JA, Babbitt PC, Jacobson MP, Almo SC. Divergent evolution in enolase superfamily: Strategies for assigning functions. JBC. 2012;287:29–34. https://doi.org/10.1074/jbc.R111.240945.
Qiao G, Wu A, Chen X, Tian Y, Lin X. Enolase 1, a moonlighting protein, as a potential target for cancer treatment. Int J Biol Sci. 2021;17:3981–92. https://doi.org/10.7150/ijbs.63556.
Angeletti A, Migliorini P, Bruschi M, Pratesi F, Candiano G, Prunotto M, et al. Anti-alpha enolase multi-antibody specificity in human diseases. Clinical significance and molecular mechanisms. Autoimmun Rev. 2021;20:102977. https://doi.org/10.1016/J.AUTREV.2021.102977.
Morales-Amparano MB, Huerta-Ocampo JÁ, Pastor-Palacios G, Teran LM. The Role of Enolases in Allergic Disease. J Allergy Clin Immunol Pract. 2021;9:3026–32. https://doi.org/10.1016/j.jaip.2021.04.005.
Breitenbach M, Simon B, Probst G, Oberkofler H, Ferreira F, Briza P, et al. Enolases are highly conserved fungal allergens. Int Arch Allergy Immunol. 1997;113:114–7. https://doi.org/10.1159/000237521.
Sližienė A, Plečkaitytė M, Zaveckas M, Juškaitė K, Rudokas V, Žvirblis G, et al. Monoclonal antibodies against the newly identified allergen β-enolase from common carp (Cyprinus carpio). Food Agri Immunol. 2022;33:129–49. https://doi.org/10.1080/09540105.2022.2028741.
Kuehn A, Hilger C, Lehners-Weber C, Codreanu-Morel F, Morisset M, Metz-Favre C, et al. Identification of enolases and aldolases as important fish allergens in cod, salmon and tuna: Component resolved diagnosis using parvalbumin and the new allergens. Clin Exp Allergy. 2013;43:811–22. https://doi.org/10.1111/cea.12117.
Kuehn A, Codreanu-Morel F, Lehners-Weber C, Doyen V, Gomez-André SA, Bienvenu F, et al. Cross-reactivity to fish and chicken meat – a new clinical syndrome. EAACI. 2016;71:1772–81. https://doi.org/10.1111/all.12968.
Tomm JM, Van Do T, Jende C, Simon JC, Treudler R, Von Bergen M, et al. Identification of New Allergens from Nile Perch (Lates niloticus) and Cod (Gadus morhua). J Investig Allergol Clin Immunol. 2013;23:159–67.
Haroun Díaz E, Martín-Pedraza L, Betancor D, Somoza ML, Blanca-López N, Vázquez de la Torre M, et al. Selective allergy to whiff (Lepidorhombus whiffiagonis): identification of enolase as a new major allergen. J Investig Allergol Clin Immunol. 2023;33:45–7. https://doi.org/10.18176/jiaci.0802.
Bernton HS, Brown H, Washington DC. Insect allergy-Preliminary studies of the cockroach. JAllergy. 1964;35:506–13. https://doi.org/10.1016/0021-8707(64)90082-6.
Chuang JG, Su SN, Chiang BL, Lee HJ, Chow LP. Proteome mining for novel IgE-binding proteins from the German cockroach (Blattella germanica) and allergen profiling of patients. Proteomics. 2010;10:3854–67. https://doi.org/10.1002/pmic.201000348.
Wang L, Xiong Q, Saelim N, Wang L, Nong W, Wan ATY, et al. Genome assembly and annotation of Periplaneta americana reveal a comprehensive cockroach allergen profile. Allergy. 2023;78:1088–103. https://doi.org/10.1111/all.15531.
Yang Y, Liu H, Zeng W, Yang Y, Zhang J, Yin J, et al. Characterization and epitope prediction of phosphopyruvate hydratase from Penaeus monodon (black tiger shrimp). J Food Sci. 2021;86:3457–66. https://doi.org/10.1111/1750-3841.15819.
Karnaneedi S, Huerlimann R, Johnston EB, Nugraha R, Ruethers T, Taki AC, et al. Novel allergen discovery through comprehensive de novo transcriptomic analyses of five shrimp species. Int J Mol Sci. 2021;22:1–24. https://doi.org/10.3390/ijms22010032.
Chang X, Zhang T, Zang J, Lv C, Zhao G. Characterization and Structural Analyses of Enolase from Shrimp (Litopenaeus vannamei). J Agric Food Chem. 2023;71:19783–90. https://doi.org/10.1021/acs.jafc.3c07135.
Madeira F, Pearce M, Tivey ARN, Basutkar P, Lee J, Edbali O, et al. Search and sequence analysis tools services from EMBL-EBI in 2022. Nucleic Acids Res Spec Publ. 2022;50:W276–9. https://doi.org/10.1093/nar/gkac240.
Abel-Fernández E, Martínez MJ, Galán T, Pineda F. Going over Fungal Allergy: Alternaria alternata and Its Allergens. JoF. 2023;9:2–15. https://doi.org/10.3390/jof9050582.
Armentia A, Martín-Armentia S, Moral A, Montejo D, Martin-Armentia B, Sastre R, et al. Molecular study of hypersensitivity to spores in adults and children from Castile & Leon. Allergol Immunopathol. 2019;47:350–6. https://doi.org/10.1016/j.aller.2018.10.002.
Twaroch TE, Curin M, Valenta R, Swoboda I. Mold allergens in respiratory allergy: From structure to therapy. AAIR. 2015;7:205–20. https://doi.org/10.4168/aair.2015.7.3.205.
Crameri R, Zeller S, Glaser AG, Vilhelmsson M, Rhyner C. Cross-reactivity among fungal allergens: A clinically relevant phenomenon? Mycoses. 2009;52:99–106. https://doi.org/10.1111/j.1439-0507.2008.01644.x.
Simon-Nobbe B, Denk U, Pöll V, Rid R, Breitenbach M. The spectrum of fungal allergy. Int Arch Allergy Immunol. 2008;145:58–86. https://doi.org/10.1159/000107578.
Sevinc MS, Kumar V, Abebe M, Casley WL, Vijay HM. Isolation and characterization of a cDNA clone encoding one IgE-binding fragment of Penicillium brevicompactum. Int Arc Allergy Immunol. 2005;138:12–20. https://doi.org/10.1159/000087353.
Chang C-Y, Chou H, Tam MF, Tang R-B, Lai H-Y, Shen H-D. Characterization of Enolase Allergen from Rhodotorula mucilaginosa. J of Biomed Sci. 2003;9:645–55. https://doi.org/10.1159/000067279.
Baldo BA, Baker RS. Inhalant allergies to fungi: reactions to bakers’ yeast (Saccharomyces cerevisiae) and identification of bakers’ yeast enolase as an important allergen. Int Arch Allergy Immun. 1988;86:201–8. https://doi.org/10.1159/000234572.
Pajno GB, Passalacqua G, Salpietro C, Vita D, Caminiti L. Looking for immunotolerance: a case of allergy to baker’s yeast (Saccharomyces cerevisiae). Ann Allergy Clin Immunol. 2005;37:271–2. https://doi.org/10.1002/9780470015902.a0000916.pub3.
Jia C, Wei Y, Shi J, Zhang H, Xiao Y, Gan Z, et al. Allergenic risk assessment of enolase leaked from Saccharomyces cerevisiae under pressurization. Food Biosci. 2023. https://doi.org/10.1016/j.fbio.2023.103399.
Pfeiffer S, Raith M, Pascal M, Munoz-Cano RM, San Bartolome C, Nöbauer K, et al. The emerging pathogen Paecilomyces variotiia novel and important fungal allergen source. Allergy: European Journal of Allergy and Clinical Immunology. 2022;77:1045–8. https://doi.org/10.1111/all.15176.
Pfeiffer S, Sandler P, Raith M, Pascal M, Munoz-Cano RM, San Bartolome C, et al. Identification of Ulocladium chartarum as an important indoor allergen source. Allergy: European Journal of Allergy and Clinical Immunology. 2021;76:3202–6. https://doi.org/10.1111/all.14999.
Ito K, Ishiguro A, Kanbe T, Tanaka K, Torii S. Detection of IgE antibody against Candida albicans enolase and its crossreactivity to Saccharomyces cerevisiae enolase. Clin Exp Allergy. 1995;25:522–8. https://doi.org/10.1111/j.1365-2222.1995.tb01089.x.
Lai H-Y, Tam MF, Tang R-B, Chou H, Chang C-Y, Tsai J, et al. cDNA Cloning and Immunological Characterization of a Newly Identified Enolase Allergen from Penicillium citrinum and Aspergillus fumigatus. Int Arch Allergy Immunol. 2002;127:181–90. https://doi.org/10.1159/000053862.
Wagner S, Breiteneder H, Simon-Nobbe B, Susani M, Krebitz M, Niggemann B, et al. Hev b 9, an enolase and a new cross-reactive allergen from Hevea latex and molds: Purification, characterization, cloning and expression. Eur J Biochem. 2000;267:7006–14. https://doi.org/10.1046/j.1432-1327.2000.01801.x.
Čelakovská J, Čermákova E, Vaňková R, Andrýs C, Krejsek J. Sensitisation to molecular components of fungi in atopic dermatitis patients, the relation to the occurrence of food hypersensitivity reactions. Food Agr Immunol. 2022;33:328–45. https://doi.org/10.1080/09540105.2022.2074968.
Hauser M, Roulias A, Ferreira F, Egger M. Panallergens and their impact on the allergic patient. AACI. 2010;6:1–14. https://doi.org/10.1186/1710-1492-6-1.
Pfeiffer S, Focke-Tejkl M, Sterflinger K, Swoboda I. Optimizing cultivation conditions for the highest expression of fungal allergens. Ann Allergy Asthma Immunol. 2023;130:479–84. https://doi.org/10.1016/j.anai.2022.11.017.
D’Amato G, Chong-Neto HJ, Monge Ortega OP, Vitale C, Ansotegui I, Rosario N, et al. The effects of climate change on respiratory allergy and asthma induced by pollen and mold allergens. EAACI. 2020;75:2219–28. https://doi.org/10.1111/all.14476.
Huerta-Ocampo JÁ, Batista-Roche LG, Morales-Amparano MB, del Robles-Burgueño M, R, Ramos-Clamont Montfort G, Vázquez-Moreno L, et al. Identification of Allergenic Proteins in Velvet Mesquite (Prosopis velutina) Pollen: An Immunoproteomics Approach. Life. 2022. https://doi.org/10.3390/life12091421.
Morales-Amparano MB, Valenzuela-Corral A, Ramos-Clamont Montfort G, Vázquez-Moreno L, Escobedo-Moratilla A, Pastor-Palacios G, et al. Immunoproteomic identification of allergenic proteins in pecan (Carya illinoinensis) pollen. J Proteom. 2021;248:10. https://doi.org/10.1016/j.jprot.2021.104348.
Jiao YX, Bin SL, Xu ZQ, Zhu DX, Yang YS, Tian M, et al. Purification and characterization of enolase as a novel allergen in Platanus acerifolia pollen. Int Immunopharmacol. 2022. https://doi.org/10.1016/j.intimp.2022.109313.
Grijincu M, Huțu I, Weber M, Babaev E, Stolz F, Valenta R, et al. Physicochemical and immunological characterization of Amb a 12, a novel ragweed (Ambrosia artemisiifolia) pollen allergen. Mol Immunol. 2023;157:18–29. https://doi.org/10.1016/j.molimm.2023.03.012.
Ito K, Ishiguro A, Kanbe T, Tanaka K, Torii S. Characterization of IgE-binding epitopes on Candida albicans enolase. Clin Exp Allergy. 1995;25:529–35. https://doi.org/10.1111/j.1365-2222.1995.tb01090.x.
Simon-Nobbe B, Probst G, Kajava AV, Oberkofler H, Susani M, Crameri R, et al. IgE-binding epitopes of enolases, a class of highly conserved fungal allergens. Journal of Allergy and Clinical Immunology. 2000;106:887–95. https://doi.org/10.1067/mai.2000.110799.
Nguyen S, Jovcevski B, Truong JQ, Pukala TL, Bruning JB. A structural model of the human plasminogen and Aspergillus fumigatus enolase complex. Proteins: Structure. Function and Bioinformatics. 2022;90:1509–20. https://doi.org/10.1002/prot.26331.
Larsen TM, Wedekind JE, Rayment I, Reed GH. A Carboxylate Oxygen of the Substrate Bridges the Magnesium Ions at the Active Site of Enolase: Structure of the Yeast Enzyme Complexed with the Equilibrium Mixture of 2-Phosphoglycerate and Phosphoenolpyruvate at 1.8 Å Resolution. Biochemistry. 1996;35:4349–58. https://doi.org/10.1021/bi952859c.
Li L, Lu H, Zhang X, Whiteway M, Wu H, Tan S, et al. Baicalein Acts against Candida albicans by Targeting Eno1 and Inhibiting Glycolysis. Microbiol Spectr. 2022. https://doi.org/10.1128/spectrum.02085-22.
Kang HJ, Jung SK, Kim SJ, Chung SJ. Structure of human α-enolase (hENO1), a multifunctional glycolytic enzyme. Acta Cryst D. 2008;64:651–7. https://doi.org/10.1107/S0907444908008561.
Rouvinen J, Jänis J, Laukkanen ML, Jylhä S, Niemi M, Päivinen T, et al. Transient Dimers of Allergens. PLoS ONE. 2017;12:e9037. https://doi.org/10.1371/journal.
Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, et al. Highly accurate protein structure prediction with AlphaFold. Nature. 2021;596:583–9. https://doi.org/10.1038/s41586-021-03819-2.
Chen VB, Arendall WB, Headd JJ, Keedy DA, Immormino RM, Kapral GJ, et al. MolProbity: All-atom structure validation for macromolecular crystallography. Acta Cryst D. 2010;66:12–21. https://doi.org/10.1107/S0907444909042073.
Funding
Research reported in this publication was supported by CONAHCYT-Mexico (grant 33269).
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Morales-Amparano, M.B., Teran, M.G., Huerta-Ocampo, J.Á. et al. Impact of Enolase in Allergic Disease. Curr Allergy Asthma Rep 24, 571–579 (2024). https://doi.org/10.1007/s11882-024-01170-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11882-024-01170-w