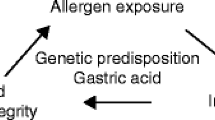
Abstract
The gastrointestinal immune system is a major component of the mucosal barrier, which provides an appropriate immunologic homeostasis between host and numerous foreign antigens, including microbial and dietary antigens. However, under certain pathological circumstances created by disturbance of the immunologic balance, allergic responses associated with the gastrointestinal tract can be triggered by abnormal immune responses against selected food protein antigens. Among the several types of immune competent cells, eosinophils are generally considered to play a central role for the development of allergic diseases in gastrointestinal tissue. Although most research has been focused on the molecular and cellular understanding of eosinophils in the peripheral tissues and lung, recent studies elucidate the unique trafficking and regulation mechanisms of eosinophils in the gastrointestinal tissues. In this review, we summarize current findings in the regulatory mechanism of gastrointestinal eosinophils. Furthermore, several unique murine models for eosinophilic gastroenteritis, which can be applied for the elucidation of underlying mechanisms of eosinophilmediated gastrointestinal allergy, and the development of new mucosal immune therapy for the control of food allergy are reviewed.
Similar content being viewed by others
References and Recommended Reading
Rothenberg ME, Mishra A, Brandt EB, Hogan SP: Gastrointestinal eosinophils. Immunol Rev 2001, 179:139–155. This is a well-prepared, comprehensive review article for the role of eosinophils in the the gastrointestinal tissues.
Kita H: The eosinophil: a cytokine-producing cell? J Allergy Clin Immunol 1996, 97:889–892.
Rothenberg ME: Eotaxin: an essential mediator of eosinophil trafficking into mucosal tissues. Am J Respir Cell Mol Biol 1999, 21:291–295.
Lewis RA, Austen KF, Soberman RJ: Leukotrienes and other products of the 5-lipoxygenase pathway: biochemistry and relation to pathobiology in human diseases. N Engl J Med 1990, 323:645–655.
Slifman NR, Loegering DA, McKean DJ, Gleich GJ: Ribonuclease activity associated with human eosinophil-derived neurotoxin and eosinophil cationic protein. J Immunol 1986, 137:2913–2917.
Rosenberg HF, Tenen DG, Ackerman SJ: Molecular cloning of the human eosinophil-derived neurotoxin: a member of the ribonuclease gene family. Proc Natl Acad Sci U S A 1989, 86:4460–4464.
Jacoby DB, Gleich GJ, Fryer AD: Human eosinophil major basic protein is an endogenous allosteric antagonist at the inhibitory muscarinic M2 receptor. J Clin Invest 1993, 91:1314–1318.
Woerly G, Roger N, Loiseau S, et al.: Expression of CD28 and CD86 by human eosinophils and role in the secretion of type 1 cytokines (interleukin 2 and interferon gamma): inhibition by immunoglobulin a complexes. J Exp Med 1999, 190:487–495.
Tamura N, Ishii N, Nakazawa M, et al.: Requirement of CD80 and CD86 molecules for antigen presentation by eosinophils. Scand J Immunol 1996, 44:229–238.
Shi HZ, Humbles A, Gerard C, et al.: Lymph node trafficking and antigen presentation by endobronchial eosinophils. J Clin Invest 2000, 105:945–953. After inhalational antigen challenge, airway eosinophils expressed MHC class II and costimulatory CD80 and CD86 proteins and acted as antigen presenting cells.
Ponath PD, Qin S, Post TW, et al.: Molecular cloning and characterization of a human eotaxin receptor expressed selectively on eosinophils. J Exp Med 1996, 183:2437–2448.
Homey B, Zlotnik A: Chemokines in allergy. Curr Opin Immunol 1999, 11:626–634.
Saavedra-Delgado AM, Metcalfe DD: Interactions between food antigens and the immune system in the pathogenesis of gastrointestinal diseases. Ann Allergy 1985, 55:694–702.
Katz AJ, Twarog FJ, Zeiger RS, Falchuk ZM: Milk-sensitive and eosinophilic gastroenteropathy: similar clinical features with contrasting mechanisms and clinical course. J Allergy Clin Immunol 1984, 74:72–78.
Kristopaitis T, Neghme C, Yong SL, et al.: Giant antral ulcer: a rare presentation of eosinophilic gastroenteritis: case report and review of the literature. Am J Gastroenterol 1997, 92:1205–1208.
Torpier G, Colombel JF, Mathieu-Chandelier C, et al.: Eosinophilic gastroenteritis: ultrastructural evidence for a selective release of eosinophil major basic protein. Clin Exp Immunol 1988, 74:404–408.
Odze RD, Wershil BK, Leichtner AM, Antonioli DA: Allergic colitis in infants. J Pediatr 1995, 126:163–170.
Walsh RE, Gaginella TS: The eosinophil in inflammatory bowel disease. Scand J Gastroenterol 1991, 26:1217–1224.
Hill SM, Milla PJ: Colitis caused by food allergy in infants. Arch Dis Child 1990, 65:132–133.
Kelly KJ, Lazenby AJ, Rowe PC, et al.: Eosinophilic esophagitis attributed to gastroesophageal reflux: improvement with an amino acid-based formula. Gastroenterology 1995, 109:1503–1512.
Brown LF, Goldman H, Antonioli DA: Intraepithelial eosinophils in endoscopic biopsies of adults with reflux esophagitis. Am J Surg Pathol 1984, 8:899–905.
Ohtsuka Y, Suzuki R, Nagata S, et al.: Chronic oral antigen exposure induces lymphocyte migration in anaphylactic mouse intestine. Pediatr Res 1998, 44:791–797.
Mishra A, Hogan SP, Lee JJ, et al.: Fundamental signals that regulate eosinophil homing to the gastrointestinal tract. J Clin Invest 1999, 103:1719–1727. This is a description of eosinophil homing into the gastrointestinal tract. Eosinophils were already found in the laminar propria of 19-day-old embryos and germ-free adult mice.
Hogan SP, Mishra A, Brandt EB, et al.: A critical role for eotaxin in experimental oral antigen-induced eosinophilic gastrointestinal allergy. Proc Natl Acad Sci U S A 2000, 97:6681–6686. This article introduces a new murine model of antigen-induced eosinophil-associated gastrointestinal allergy. Eotaxin was identified as a critical regulator of antigen-induced eosinophil accumulation in the small intestine.
Hogan SP, Mishra A, Brandt EB, et al.: A pathological function for eotaxin and eosinophils in eosinophilic gastrointestinal inflammation. Nat Immunol 2001, 2:353–360. This article describes how the oral exposure of enteric coated antigen provokes the eosinophilic allergic reaction in the esophagus, stomach, small intestine, and Peyer’s patches. Eosinophil-mediated allergic mice also develop gastric dysmotility, gastromegaly, and cachexia.
Mishra A, Hogan SP, Brandt EB, Rothenberg ME: Peyer’s patch eosinophils: identification, characterization, and regulation by mucosal allergen exposure, interleukin-5, and eotaxin. Blood 2000, 96:1538–1544.
Mishra A, Hogan SP, Brandt EB, Rothenberg ME: An etiological role for aeroallergens and eosinophils in experimental esophagitis. J Clin Invest 2001, 107:83–90.
Kweon MN, Yamamoto M, Kajiki M, et al.: Systemically derived large intestinal CD4+ Th2 cells play a central role in STAT6-mediated allergic diarrhea. J Clin Invest 2000, 106:199–206. This paper introduces a new murine model for the antigen-induced mucosal allergy. Repeated administration of oral antigen induced severe diarrhea in systemically primed mice. A unique finding reported in this paper is that antigen-specific allergic reactions selectively occur in the large intestine but not the small intestine.
Yamaguchi Y, Suda T, Suda J, et al.: Purified interleukin-5 supports the terminal differentiation and proliferation of murine eosinophilic precursors. Exp Med 1988, 167:43–56.
Weller PF: The immunobiology of eosinophils. N Engl J Med 1991, 324:1110–1118.
Foster PS, Hogan SP, Ramsay AJ, et al.: Interleukin 5 deficiency abolishes eosinophilia, airways hyperreactivity, and lung damage in a mouse asthma model. J Exp Med 1996, 183:195–201.
Mould AW, Matthaei KI, Young IG, Foster PS: Relationship between interleukin-5 and eotaxin in regulating blood and tissue eosinophilia in mice. J Clin Invest 1997, 99:1064–1071.
Rothenberg ME, MacLean JA, Pearlman E, et al.: Targeted disruption of the chemokine eotaxin partially reduces antigeninduced tissue eosinophilia. J Exp Med 1997, 185:785–790.
Matthews AN, Friend DS, Zimmermann N, et al.: Eotaxin is required for the baseline level of tissue eosinophils. Proc Natl Acad Sci U S A 1998, 95:6273–6278.
Vandezande LM, Wallaert B, Desreumaux P, et al.:Interleukin-5 immunoreactivity and mRNA expression in gut mucosa from patients with food allergy. Clin Exp Allergy 1999, 29:652–659.
Talley NJ, Shorter RG, Phillips SF, Zinsmeister AR: Eosinophilic gastroenteritis: a clinicopathological study of patients with disease of the mucosa, muscle layer, and subserosal tissues. Gut 1990, 31:54–58.
Mishra A, Hogan SP, Brandt EB, et al.: Enterocyte expression of the eotaxin and interleukin-5 transgenes induces compartmentalized dysregulation of eosinophil trafficking. J Biol Chem 2002, 277:4406–4412.
Kunkel EJ, Butcher EC: Chemokines and the tissue-specific migration of lymphocytes. Immunity 2002, 16:1–4.
Resnick MB, Weller PF: Mechanisms of eosinophil recruitment. Am J Respir Cell Mol Biol 1993, 8:349–355.
Henriques GM, Miotta JH, Cordeiro SB, et al.: Selectins mediate eosinophil recruitment in vivo: a comparison with their role in neutrophil influx. Blood 1996, 87:5297–5304
Walsh GM, Mermod JJ, Hartnell A, et al.: Human eosinophil, but not neutrophil, adherence to IL-1-stimulated human umbilical vascular endothelial cells is alpha 4 beta 1 (very late antigen-4) dependent. J Immunol 1991, 146:3419–3423.
Weller PF, Rand TH, Goelz SE, et al.: Human eosinophil adherence to vascular endothelium mediated by binding to vascular cell adhesion molecule 1 and endothelial leukocyte adhesion molecule 1. Proc Natl Acad Sci U S A. 1991, 88:7430–7433.
Gonzalo JA, Lloyd CM, Kremer L, et al.: Eosinophil recruitment to the lung in a murine model of allergic inflammation: the role of T cells, chemokines, and adhesion receptors. J Clin Invest 1996, 98:2332–2345.
Bochner BS, Sterbinsky SA, Knol EF, et al.: Function and expression of adhesion molecules on human basophils. J Allergy Clin Immunol 1994, 94:1157–1162.
Artis D, Humphreys NE, Potten CS, et al.: Beta7 integrin-deficient mice: delayed leukocyte recruitment and attenuated protective immunity in the small intestine during enteric helminth infection. Eur J Immunol 2000, 30:1656–1664.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kweon, MN., Kiyono, H. Eosinophilic gastroenteritis: A problem of the mucosal immune system?. Curr Allergy Asthma Rep 3, 79–85 (2003). https://doi.org/10.1007/s11882-003-0016-7
Issue Date:
DOI: https://doi.org/10.1007/s11882-003-0016-7