Abstract
Purpose of Review
The human commensal microbiota is now widely accepted as a key regulator of human health and disease. The composition of the mucosal associated microbiota has been shown to play a critical role in the lung health. The role of the mucosal microbiota in the development and severity of allergy, asthma, and occupational lung disease is only beginning to take shape. However, advances in our understanding of these links have tremendous potential to led to new clinical interventions to reduce allergy, asthma, and occupational lung disease morbidity.
Recent Findings
We review recent work describing the relationship and role of the commensal microbiota in the development of allergy, asthma, and occupational lung disease. Our review primarily focuses on occupational exposures and the effects of the microbiome, both in composition and function. Data generated from these studies may lead to the development of interventions targeted at establishing and maintaining a healthy microbiota. We also highlight the role of environmental exposures and the effects on the commensal microbial community and their potential association with occupational lung disease.
Summary
This review explores the current research describing the role of the human microbiome in the regulation of pulmonary health and disease, with a specific focus on the role of the mucosal microbiota in the development of allergy, asthma, and occupational lung disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One of the very first descriptions of the microbiome dates to the late 1800s when Theodore Escherich published his thesis titled “The Intestinal Bacteria of the Infant and Their Relation to the Physiology of Digestion [1].” The study of the human microbiome has since undergone a massive paradigm shift. Within the past decade alone, more than US$1.7 billion has been allocated for human microbiome research [2], demonstrating just how imperative it is to gain a better understanding of the microbiome and how it interacts with its surroundings. Specifically, the meta-organism concept or holobiont theory has become universally accepted as numerous preclinical and clinical studies have demonstrated a critical role of commensal microorganisms in human health [3,4,5,6,7,8,9,10,11,12,13,14]. Understanding the mechanisms that mediate crosstalk between the mucosal microbial communities and the lungs and how this interaction facilitates optimal lung health is a rapidly growing area of research. The role of the GI microbiota on mediating, maintaining, and regulating the health of multi-organ systems is an expanding area of research that has massive potential to aid in the development of novel treatment and management strategies for lung disease.
The Role of the Microbiome in the Development of Allergy
A vital role between various exposures and the composition of the mucosal microbiota and subsequent development of allergic disease has been established by multiple groups [14,15,16,17,18,19,20,21,22,23,24,25,26,27,28]. Specifically, maternal prenatal exposure to pets, vaginal vs. c-section modes of delivery, childhood environmental exposures, and childhood exposure to pets have all been associated with a reduced allergy risk. In addition, breastfeeding, early exposure to antibiotics and the development of oral tolerance to different antigens all play a critical role in the development of allergy. Importantly the dysregulation of resident microbial communities due to alterations in these processes may represent a key component that drives allergy risk. For instance, changes in the cutaneous microbiome poses a risk for atopic dermatitis (AD) flare-ups, giving biofilm-producing Staphylococcus aureus the opportunity to overgrow. Additionally, it was demonstrated that individuals with AD lack mucin-producing bacteria that provide sustenance for beneficial commensal gut microbes. In patients with allergic rhinitis (AR), dysbiosis of the nasal bacteriome is a novel rising area of interest. Furthermore, it has been established that patients with distinct food allergies have gut dysbiosis, leading to a decrease in short chain fatty acids (SCFAs). Interestingly, in all these conditions, residential microbiome colonization and antibiotic use are extraordinarily important during the first few years of life. Microbial transplants and supplementation with commensal microbes have shown promising results across various allergies. This topic has been subject to many review articles [14, 28,29,30], as such we will not focus on this topic. However, Table 1 provides an overview of the changes in the microbiota associated with the development of allergies [31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52].
The Role of the Microbiome in the Development of Asthma
The composition of the GI and lung microbiota and the subsequent development of asthma is also well established [15, 16, 53,54,55,56,57]. Specifically, maternal prenatal exposure to pets, vaginal vs. c-section modes of delivery, childhood environments exposures, and childhood exposure to pets have all been associated with a reduced asthma risk. Particularly, dysbiosis of the airway can give rise to favorable growth conditions for Proteobacteria associated with viral respiratory infections. This dysbiosis dysregulates the crosstalk between the gut-lung axis and poses a serious risk for asthma development. Like allergy and the microbiome, the role of the microbiome in the development of asthma has been subject to many review articles [14, 15, 30, 55]. However, Table 1 provides an overview of the microbiota changes associated with the development of asthma [31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52].
The Role of the Microbiome in Occupational Lung Disease
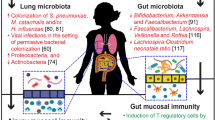
Our understanding of the composition of commensal microbiota and the subsequent development of occupational lung disease is limited. This section will review our current understanding of the effects of occupational exposures on the composition of the microbiota and their potential impact in the development of occupational lung disease. Figure 1 summarizes the current data regarding occupational exposures and the subsequent impact on commensal microbial populations.
Agricultural Exposures and the Microbiome
Animals/Animal Products
One of the best studied occupational exposures relative to the microbiome comes from studies involving agriculture workers exposed to various animals and animal products. Early studies demonstrated a significant association between bacterial communities in air samples from swine barns with the nasopharyngeal flora of the workers in contact with pigs. These similarities were not observed in non-exposed control groups [58]. Similarly, other studies demonstrated seasonal variability in the microbes comprising the air of pig barns and the correlation with fluctuation in the nasal microbiota of workers [59]. Additionally, shared microbial diversity seemed to be, in part, dependent on contact time with the animals, as the air-microbiota from pig farms and the nasal samples from pig farmers had 31.7% shared OTUs, which was significantly higher than the 23.4% shared OTUs between the bioaerosols in pig slaughterhouses and slaughterhouse workers [60]. Finally, other studies have demonstrated a higher similarity in gut microbiota between farmers and pigs, than between farmers and non-exposed human controls [61]. Additionally, pig farmers had less microbiota species diversity when compared to non-exposed villagers, which may suggest a higher health risk. These data were further confirmed by a longitudinal investigation of the impact of the swine farm on the gut microbial composition [62]. Specifically, veterinary students who had 3-month internships at a swine farm, exhibited a significant change their gut microbiota, which was observed to be more similar in composition to full-time farm workers’. Interestingly, these changes only partially reverted following 6-months of no exposure [63]. The composition of the nasal microbiome in subjects during and after long- and short-term exposure to livestock-associated MRSA showed similar trends in microbial imbalance. Nasal samples from all the tested pig farm workers showed the presence of LA-MRSA CC398. Further, all nasal samples collected immediately after a short-term exposure to the farm were positive for MRSA, which is particularly relevant as all the nasal samples collected prior to the farm visit were MRSA-negative [64]. In addition, to changes associated with swine operations, the species richness in the nasal microbiota of dairy farmers compared to non-dairy farmers was significantly higher. Further, dairy farmers had lower nasal carriage of Staphylococcus spp [65]. Additionally, higher levels of Proteobacteria, but lower levels of Actinobacteria, were observed on the forearm skin microbiota of farmworkers. Farm animal operations were also associated with a reduction in the prevalence of Staphylococcus and Streptococcus on the skin [66].
Pesticides
The effects of agricultural pesticide exposure on the microbiome have only been assessed in one study. The microbial composition of buccal (oral) microbiota was found to be significantly associated with blood concentration of the insecticide azinphos methyl. Specifically, there was a significant reduction in the abundance of Streptococcus [67]. In addition to human studies, there has been one animal study investigating the relationship between intestinal dysbiosis and pesticide exposure. Using a rat model of chlorpyrifos exposure, researchers found that exposed rats had significant changes in their gut microbiota, as well as changes in the metabolic capacity of the microbiota, all of which lead to an increased incident of obesity when compared to nonexposed rats [68].
Industrial Exposures and the Microbiome
Metalworking Fluid Exposure
Metalworking fluid is a cooling and lubricating fluid that is frequently colonized by microorganisms such as Pseudomonas. Interestingly, lung biopsies from symptomatic (pulmonary symptoms) workers exposed to metalworking fluid demonstrated an increased abundance of bacteria species typically isolated from the metalworking fluid. The pulmonary condition associated with metalworking fluids was characterized by lymphocytic bronchiolitis and alveolar ductitis with B-cell follicles and emphysema [69]. All symptomatic workers had an increased abundance of Pseudomonas in the lung, skin, and nasal samples of exposed workers, which was also found in metalworking fluid, suggesting that pulmonary Pseudomonas may have been derived from the metal working fluid. The Pseudomonas isolate was not identified in air samples from the metalworking shops, which suggests that Pseudomonas is being transmitted via contact with the metalworking fluid and not the the air [70]. Several additional studies have evaluated the effects heavy metal exposure on the microbial composition of the GI tract. For example, in both human and animal studies arsenic exposure alters the gut microbiome [71, 72]. Specifically, a higher abundance of Proteobacteria was observed in children with high arsenic exposure when compared to the low arsenic exposure controls [72]. Furthermore, WGS sequencing demonstrated that genes involved in virulence and multidrug resistance were positively correlated with arsenic levels. In mice, exposure to 100 ppb arsenic for 13 weeks significantly alters the composition and functional capacity of the GI microbiota compared to controls [71].
Dust Exposure
A limited number of papers have investigated the effects of dust (silica and ceramic dust) exposures on the microbiome. Subjects with early-stage pulmonary fibrosis due to silica exposure had significantly lower levels of Firmicutes and Actinobacteria in their GI microbial communities than healthy controls [73]. Further, the composition of the nasal microbiota in workers exposed to dust in ceramic factories was significantly altered. Specifically, there were marked increases in the abundance of Haemophilus spp., which was accompanied by a significant decrease in the abundance of Actinobacteria and Bacteroidetes, when compared to health non-exposed controls [74].
Deployment Exposures and the Microbiome
Chemical Exposure
Few studies have examined the microbiome in military personnel in relationship to deployment related exposures. One longitudinal study evaluated the respiratory tract microbial communities of healthy military personnel. This study found that Staphylococcus, Corynebacterium, and Propionibacterium accounted for ~75% of all microbial species in the nasal and nasopharyngeal microbiota, while Streptococcus was the only dominant bacterial genus in the oropharynx [75]. However, this study did not include a control group, which makes it difficult to evaluate if military exposures were associated with any changes in microbial composition. Similarly, in a mouse model of Gulf War illness, exposure to Gulf War chemicals were associated with GI microbial dysbiosis and decreased GI barrier function [76]. Interestingly, a follow-up study found that oral butyrate supplementation ameliorated many the associated effects of the chemical exposure in mice, suggesting that restoration of GI microbial functions may be an important factor in treating exposed personnel [77].
Dust/Burn Pits Exposure
While a large ongoing longitudinal project (the US Veteran Microbiome Project, [78, 79]) has been collecting samples since 2015, no studies have investigated the relationship between military deployment related exposures and the microbiome to our knowledge.
Chemical/Gas/Smoke Exposures and the Microbiome
Gas Exposure During Occupational Diving
To date only one study has evaluated the effects of helium–oxygen saturation diving on the GI microbial community. Specifically, there was a marked decrease in the abundance of Bifidobacterium Fusicatenibacter, Faecalibacterium, and Anaerostipes following saturation diving. The prevalence of Lactococcus, Actinomyces, Peptoclostridium, Butyricimonas, Streptococcus, and Porphyromonas increased post-dive in comparison to samples collected pre-saturation diving [80].
Smoke (Forest Fire, Wood Smoke) and Vehicle Emissions
Polychlorinated dibenzo-p-dioxins are formed and found naturally in the environment through volcanic eruptions and forest fires. Yet, the manufacturing of pesticides and burning organic materials (i.e., garbage) are also a major source of occupational exposure and environmental contamination with dioxins. The two major dioxins that have been studied are 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and 2,3,7,8-tetrachlorodibenzofuran (TCDF). To date we only have data from animal models and in vitro culture experiments on the effects of dioxin exposure on the microbiota. Specifically, conventional female mice treated with TCDD showed increased Enterobacteriaceae, as well as in increase in the reservoir of antibiotic-resistant genes when compared with control mice [81]. Finally, an in vitro study cultured human fecal suspensions with TCDD. Following treatment, fecal supernatants were incubated with intestinal epithelial cells and IL-8 secretion from intestinal epithelial cells was assessed [82]. TCDF exposure increased IL-8 only in the presence of microbial products, which suggest that dioxin-mediated dysbiosis may promote inflammation [82]. Similarly, rats exposed to smoke from either smoldered sawdust or motor vehicle exhaust over 4 weeks exhibited significance differences in the microbial diversity of the respiratory microbiota. Specifically, rats exposed to smoke or vehicle emissions exhibited a significant loss of Proteobacteria in the lungs, which correlated with significant changes in innate immune function [83]. Finally, mice exposed to concentrated ambient air (Chicago area) exhibited a significant increase in the diversity of the intestinal microbiota compared to controls. However, exposed mice exhibited a significant decrease in the abundance of Firmicutes [84].
Textile Exposures and the Microbiome
Exposure to dust in textile factories (cotton and/or flax) was one of the first occupational exposures thought to be associated with lung disease, namely asthma and COPD. This is due to the fact that the highest levels of airborne endotoxin were historically found recorded in textile factories, such as cotton mills [85]. Following this observation, several research studies linked endotoxin in organic dust to both asthma and COPD development [86, 87]. However, the literature on inhaled endotoxin exposure has been incongruent. Environmental endotoxin exposure on the development of childhood asthma has been shown to be protective [56, 57], while other studies have observed either no effect [88] or even a harmful effect [89, 90]. To our knowledge, no studies have evaluated the effects of textile dust exposure or inhaled endotoxin on microbiota diversity.
Conclusions
While the evidence for microbiome-mediated effects on the severity and development of allergy and asthma continue to grow, there remains a paucity of data regarding the functional consequences of microbial dysbiosis associated with occupational exposure. Data from both human and animal studies clearly describe marked differences in the composition, function, and immune phenotype of the microbial communities from multiple body sites following occupational exposure. Yet, to our knowledge, no studies have examined the functional consequences of the changes in the microbial communities with the development of occupational lung disease or occupational diseases in general. These knowledge gaps are an area primed for new investigation and will most likely significantly enhance our understanding of occupational exposures and the subsequent development of disease.
Data Availability
No datasets were generated or analysed during the current study.
References
Escherich T. Die darmbakterien des säuglings und ihre beziehungen zur physiologie der Verdauung. Enke; 1886.
Proctor L. Priorities for the next 10 years of human microbiome research. Nature. 2019;569:623–5.
Aarnoutse R, Ziemons J, de Vos-Geelen J, Valkenburg-van Iersel L, Wildeboer ACL, Vievermans A, Creemers GM, Baars A, Vestjens H, Le GN, et al. The role of intestinal microbiota in metastatic colorectal cancer patients treated with capecitabine. Clin Colorectal Cancer. 2022;21:e87–97. https://doi.org/10.1016/j.clcc.2021.10.004.
Abdul Rahim MBH, Chilloux J, Martinez-Gili L, Neves AL, Myridakis A, Gooderham N, Dumas ME. Diet-induced metabolic changes of the human gut microbiome: importance of short-chain fatty acids, methylamines and indoles. Acta Diabetol. 2019;56:493–500. https://doi.org/10.1007/s00592-019-01312-x.
Alsharairi NA. The role of short-chain fatty acids in the interplay between a very low-calorie ketogenic diet and the infant gut microbiota and its therapeutic implications for reducing asthma. Int J Mol Sci. 2020. https://doi.org/10.3390/ijms21249580.
Amabebe E, Anumba DOC. Female gut and genital tract microbiota-induced crosstalk and differential effects of short-chain fatty acids on immune sequelae. Front Immunol. 2020;11:2184. https://doi.org/10.3389/fimmu.2020.02184.
Baedke J, Fabregas-Tejeda A, Nieves Delgado A. The holobiont concept before Margulis. J Exp Zool B Mol Dev Evol. 2020;334:149–55. https://doi.org/10.1002/jez.b.22931.
Baxter NT, Schmidt AW, Venkataraman A, Kim KS, Waldron C, Schmidt TM. Dynamics of human gut microbiota and short-chain fatty acids in response to dietary interventions with three fermentable fibers. mBio. 2019. https://doi.org/10.1128/mBio.02566-18.
Bojovic K, Ignjatovic Eth I, Sokovic Bajic S, Vojnovic Milutinovic D, Tomic M, Golic N, Tolinacki M. Gut microbiota dysbiosis associated with altered production of short chain fatty acids in children with neurodevelopmental disorders. Front Cell Infect Microbiol. 2020;10:223. https://doi.org/10.3389/fcimb.2020.00223.
Castillo-Alvarez F, Marzo-Sola ME. Disease of the holobiont, the example of multiple sclerosis. Med Clin (Barc). 2019;152:147–53. https://doi.org/10.1016/j.medcli.2018.08.019.
Chambers ES, Preston T, Frost G, Morrison DJ. Role of gut microbiota-generated short-chain fatty acids in metabolic and cardiovascular health. Curr Nutr Rep. 2018;7:198–206. https://doi.org/10.1007/s13668-018-0248-8.
Chu H, Duan Y, Yang L, Schnabl B. Small metabolites, possible big changes: a microbiota-centered view of non-alcoholic fatty liver disease. Gut. 2019;68:359–70. https://doi.org/10.1136/gutjnl-2018-316307.
Dickson I. Gut microbiota: Diagnosing IBD with the gut microbiome. Nat Rev Gastroenterol Hepatol. 2017;14:195. https://doi.org/10.1038/nrgastro.2017.25.
Samuelson DR, Welsh DA, Shellito JE. Regulation of lung immunity and host defense by the intestinal microbiota. Front Microbiol. 2015;6:1085. https://doi.org/10.3389/fmicb.2015.01085.
Song XL, Liang J, Lin SZ, Xie YW, Ke CH, Ao D, Lu J, Chen XM, He YZ, Liu XH, et al. Gut-lung axis and asthma: A historical review on mechanism and future perspective. Clin Transl Allergy. 2024;14:e12356. https://doi.org/10.1002/clt2.12356.
Kallio S, Jian C, Korpela K, Kukkonen AK, Salonen A, Savilahti E, Kuitunen M, M. de Vos W. Early-life gut microbiota associates with allergic rhinitis during 13-year follow-up in a Finnish probiotic intervention cohort. Microbiol Spectr. 2024. https://doi.org/10.1128/spectrum.04135-23.
Hara M, Suzuki H, Hayashi D, Morii W, Nakamura T, Kiyoki K, Hara H, Ishii R, Noguchi E, Takada H. Gut microbiota of one-and-a-half-year-old food-allergic and healthy children. Allergol Int. 2024. https://doi.org/10.1016/j.alit.2024.03.004.
Vijayan S, Kandi V, Palacholla PS, Rajendran R, Jarugu C, Ca J, Pravallika M, Reddy SC, Sucharitha AS. Probiotics in allergy and immunological diseases: a comprehensive review. Cureus. 2024;16:e55817. https://doi.org/10.7759/cureus.55817.
Gourbeyre P, Denery S, Bodinier M. Probiotics, prebiotics, and synbiotics: impact on the gut immune system and allergic reactions. J Leukoc Biol. 2011;89:685–95. https://doi.org/10.1189/jlb.1109753.
Rodriguez B, Prioult G, Bibiloni R, Nicolis I, Mercenier A, Butel MJ, Waligora-Dupriet AJ. Germ-free status and altered caecal subdominant microbiota are associated with a high susceptibility to cow’s milk allergy in mice. FEMS Microbiol Ecol. 2011;76:133–44. https://doi.org/10.1111/j.1574-6941.2010.01035.x.
Van Zwol A, Van Den Berg A, Knol J, Twisk JW, Fetter WP, Van Elburg RM. Intestinal microbiota in allergic and nonallergic 1-year-old very low birth weight infants after neonatal glutamine supplementation. Acta Paediatr. 2010;99:1868–74. https://doi.org/10.1111/j.1651-2227.2010.01934.x.
del Giudice MM, Leonardi S, Maiello N, Brunese FP. Food allergy and probiotics in childhood. J Clin Gastroenterol. 2010;44(Suppl 1):S22-25. https://doi.org/10.1097/MCG.0b013e3181e102a7.
Shimada T, Kondoh M, Motonaga C, Kitamura Y, Cheng L, Shi H, Enomoto T, Tsuruta D, Ishii M, Kobayashi H. Enhancement of anti-allergic effects mediated by the Kampo medicine Shoseiryuto (Xiao-Qing-Long-Tang in Chinese) with lysed Enterococcus faecalis FK-23 in mice. Asian Pac J Allergy Immunol. 2010;28:59–66.
Ozdemir O. Any benefits of probiotics in allergic disorders? Allergy Asthma Proc. 2010;31:103–11. https://doi.org/10.2500/aap.2010.31.3313.
Shreiner A, Huffnagle GB, Noverr MC. The “Microflora Hypothesis” of allergic disease. Adv Exp Med Biol. 2008;635:113–34. https://doi.org/10.1007/978-0-387-09550-9_10.
Noverr MC, Noggle RM, Toews GB, Huffnagle GB. Role of antibiotics and fungal microbiota in driving pulmonary allergic responses. Infect Immun. 2004;72:4996–5003. https://doi.org/10.1128/IAI.72.9.4996-5003.2004.
Kalliomaki M, Isolauri E. Role of intestinal flora in the development of allergy. Curr Opin Allergy Clin Immunol. 2003;3:15–20. https://doi.org/10.1097/00130832-200302000-00003.
Kirjavainen PV, Gibson GR. Healthy gut microflora and allergy: factors influencing development of the microbiota. Ann Med. 1999;31:288–92. https://doi.org/10.3109/07853899908995892.
Zubeldia-Varela E, Barker-Tejeda TC, Obeso D, Villasenor A, Barber D, Perez-Gordo M. Microbiome and allergy: new insights and perspectives. J Investig Allergol Clin Immunol. 2022;32:327–44. https://doi.org/10.18176/jiaci.0852.
Noverr MC, Huffnagle GB. Does the microbiota regulate immune responses outside the gut? Trends Microbiol. 2004;12:562–8. https://doi.org/10.1016/j.tim.2004.10.008.
Aguilera AC, Dagher IA, Kloepfer KM. Role of the microbiome in allergic disease development. Curr Allergy Asthma Rep. 2020;20:44. https://doi.org/10.1007/s11882-020-00944-2. Current overview of prevalent allergies, their development, their interplay with resident microbiomes, and potential treatments.
Amedei A, Codolo G, Del Prete G, de Bernard M, D’Elios MM. The effect of Helicobacter pylori on asthma and allergy. J Asthma Allergy. 2010;3:139–47. https://doi.org/10.2147/JAA.S8971.
D’Elios MM, Codolo G, Amedei A, Mazzi P, Berton G, Zanotti G, Del Prete G, de Bernard M. Helicobacter pylori, asthma and allergy. FEMS Immunol Med Microbiol. 2009;56:1–8. https://doi.org/10.1111/j.1574-695X.2009.00537.x.
Huang YJ, Boushey HA. The microbiome in asthma. J Allergy Clin Immunol. 2015;135:25–30. https://doi.org/10.1016/j.jaci.2014.11.011.
Karimkhani C, Silverberg JI, Dellavalle RP. Defining intrinsic vs. extrinsic atopic dermatitis. Dermatol Online J. 2015;21(6):13030/qt14p8p404. PMID: 26158358.
Sokolowska M, Frei R, Lunjani N, Akdis CA, O’Mahony L. Microbiome and asthma. Asthma Res Pract. 2018;4:1. https://doi.org/10.1186/s40733-017-0037-y.
Spacova I, Van Beeck W, Seys S, Devos F, Vanoirbeek J, Vanderleyden J, Ceuppens J, Petrova M, Lebeer S. Lactobacillus rhamnosus probiotic prevents airway function deterioration and promotes gut microbiome resilience in a murine asthma model. Gut Microbes. 2020;11:1729–44. https://doi.org/10.1080/19490976.2020.1766345.
Bunyavanich S, Berin MC. Food allergy and the microbiome: current understandings and future directions. J Allergy Clin Immunol. 2019;144:1468–77. https://doi.org/10.1016/j.jaci.2019.10.019.
Ho HE, Chun Y, Jeong S, Jumreornvong O, Sicherer SH, Bunyavanich S. Multidimensional study of the oral microbiome, metabolite, and immunologic environment in peanut allergy. J Allergy Clin Immunol. 2021;148:627-632 e623. https://doi.org/10.1016/j.jaci.2021.03.028.
Al-Muhsen S, Clarke AE, Kagan RS. Peanut allergy: an overview. CMAJ. 2003;168:1279–85.
Caubet JC, Wang J. Current understanding of egg allergy. Pediatr Clin North Am. 2011;58:427–43. https://doi.org/10.1016/j.pcl.2011.02.014.
Chiang TY, Yang YR, Zhuo MY, Yang F, Zhang YF, Fu CH, Lee TJ, Chung WH, Chen L, Chang CJ. Microbiome profiling of nasal extracellular vesicles in patients with allergic rhinitis. World Allergy Organ J. 2022;15:100674. https://doi.org/10.1016/j.waojou.2022.100674.
Choi BY, Han M, Kwak JW, Kim TH. Genetics and epigenetics in allergic rhinitis. Genes (Basel). 2021. https://doi.org/10.3390/genes12122004.
Chong AC, Visitsunthorn K, Ong PY. Genetic/environmental contributions and immune dysregulation in children with atopic dermatitis. J Asthma Allergy. 2022;15:1681–700. https://doi.org/10.2147/JAA.S293900.
Giovannini M, Beken B, Buyuktiryaki B, Barni S, Liccioli G, Sarti L, Lodi L, Pontone M, Bartha I, Mori F, et al. IgE-mediated shellfish allergy in children. Nutrients. 2023. https://doi.org/10.3390/nu15122714.
Kanchongkittiphon W, Nopnipa S, Mathuranyanon R, Nonthabenjawan N, Sritournok S, Manuyakorn W, Wanapaisan P. Characterization of gut microbiome profile in children with confirmed wheat allergy. Asian Pac J Allergy Immunol. 2024. https://doi.org/10.12932/AP-080623-1626.
Miao P, Jiang Y, Jian Y, Shi J, Liu Y, Piewngam P, Zheng Y, Cheung GYC, Liu Q, Otto M, et al. Exacerbation of allergic rhinitis by the commensal bacterium Streptococcus salivarius. Nat Microbiol. 2023;8:218–30. https://doi.org/10.1038/s41564-022-01301-x.
Suther C, Moore MD, Beigelman A, Zhou Y. The gut microbiome and the big eight. Nutrients. 2020. https://doi.org/10.3390/nu12123728. This review provides an in-depth and up-to-date review of the “big eight” food allergens, including the potential mechanisms, risks, and known interactions between the gut microbiome.
Watts AM, West NP, Zhang P, Smith PK, Cripps AW, Cox AJ. The gut microbiome of adults with allergic rhinitis is characterised by reduced diversity and an altered abundance of key microbial taxa compared to controls. Int Arch Allergy Immunol. 2021;182:94–105. https://doi.org/10.1159/000510536.
Zhang Q, Wang Y, Fu L. Application of (multi-) omics approaches for advancing food allergy: an updated review. Curr Opin Food Sci. 2022;46:100854. Summarization of current studies using high-throughput technologies to observe gene expression, biologic markers, and microbiome composition of individuals with food allergies. This review also highlights potential therapeutic and preventative strategies to mitigate food allergies.
Kantor R, Silverberg JI. Environmental risk factors and their role in the management of atopic dermatitis. Expert Rev Clin Immunol. 2017;13:15–26. https://doi.org/10.1080/1744666X.2016.1212660.
Wang DY. Risk factors of allergic rhinitis: genetic or environmental? Ther Clin Risk Manag. 2005;1:115–23. https://doi.org/10.2147/tcrm.1.2.115.62907.
Sampaio Dotto Fiuza B, Machado de Andrade C, Meirelles PM, Santos da Silva J, de Jesus Silva M, Vila Nova Santana C, Pimentel Pinheiro G, Mpairwe H, Cooper P, Brooks C, et al. Gut microbiome signature and nasal lavage inflammatory markers in young people with asthma. J Allergy Clin Immunol Glob. 2024;3:100242. https://doi.org/10.1016/j.jacig.2024.100242.
Huang YJ, Nelson CE, Brodie EL, Desantis TZ, Baek MS, Liu J, Woyke T, Allgaier M, Bristow J, Wiener-Kronish JP, et al. Airway microbiota and bronchial hyperresponsiveness in patients with suboptimally controlled asthma. J Allergy Clin Immunol. 2011;127(372–381):e371-373. https://doi.org/10.1016/j.jaci.2010.10.048.
Huffnagle GB. The microbiota and allergies/asthma. PLoS Pathog. 2010;6:e1000549. https://doi.org/10.1371/journal.ppat.1000549.
Douwes J, van Strien R, Doekes G, Smit J, Kerkhof M, Gerritsen J, Postma D, de Jongste J, Travier N, Brunekreef B. Does early indoor microbial exposure reduce the risk of asthma? The prevention and incidence of asthma and mite allergy birth cohort study. J Allergy Clin Immunol. 2006;117:1067–73. https://doi.org/10.1016/j.jaci.2006.02.002.
Braun-Fahrlander C, Riedler J, Herz U, Eder W, Waser M, Grize L, Maisch S, Carr D, Gerlach F, Bufe A, et al. Environmental exposure to endotoxin and its relation to asthma in school-age children. N Engl J Med. 2002;347:869–77. https://doi.org/10.1056/NEJMoa020057.
Mbareche H, Veillette M, Pilote J, Letourneau V, Duchaine C. Bioaerosols play a major role in the nasopharyngeal microbiota content in agricultural environment. Int J Environ Res Public Health. 2019. https://doi.org/10.3390/ijerph16081375.
Kraemer JG, Aebi S, Oppliger A, Hilty M. The indoor-air microbiota of pig farms drives the composition of the pig farmers’ nasal microbiota in a season-dependent and farm-specific manner. Appl Environ Microbiol. 2019. https://doi.org/10.1128/AEM.03038-18.
Wu JY, Zhu YS, Guo C, Xia Y, Guo ZM, Li QL, Lu JH. A comparative study of associated microbiota between pig farm and pig slaughterhouse in Guangdong, China. Curr Microbiol. 2020;77:3310–20. https://doi.org/10.1007/s00284-020-02187-w.
Tan SC, Chong CW, Yap IKS, Thong KL, Teh CSJ. Comparative assessment of faecal microbial composition and metabonome of swine, farmers and human control. Sci Rep. 2020;10:8997. https://doi.org/10.1038/s41598-020-65891-4. This study demonstrates that long term occupational exposure to swine and farm environment effects the gut bacterial composition of farmers.
Sun J, Huang T, Chen C, Cao TT, Cheng K, Liao XP, Liu YH. Comparison of fecal microbial composition and antibiotic resistance genes from swine, farm workers and the surrounding villagers. Sci Rep. 2017;7:4965. https://doi.org/10.1038/s41598-017-04672-y.
Sun J, Liao XP, D’Souza AW, Boolchandani M, Li SH, Cheng K, Luis Martinez J, Li L, Feng YJ, Fang LX, et al. Environmental remodeling of human gut microbiota and antibiotic resistome in livestock farms. Nat Commun. 2020;11:1427. https://doi.org/10.1038/s41467-020-15222-y. This study examined the impact of the swine farm environments on temporal changes in the gut microbiome and resistome. This study found that acute changes in a human’s living environment persistently shape their gut microbiota and antibiotic resistome.
Islam MZ, Johannesen TB, Lilje B, Urth TR, Larsen AR, Angen O, Larsen J. Investigation of the human nasal microbiome in persons with long- and short-term exposure to methicillin-resistant Staphylococcus aureus and other bacteria from the pig farm environment. PLoS ONE. 2020;15:e0232456. https://doi.org/10.1371/journal.pone.0232456.
Shukla SK, Ye Z, Sandberg S, Reyes I, Fritsche TR, Keifer M. The nasal microbiota of dairy farmers is more complex than oral microbiota, reflects occupational exposure, and provides competition for staphylococci. PLoS ONE. 2017;12:e0183898. https://doi.org/10.1371/journal.pone.0183898.
Peng M, Biswas D. Environmental influences of high-density agricultural animal operation on human forearm skin microflora. Microorganisms. 2020. https://doi.org/10.3390/microorganisms8101481.
Stanaway IB, Wallace JC, Shojaie A, Griffith WC, Hong S, Wilder CS, Green FH, Tsai J, Knight M, Workman T, et al. Human oral buccal microbiomes are associated with farmworker status and azinphos-methyl agricultural pesticide exposure. Appl Environ Microbiol. 2017. https://doi.org/10.1128/AEM.02149-16.
Fang B, Li JW, Zhang M, Ren FZ, Pang GF. Chronic chlorpyrifos exposure elicits diet-specific effects on metabolism and the gut microbiome in rats. Food Chem Toxicol. 2018;111:144–52. https://doi.org/10.1016/j.fct.2017.11.001.
Cummings KJ, Stanton ML, Nett RJ, Segal LN, Kreiss K, Abraham JL, Colby TV, Franko AD, Green FHY, Sanyal S, et al. Severe lung disease characterized by lymphocytic bronchiolitis, alveolar ductitis, and emphysema (BADE) in industrial machine-manufacturing workers. Am J Ind Med. 2019;62:927–37. https://doi.org/10.1002/ajim.23038.
Wu BG, Kapoor B, Cummings KJ, Stanton ML, Nett RJ, Kreiss K, Abraham JL, Colby TV, Franko AD, Green FHY, et al. Evidence for environmental-human microbiota transfer at a manufacturing facility with novel work-related respiratory disease. Am J Respir Crit Care Med. 2020;202:1678–88. https://doi.org/10.1164/rccm.202001-0197OC.
Chi L, Bian X, Gao B, Tu P, Ru H, Lu K. The effects of an environmentally relevant level of arsenic on the gut microbiome and its functional metagenome. Toxicol Sci. 2017;160:193–204. https://doi.org/10.1093/toxsci/kfx174.
Dong X, Shulzhenko N, Lemaitre J, Greer RL, Peremyslova K, Quamruzzaman Q, Rahman M, Hasan OS, Joya SA, Golam M, et al. Arsenic exposure and intestinal microbiota in children from Sirajdikhan, Bangladesh. PLoS One. 2017;12:e0188487. https://doi.org/10.1371/journal.pone.0188487.
Zhou Y, Chen L, Sun G, Li Y, Huang R. Alterations in the gut microbiota of patients with silica-induced pulmonary fibrosis. J Occup Med Toxicol. 2019;14:5. https://doi.org/10.1186/s12995-019-0225-1.
Ahmed N, Mahmoud NF, Solyman S, Hanora A. Human nasal microbiome as characterized by metagenomics differs markedly between rural and industrial communities in Egypt. OMICS. 2019;23:573–82. https://doi.org/10.1089/omi.2019.0144.
Hang J, Zavaljevski N, Yang Y, Desai V, Ruck RC, Macareo LR, Jarman RG, Reifman J, Kuschner RA, Keiser PB. Composition and variation of respiratory microbiota in healthy military personnel. PLoS ONE. 2017;12:e0188461. https://doi.org/10.1371/journal.pone.0188461.
Alhasson F, Das S, Seth R, Dattaroy D, Chandrashekaran V, Ryan CN, Chan LS, Testerman T, Burch J, Hofseth LJ, et al. Altered gut microbiome in a mouse model of Gulf War Illness causes neuroinflammation and intestinal injury via leaky gut and TLR4 activation. PLoS ONE. 2017;12:e0172914. https://doi.org/10.1371/journal.pone.0172914.
Seth RK, Kimono D, Alhasson F, Sarkar S, Albadrani M, Lasley SK, Horner R, Janulewicz P, Nagarkatti M, Nagarkatti P, et al. Increased butyrate priming in the gut stalls microbiome associated-gastrointestinal inflammation and hepatic metabolic reprogramming in a mouse model of Gulf War Illness. Toxicol Appl Pharmacol. 2018;350:64–77. https://doi.org/10.1016/j.taap.2018.05.006.
Stanislawski MA, Stamper CE, Stearns-Yoder KA, Hoisington AJ, Brostow DP, Forster JE, Postolache TT, Lowry CA, Brenner LA. Characterization of the gut microbiota among Veterans with unique military-related exposures and high prevalence of chronic health conditions: A United States-Veteran Microbiome Project (US-VMP) study. Brain Behav Immun Health. 2021;18:100346. https://doi.org/10.1016/j.bbih.2021.100346. This study examined the relationship between military-related exposures and chronic health conditions. This study also sets the ground work to evaluate if microbiota related changes are mediating or associated with exposures and health outcomes.
Brenner LA, Hoisington AJ, Stearns-Yoder KA, Stamper CE, Heinze JD, Postolache TT, Hadidi DA, Hoffmire CA, Stanislawski MA, Lowry CA. Military-related exposures, social determinants of health, and dysbiosis: The United States-Veteran Microbiome Project (US-VMP). Front Cell Infect Microbiol. 2018;8:400. https://doi.org/10.3389/fcimb.2018.00400.
Yuan Y, Zhao G, Ji H, Peng B, Huang Z, Jin W, Chen X, Guan H, Tang G, Zhang H, et al. Changes in the gut microbiota during and after commercial helium-oxygen saturation diving in China. Occup Environ Med. 2019;76:801–7. https://doi.org/10.1136/oemed-2019-106031.
Stedtfeld RD, Stedtfeld TM, Fader KA, Williams MR, Bhaduri P, Quensen J, Zacharewski TR, Tiedje JM, Hashsham SA. TCDD influences reservoir of antibiotic resistance genes in murine gut microbiome. FEMS Microbiol Ecol. 2017. https://doi.org/10.1093/femsec/fix058.
Defois C, Ratel J, Garrait G, Denis S, Le Goff O, Talvas J, Mosoni P, Engel E, Peyret P. Food chemicals disrupt human gut microbiota activity and impact intestinal homeostasis as revealed by in vitro systems. Sci Rep. 2018;8:11006. https://doi.org/10.1038/s41598-018-29376-9.
Li N, He F, Liao B, Zhou Y, Li B, Ran P. Exposure to ambient particulate matter alters the microbial composition and induces immune changes in rat lung. Respir Res. 2017;18:143. https://doi.org/10.1186/s12931-017-0626-6.
Mutlu EA, Comba IY, Cho T, Engen PA, Yazici C, Soberanes S, Hamanaka RB, Nigdelioglu R, Meliton AY, Ghio AJ, et al. Inhalational exposure to particulate matter air pollution alters the composition of the gut microbiome. Environ Pollut. 2018;240:817–30. https://doi.org/10.1016/j.envpol.2018.04.130.
Olenchock SA, Christiani DC, Mull JC, Ye TT, Lu PL. Endotoxins in baled cottons and airborne dusts in textile mills in the People’s Republic of China. Appl Environ Microbiol. 1983;46:817–20. https://doi.org/10.1128/aem.46.4.817-820.1983.
Vogelzang PF, van der Gulden JW, Folgering H, Kolk JJ, Heederik D, Preller L, Tielen MJ, van Schayck CP. Endotoxin exposure as a major determinant of lung function decline in pig farmers. Am J Respir Crit Care Med. 1998;157:15–8. https://doi.org/10.1164/ajrccm.157.1.9703087.
Lai PS, Christiani DC. Long-term respiratory health effects in textile workers. Curr Opin Pulm Med. 2013;19:152–7. https://doi.org/10.1097/MCP.0b013e32835cee9a.
Rullo VE, Arruda LK, Cardoso MR, Valente V, Zampolo AS, Nobrega F, Naspitz CK, Sole D. Respiratory infection, exposure to mouse allergen and breastfeeding: role in recurrent wheezing in early life. Int Arch Allergy Immunol. 2009;150:172–8. https://doi.org/10.1159/000218120.
Perzanowski MS, Miller RL, Thorne PS, Barr RG, Divjan A, Sheares BJ, Garfinkel RS, Perera FP, Goldstein IF, Chew GL. Endotoxin in inner-city homes: associations with wheeze and eczema in early childhood. J Allergy Clin Immunol. 2006;117:1082–9. https://doi.org/10.1016/j.jaci.2005.12.1348.
Thorne PS, Kulhankova K, Yin M, Cohn R, Arbes SJ Jr, Zeldin DC. Endotoxin exposure is a risk factor for asthma: the national survey of endotoxin in United States housing. Am J Respir Crit Care Med. 2005;172:1371–7. https://doi.org/10.1164/rccm.200505-758OC.
Acknowledgements
Figures were created with BioRender.com.
Funding
Dr. Samuelson is supported by National Institutes of Health, the National Institute on Alcohol Abuse and Alcoholism Grant #P50-AA030407; National Institutes of Health; and the National Institute of Diabetes and Digestive and Kidney Diseases Grant #R01-DK131990-01.
Author information
Authors and Affiliations
Contributions
Ashley Peer drafted the manuscript and prepared the figures. Dr. Samuelson conceived of the work and critically reviewed the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Peer, A., Samuelson, D.R. The Role of the Microbiome in Allergy, Asthma, and Occupational Lung Disease. Curr Allergy Asthma Rep (2024). https://doi.org/10.1007/s11882-024-01156-8
Accepted:
Published:
DOI: https://doi.org/10.1007/s11882-024-01156-8