Abstract
Air pollution is an environmental factor involved in neuroinflammation, which leads to the progressive neuronal damage that appears in various neurodegenerative diseases. This article reviews the impact on Amyotrophic Lateral Sclerosis (ALS), the most common degenerative motor neuron disease, of exposure to BTEX (benzene, toluene, ethylbenzene, and xylene), aromatic hydrocarbons capable of crossing the blood-brain barrier, with documented neurotoxic effects. Results show that occupational exposure to BTEX has been linked to the incidence of ALS, while the relationship with the exposure in residential environments with high levels of these toxins in outdoor air is not conclusive; sources of BTEX emissions often lead to mixed toxic exposure, making it challenging to assess the specific impact of this group of pollutants. Under the commonly accepted hypothesis that ALS is a disease triggered by the accumulation of multiple steps, BTEX could be the step causing toxic insult, or alternatively, BTEX might play a role in the disease’s progression. However new studies are necessary to determine its involvement in the disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The role of environmental factors in the neuroinflammation that leads to many Central Nervous System (CNS) diseases has been previously described in the literature, among these, exposure to air pollution is considered the primary culprit for the inflammation and oxidative stress (Block and Calderón-Garcidueñas 2009; Costa et al. 2020).
When exposed to air pollution, the microglia (a type of cells of the central nervous system that function as elements of the immune system, protecting the organism from external and internal aggressions) activate and release cytokines and Reactive Oxygen Species (ROS) that are involved in the progressive neuronal damage that appears in various neurodegenerative diseases. Specifically, the MAC1-NOX2 pathway has been identified as a mechanism through which microglia respond to different forms of air pollution (Jayaraj et al. 2017). Regarding the functioning of this pathway, phagocytic NADPH oxidase (PHOX) is involved in chronic neurodegeneration mediated by oxidative stress. PHOX is generally inactive in microglia, but upon certain stimuli (such as some atmospheric pollutants), it can become activated and act on the microglial MAC1 receptor. MAC1 can recognize damage-associated molecular patterns (DAMPs) (released from damaged or dead neurons) and activate downstream NADPH oxidase (NOX2) to produce superoxide anions and associated ROS, such as hydrogen peroxide, a compound which play a critical role in reactive microgliosis and drive chronic neurodegeneration (Chen et al. 2016; Gao et al. 2012).
Along with these, different studies have linked high levels of air pollution to a wide variety of degenerative neurological diseases such as Alzheimer’s disease, Parkinson’s disease, as well as other non-degenerative diseases such as strokes (Costa et al. 2020; Murata et al. 2022; Royé et al., 2019).
In the specific case of Amyotrophic Lateral Sclerosis (ALS), results from different studies published so far are conflicting. Some authors have described a relationship between long-term exposure to poor air quality and the incidence of ALS (Malek et al., 2023; Saucier et al. 2023; Seelen et al. 2017), but these results have not been reproduced in other areas (Filippini et al. 2021; Parks et al. 2022). Additionally, exposure to increased levels of air pollution (from particulate matter, carbon monoxide and sulfur dioxide) has been found to be associated with increased emergency department visits (Myung et al. 2019) and to have a positive correlation with the clinical deterioration of patients with neurodegenerative diseases (Nunez et al. 2021).
Generically, analysis of air pollution takes a dual approach, depending on whether it is indoor pollution or outdoor environmental pollution. The studies mentioned above use air quality measurements in outdoor environments. However, in a recent study Kotzias (2022) concluded that, when exposure to poor air quality is being addressed, indoor environments are critical due to simultaneously the amount of time individuals spend indoors (between home, workplaces, visits to public spaces, school, etc., Kotzias estimated to be more than 85% of the day) and the concentration accumulate of air pollutants. The WHO estimates that 4.3 million people die annually from exposure to air pollutants in the home (WHO, 2014). Furthermore, in enclosed environments, it is more common for toxins to concentrate, and it is easier to quantify individual human exposure than in outdoor environments where meteorological factors play a significant role in their dispersion (Di Bernardino et al. 2021). For these reasons, there are increasingly more studies are aimed at assessing the impact of indoor air quality (IAC) on many diseases (Kumar et al. 2023).
In indoor environments, Volatile Organic Compounds (VOCs), hydrocarbons that are present in a gaseous state at normal ambient temperature or are very volatile at that temperature, come from both internal and external sources and have a natural (biogenic VOCs) and anthropogenic (due to evaporation of organic solvents, burning of fuels, transportation, etc.) origin. Although there are many VOCs, the study of BTEX (benzene, toluene, ethylbenzene and the three isomers of xylene [o-xylene and (m, p)-xylene]) has gained special prominence due to their extreme toxicity (Dehghani et al. 2018; Shrubsole et al. 2019), role in tropospheric chemistry (specifically in the formation of ozone), and their abundance in the air in urban environments.
In this article we will carry a narrative literature review of the effects on ALS of air quality, focusing specifically on exposure to BTEX volatile organic compounds (VOCs).
Amyotrophic lateral sclerosis (ALS)
ALS is the most common degenerative disease of the motor neurons (MNs), with an annual incidence rate ranging from one to three cases per 100,000 inhabitants (Masrori et al., 2020; Riancho et al. 2016). This disease is characterized by progressive degeneration of the MNs, which translates into a progressive amyotrophy that leads to the patient’s death in an average time of about two to three years from time of diagnosis.
From a clinical point of view, ALS is a very heterogeneous disease that can debut in different ways. The two most frequent forms of presentation are the spinal onset in which the patient starts with weakness, fasciculations and muscle atrophy affecting the arm or the leg that subsequently spreads to the rest of the limbs, and the bulbar onset form in which patients have a progressive bulbar involvement typically characterized by swallowing difficulties (dysphagia), language articulation impairment and ventilatory disorders (Feldman et al. 2022).
ALS lacks a specific diagnostic test. Over time, different diagnostic criteria have appeared based on clinical and neurophysiological aspects that should prove involvement of both the upper motor neuron (neuron connecting the motor cortex with the motor nuclei of the brainstem or the anterior horn of the spinal cord) and the lower motor neuron (neuron that connects the motor nuclei of the brainstem or the anterior horn of the spinal cord with the muscles) (Shefner et al. 2020). In addition, other diseases that may mimic ALS should be rule out. In this sense patients are commonly performed several ancillary tests including (blood analysis and neuroimaging studies) (Yedavalli et al. 2018).
A small percentage of ALS cases have a genetic origin (familial ALS, fALS) and are due to mutations in specific genes. By contrast, the vast majority of cases are presumed to have a sporadic origin (sALS) and the role of environmental factors could be key in these cases (Al Chalabi et al., 2014; Riancho et al. 2021). Although the pathogenesis of ALS has not been fully clarified, in recent decades degeneration of the MNs has been associated with different mechanisms, among which are found alterations in gene processing, increased levels of oxidative stress, disorders in protein metabolism, mitochondrial and cellular bioenergetic dysfunction, alterations in axonal transport or glial cell dysfunction (López-Pingarrón et al. 2023; Motataianu et al. 2022).
Even though the precise factors that trigger the disease have not been clarified, it is assumed that the vast majority of ALS cases are the result of the interaction between environmental risk factors/exposures, genetic factors, and aging. Under this theory, the disease would occur in people in whom the sum of these three components reached a certain threshold (regardless of the particular magnitude of each one of them), from which mechanisms would be activated that perpetuate the disease. Along this line of thought, certain authors have published that ALS could be a “step-by-step” disease, with a series of successive events being needed before it develops (Al Chalabi et al., 2014). In this context, exposure to different environmental factors would condition different “steps” of the equation. In recent decades, various studies have pointed to a striking increase in the incidence of ALS (Fang et al. 2009; GBD 2016 Motor Neuron Disease Collaborators, 2018; Mata et al. 2023). In the conception of ALS described above, this increase in cases does not seem to be due to changes in the genetic structure of the population (which occur slowly over many generations) but rather to progressive aging of the population and changes in the environmental factors to which the population has been exposed (Riancho et al. 2016).
Benzene, toluene, ethylbenzene, and xylenes (BTEX)
BTEX belong to the aromatic hydrocarbon family, are lipophilic, so they can cross the blood-brain barrier. Acute exposure to high doses has been shown to be neurotoxic, and chronic exposure has been associated with encephalopathy and cognitive deficit (Dickerson et al. 2020; Sainio 2015; Słomińska et al., 2014).
All the compounds that make up the BTEX group have effects on the neurological system separately. Nevertheless, in environments where people are exposed to these compounds they are usually found together and the impact of mixed exposure is more severe on health (ATSDR, 2004).
The main entryway of BTEX into the organism is through inhalation and this is determined by the concentration of these compounds in indoor and outdoor spaces (Kamani et al. 2023).
BTEX are found naturally in crude oil and seawater where gas and oil deposits are extracted. In addition, BTEX are commonly used at the industrial level because of their high solvent power, and they are present in tobacco smoke and in various products used in the home (paints, solvents, insecticides, air fresheners, detergents, etc.). In outdoor environments, the main sources of emission are motor vehicles and gas stations. In indoor spaces, exposure is found in occupational locations as well as in housing (Bakhtiari et al. 2018; Latif et al. 2019).
BTEX and ALS
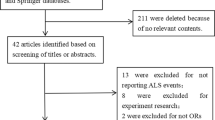
The studies examining the correlation between exposure to VOCs, particularly BTEX, and ALS are extremely limited. Currently, there is no published research analyzing this association based on direct measurements conducted inside homes. Below, we present the findings related to BTEX exposure, whether measured in outdoor residential environments or workplaces. Table 1 provides a summary of the results from these referenced studies.
Outdoor environment
Only two published studies have examined the relationship between exposure to “aromatic solvents” (including 2,4-dinitrotoluene, benzene, ethylbenzene, styrene, toluene, and mixed isomers of xylenes) in outdoor environments and ALS. These two studies, employing similar methodologies, yield different results. The first study, conducted by Malek et al. in 2015, involved a sample of 51 patients diagnosed with ALS and 51 controls. They found that residential exposure to aromatic solvents significantly increased the risk of ALS among cases compared to controls in 2002 (OR = 5.03, 95% CI: 1.29, 19.53) and 1999 (OR = 4.27, 95% CI: 1.09, 16.79) after adjusting for education, smoking, and other exposure groups. Moreover, they didn’t find an association with metals, pesticides, and other HAPs (Malek et al. 2015).
The second study, recently published by Wu et al., examined a sample of 267 ALS patients and 267 controls. Exposure assessment for 34 ambient air toxicants was conducted, but no association with aromatic solvents was found (Wu et al. 2024).
Workplace environment
When analyzing the link between exposure to BTEX and ALS, most authors have concentrated on occupational exposure. For example, Dickerson et al. (2020) reviewed cases of ALS in Denmark from 1982 to 2013, and estimated the cumulative exposure to solvents through the work history through a comparison with controls. They included 1,639 ALS cases and 151,974 controls and found that suffering from ALS is more likely among men exposed to benzene (aOR [adjusted odds of ALS] = 1.20; 95% CI 1.02–1.41, p = 0.03) and methylene chloride (aOR = 1.23; 95% CI 1.07–1.42, p = 0.003).
Ratner et al. (2018) published a case report study design on where a patient was diagnosed with ALS after being exposed in his workplace by a job in which different VOCs were used, emphasizing the high levels of toluene and xylene.
For their part, Goutman et al. (2022), performed a case- control study in Michigan; they included 381 ALS and 272 control participants and found that occupational exposure to VOCs was a risk factor for ALS (OR = 1.22, 95% CI 1.02–1.45, p = 0.029); however, in a second phase of the work they create a multivariable model and exposure to VOCs no longer had a significant relationship; only occupational exposure to metals remained significant risk (OR = 1.56, 95% CI 1.11–2.20, p = 0.011).
It is worth noting that in many work environments the pollution is mixed and its effects may result from joint exposure to different pollutants (Re et al. 2022). Additionally, in the studies mentioned above, specific measurements of the levels of pollutants in the workplace were not made, but rather these have been estimated based on the characteristics of the environment and taking into account the known primary sources of exposure.
In relation to mixed exposure, the case of road traffic is noteworthy. A study published by Pamphlett and Rikard-Bell (2013), whose objective was to analyze whether occupational exposure to toxins played a role in the pathogenesis of ALS, assessed the work context of 611 patients diagnosed with sporadic ALS and 775 healthy controls. Of all occupations, only truck drivers, where exposure to diesel exhaust is common, maintained a higher risk for sporadic ALS. It should be taken into account that truck drivers are exposed to numerous polluting substances, among them BTEX (Amaral et al. 2016).
Pathophysiology
Even though it is not possible to identify in the studies published a compound in isolation as a risk factor for Motataianu et al. (2022) have tried to explain through the pathophysiology of the disease why exposure to BTEX, and especially toluene and xylene, could be considered a risk factor for ALS. In their study they explain that toluene and xylene are frequently involved in deterioration of how the CNS performs because of the production of oxidative stress due to glutathione (GSH) depletion, and GSH levels decrease as ALS progresses. This would support the hypothesis that exposure to VOCs could contribute to inducing latent ALS. These compounds also deplete mitochondrial ATP, which leads to abnormal functioning of ATP-dependent cellular processes and, eventually, apoptosis. Moreover, constant exposure to VOCs is associated with increased neuronal excitation. Given the involvement of motor neuron hyperexcitability in the pathogenesis of neuronal damage, this latter mechanism would support the potential role of VOCs in the progression of ALS. In addition, exposure to toluene has been shown to interfere with axonal transport by lowering the levels of microtubule-associated protein 2 (MAP2), which contributes to the loss of neurons from the anterior horn in patients.
BTEX in biological samples
Given the importance of quantifying toxins in biological samples to directly assess individual exposure (Viegas et al. 2020), a generic analysis of studies quantifying BTEX is presented below, with a deeper dive into the measurements conducted in individuals with neurological diseases and the only case where its analysis has been published in relation to ALS in Sect. 3.4.1.
The methodology to analyze BTEX in biological samples has gradually improved (Kohn et al., 2022; Blount et al., 2006) and some studies have evaluated the concentration levels of these toxins in biological samples (mainly in urine) in relation to occupational exposure; for example, Moridzadeh et al. (2020) quantified BTEX in urine in order to do biomonitoring on workers in a gas field in Iran, concluding that it was a reliable biomarker for exposure. Along these lines, Hoseini et al. (2023) carried out a review to evaluate BTEX concentrations in biological samples from different professionals and found higher urinary concentrations of BTEX in people whose work involved exposure to toxins (mainly among painters and police officers).
It was previously explained that these toxins have very diverse emission sources. However, Chambers et al. (2018) posited that the blood levels of the different compounds could make it easier to know the origin of the exposure. Considering the toxicokinetics of the compounds, during the first phase, absorption, the toxins enter the bloodstream (later they are excreted, deposited, biotransformed, or act directly on the target organs). For this reason, blood samples provide information about the most recent exposure and allow tracing the nearest sources with which the individual has come into contact (Gehring and Merwe 2014).
In the framework of occupational medicine, biomonitoring of toxins is usually done on blood and urine samples, allowing information on recent exposure to be deduced (Angerer et al. 2007), and hair analysis provides information on chronic or older exposures (Kintz 2018). Although the presence of BTEX in the air and in certain products can lead to it being deposited in hair from outside, which must be taken into account as it would contaminate the sample (Kaikiti et al. 2022), the technique for its biomonitoring in hair has been fine-tuned (Es’haghi et al. 2011); nevertheless, no studies have been found that make use of this biological sample to assess exposure.
Analyzing BTEX in biological samples in neurological patients
Regarding biomonitoring and neurological effects, Werder et al. (2019) analyzed the presence of BTEX in blood samples from 690 U.S. Gulf coast residents and evaluated the relationship with their self-reported feelings of the CNS or peripheral nervous system (PNS) being affected. In their analysis they detected a higher prevalence of CNS symptoms among those with higher blood benzene concentration, and symptoms related to the PNS being affected increased when toluene was concomitantly detected.
Some authors have suggested that the measurement of VOCs in biological samples could be a biomarker for ALS (Jiang et al. 2015). A biomarker is a biological observation that serves as a substitute for, and ideally predicts, a clinically relevant endpoint or intermediate outcome that is more difficult to observe (Aronson and Ferner 2017). However, for a measurement of toxins in biological samples to be considered a biomarker, it must guarantee its specific involvement in the disease; in relation with BTEX, only one published study, a clinical case, in which the presence of BTEX in biological samples was analyzed, has been found in the literature reviewed. In the work, the presence of toluene (and other toxic compounds) was examined in biological samples of a patient diagnosed with ALS who had worked professionally as a furniture maker and had worked with paints, some diluted in organic solvents, for 30 years. The patient was also regularly exposed to insecticides because the workshop was fumigated monthly. To varnish the furniture, he used preparations containing, among other substances, toluene and xylene. Although the patient had never shown clinical signs of acute poisoning, organochlorines (DDT) and organophosphates (OP) were detected in his hair samples and n-hexane and toluene were detected in his blood (Kanavouras et al. 2011).
Discussion and conclusions
ALS is a complex disease with a very short life expectancy from the moment it is diagnosed. Although a large number of alterations/cellular pathways have been involved in degeneration of the motor neurons, the pathogenesis has not been fully clarified. Within the pathogenesis of the disease, one of the main disorders is increased levels of oxidative stress, which through different mechanisms translate into progressive damage both at the neuronal and non-neuronal levels by means of the oxidation of different components, among which are nucleic acids, proteins and lipids (Sienes Bailo et al. 2022).
As discussed above, although the pathogenesis of ALS has not been fully elucidated, in recent years numerous cellular pathways have been implicated in motor neuron degeneration (Riancho et al. 2019). Among these, it is widely documented that ALS patients have abnormal oxidative stress responses (Zufiria et al., 2016). It is possible that these increased levels of oxidative stress appear as a result of a primary disorder but also as a secondary event as the disease progresses (Scarian et al. 2024). In relation to primary damage, mutations in the SOD1 gene are the second most frequent cause of genetic ALS (Vázquez-Costa et al. 2022). The SOD1 gene encodes for the superoxide dismutase 1, an enzyme involved in the protection against oxidative stress through the elimination of free radicals (Ruiz-Soto et al., 2020). In a complementary manner, different authors have documented increased levels of oxidative stress and in a context of global degeneration, with mitochondrial dysfunction and a cellular stress response (Scarian et al. 2024).
Based on this, one of the main treatment strategies has focused on identification of therapeutic agents capable of inhibiting this oxidative stress and decreasing neuroinflammation (Park and Yang 2021).
Atmospheric pollutants and specifically BTEX produce oxidative stress, and some epidemiological studies link exposure to these compounds with ALS.
In the multiple-step model needed to trigger ALS, proposed by Al Chalabi et al. (2014), BTEX could be the step that causes toxic aggression (although other toxic agents could activate the same process). Alternatively, BTEX could play a role in the progression of ALS, Fig. 1.
Measuring daily exposure of patients to BTEX is complex, but especially in regard to deducing past exposure. In this sense, most epidemiological studies are focused on occupational exposure and do not carry out direct measurements. Moreover, While the harmful effects of BTEX on human health are widely known, further studies are still needed to clarify the involvement of BTEX in ALS. From an epidemiological perspective, the different sources of BTEX and the simultaneous presence with other toxins make it difficult to assess their impact. The Scientific Committee on Health and Environmental Risks (SCHER) of the European Commission (2007) explained that some air pollutants can interact with each other, and that several chemicals acting together can cause more harmful effects than the sum of the effects caused by each chemical separately. Additionally, there is no accepted strategy for assessing health risks arising from exposure to a mixture of pollutants because the mechanisms of action of pollutants acting together to estimate toxicity are not known (SCHER, 2007). In this line, Fazakas et al. published a review in 2023 in which the emphasized that there were few studies addressing the health effects of air pollutants as mixtures and there is a gap in knowledge regarding the health effects associated with these mixtures.
Measurement of BTEX in biological samples could be a good indicator of regular exposure, and not only professional. But even though the techniques to quantify the levels are highly developed, only one published work has been found, regarding a clinical case, that analyzes toluene levels in a patient’s urine samples. Specifically, hair analysis could be a tool to deduce past exposure or exposure that has happened over time.
This review has two main limitations. First, there is a limitation in terms of methodology, as a systematic review would have been preferable but was not feasible due to the limited available literature, the excessive diversity of knowledge domains involved, and the multitude of terms used to refer to pollutants. Second, since different authors tend to arrive at different conclusions on this issue, and due to the fact, the published data do not precisely measure individual exposure, new studies are necessary in order to gain valuable insights.
Finally, we believe that while this article has primarily focused on analyzing the potential correlation between BTEX compounds and the pathogenesis of ALS, these compounds might also play a role in the pathogenesis of various other neurodegenerative diseases. It is widely acknowledged that prevalent diseases in our society, such as Alzheimer’s disease or Parkinson’s disease, have an environmental component that remains incompletely understood (Sakowski et al. 2024). Should the relationship between BTEX exposure and ALS be confirmed, whether as a triggering factor or as a pathogenic element in its own right, it could signify a groundbreaking milestone in establishing public health interventions to manage this disease.
Moreover, considering the shared disease mechanisms among the majority of neurodegenerative diseases (Klemmensen et al. 2024), their potential influence on other conditions could pave the way for widespread prevention strategies, both in occupational settings and households, aiming to mitigate the incidence of this spectrum of diseases.
Data availability
Not applicable.
Abbreviations
- ALS:
-
Amyotrophic Lateral Sclerosis
- fALS:
-
familial ALS
- sALS:
-
sporadic ALS
- BTEX:
-
Benzene, toluene, ethylbenzene, and xylenes
- CNS:
-
Central Nervous System
- IAC:
-
indoor air quality
- ROS:
-
Reactive Oxygen Species
- VOCs:
-
Volatile Organic Compounds
References
Al-Chalabi A, Calvo A, Chio A, Colville S, Ellis CM, Hardiman O, Heverin M, Howard RS, Huisman MHB, Keren N, Leigh PN, Mazzini L, Mora G, Orrell RW, Rooney J, Scott KM, Scotton WJ, Seelen M, Shaw CE, Sidle KS, Swingler R, Tsuda M, Veldink JH, Visser AE, van den Berg LH, Pearce N (2014) Analysis of amyotrophic lateral sclerosis as a multistep process: a population-based modelling study. Lancet Neurol 13(11):1108–1113. https://doi.org/10.1016/S1474-4422(14)70219-4
Amaral BS, Novaes FJM, Ramos MDCKV, de Aquino Neto FR, Gioda A (2016) Comparative profile of pollutants generated by a stationary engine fueled with diesel, biodiesel, and ethanol. J Aerosol Sci 100:155–163. https://doi.org/10.1016/j.jaerosci.2016.07.009
Angerer J, Ewers U, Wilhelm M (2007) Human biomonitoring: state of the art. Int J Hyg Environ Health 210(3–4):201–228. https://doi.org/10.1016/j.ijheh.2007.01.024
Aronson JK, Ferner RE (2017) Biomarkers-A General Review. Curr Protoc Pharmacol 76. 9.23.1–9.23.17
ATSDR Agency for Toxic Substances and Disease Registry. Interaction profile for
Bakhtiari R, Hadei M, Hopke PK, Shahsavani A, Rastkari N, Kermani M, Yarahmadi M, Ghaderpoori A (2018) Investigation of in-cabin volatile organic compounds (VOCs) in taxis; influence of vehicle’s age, model, fuel, and refueling. Environ Pollut 237:348–355
Benzene T Ethylbenzene, and Xylenes (BTEX). Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service. https://www.atsdr.cdc.gov/interactionprofiles/ip05.html
Block ML, Calderón-Garcidueñas L (2009) Air pollution: mechanisms of neuroinflammation and CNS disease. Trends Neurosci. 2009;32(9):506 – 16. https://doi.org/10.1016/j.tins.2009.05.009
Chen SH, Oyarzabal EA, Hong JS (2016) Critical role of the Mac1/NOX2 pathway in mediating reactive microgliosis-generated chronic neuroinflammation and progressive neurodegeneration. Curr Opin Pharmacol 26:54–60. https://doi.org/10.1016/j.coph.2015.10.001
Costa LG, Cole TB, Dao K, Chang YC, Coburn J, Garrick JM (2020) Effects of air pollution on the nervous system and its possible role in neurodevelopmental and neurodegenerative disorders. Pharmacol Ther 210:107523. https://doi.org/10.1016/j.pharmthera.2020.107523
Dehghani M, Fazlzadeh M, Sorooshian A, Tabatabaee HR, Miri M, Baghani AN, Delikhoon M, Mahvi AH, Rashidi M (2018) Characteristics and health effects of BTEX in a hot spot for urban pollution. Ecotoxicol Environ Saf 155:133–143. https://doi.org/10.1016/j.ecoenv.2018.02.065
Di Bernardino A, Iannarelli AM, Casadio S, Perrino C, Barnaba F, Tofful L, Campanelli M, Di Liberto L, Mevi G, Siani AM, Cacciani M (2021) Impact of synoptic meteorological conditions on air quality in three different case studies in Rome. Italy Atmos Pollut Res 12:76–88. https://doi.org/10.1016/j.apr.2021.02.019
Dickerson AS, Hansen J, Thompson S, Gredal O, Weisskopf MG (2020) A mixtures approach to solvent exposures and amyotrophic lateral sclerosis: a population-based study in Denmark. Eur J Epidemiol 35(3):241–249. https://doi.org/10.1007/s10654-020-00624-5
Es’haghi Z, Ebrahimi M, Hosseini MS, J Chromatogr A (2011) Optimization of a novel method for determination of benzene, toluene, ethylbenzene, and xylenes in hair and waste water samples by carbon nanotubes reinforced sol-gel based hollow fiber solid phase microextraction and gas chromatography using factorial experimental design. 1218(21):3400–3406. https://doi.org/10.1016/j.chroma.2011.03.043
Fang F, Valdimarsdóttir U, Bellocco R, Ronnevi LO, Sparén P, Fall K, Ye W (2009) Amyotrophic lateral sclerosis in Sweden, 1991–2005. Arch Neurol 66(4):515–519. https://doi.org/10.1001/archneurol.2009.13
Fazakas E, Neamtiu IA, Gurzau ES (2023) Health effects of air pollutant mixtures (volatile organic compounds, particulate matter, sulfur and nitrogen oxides) - a review of the literature. Rev Environ Health. https://doi.org/10.1515/reveh-2022-0252
Feldman EL, Goutman SA, Petri S, Mazzini L, Savelieff MG, Shaw PJ, Sobue G (2022) Amyotrophic lateral sclerosis. Lancet 400(10360):1363–1380. https://doi.org/10.1016/S0140-6736(22)01272-7
Filippini T, Mandrioli J, Malagoli C, Costanzini S, Cherubini A, Maffeis G, Vinceti M (2021) Risk of Amyotrophic Lateral Sclerosis and Exposure to Particulate Matter from Vehicular Traffic: A Case-Control Study. Int J Environ Res Public Health. 22;18(3):973. doi: 10.3390/ijerph18030973.GBD 2016 Motor Neuron Disease Collaborators, 2018. Global, regional, and national burden of motor neuron diseases 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 17(12):1083–1097. https://doi.org/10.1016/S1474-4422(18)30404-6
Gao HM, Zhou H, Hong JS (2012) NADPH oxidases: novel therapeutic targets for neurodegenerative diseases. Trends Pharmacol Sci 33(6):295–303. https://doi.org/10.1016/j.tips.2012.03.008
Gehring R, Merwe D (2014) Chapter 8 - Toxicokinetic-toxicodynamic modeling. Biomarkers in Toxicology. Academic Press. Pages 149–153. ISBN 9780124046306, https://doi.org/10.1016/B978-0-12-404630-6.00008-7
Goutman SA, Boss J, Godwin C, Mukherjee B, Feldman EL, Batterman SA (2022) Associations of self-reported occupational exposures and settings to ALS: a case-control study. Int Arch Occup Environ Health 95(7):1567–1586. https://doi.org/10.1007/s00420-022-01874-4
Hoseini M, Samaei MR, Shahesmaeili A, Martínez SS, Amiri H (2023) Using biomonitoring as a complementary approach in BTEX exposure assessment in the general population and occupational settings: a systematic review and meta-analysis. Rev Environ Health. 2022;38(3):493–510. https://doi.org/10.1515/reveh-2022-0042
Jayaraj RL, Rodriguez EA, Wang Y, Block ML (2017) Outdoor Ambient Air Pollution and neurodegenerative diseases: the Neuroinflammation hypothesis. Curr Environ Health Rep 4(2):166–179. https://doi.org/10.1007/s40572-017-0142-3
Jiang H, Wang C, Ren M, Yin X, Chi C, Guo L, Ke C, Feng H, Li E (2015) Blood volatile organic compounds as potential biomarkers for amyotrophic lateral sclerosis: an animal study in the SOD1 G93A mouse. J Mol Neurosci. 2015;55(1):167–173. https://doi.org/10.1007/s12031-014-0297-4
Julian TH, Boddy S, Islam M, Kurz J, Whittaker KJ, Moll T, Harvey C, Zhang S, Snyder MP, McDermott C, Cooper-Knock J, Shaw PJ (2022) A review of mendelian randomization in amyotrophic lateral sclerosis. Brain 145(3):832–842. https://doi.org/10.1093/brain/awab420
Kaikiti C, Stylianou M, Agapiou A (2022) TD-GC/MS analysis of indoor air pollutants (VOCs, PM) in hair salons. Chemosphere 294:133691. https://doi.org/10.1016/j.chemosphere.2022.133691
Kamani H, Baniasadi M, Abdipour H, Mohammadi L, Rayegannakhost S, Moein H, Azari A (2023) Health risk assessment of BTEX compounds (benzene, toluene, ethylbenzene and xylene) in different indoor air using Monte Carlo simulation in zahedan city. Iran Heliyon 9(9):e20294. https://doi.org/10.1016/j.heliyon.2023.e20294
Kanavouras K, Tzatzarakis MN, Mastorodemos V, Plaitakis A, Tsatsakis AM (2011) A case report of motor neuron disease in a patient showing significant level of DDTs, HCHs and organophosphate metabolites in hair as well as levels of hexane and toluene in blood. Toxicol Appl Pharmacol 256(3):399–404. https://doi.org/10.1016/j.taap.2011.07.022
Kintz P (2018) Hair analysis in forensic toxicology. WIREs Forensic Sci 1(1). https://doi.org/10.1002/wfs2.1196
Klemmensen MM, Borrowman SH, Pearce C, Pyles B, Chandra B (2024) Mitochondrial dysfunction in neurodegenerative disorders. Neurotherapeutics 21(1):e00292. https://doi.org/10.1016/j.neurot.2023.10.002
Kotzias D (2022) Exposure to volatile organic compounds in indoor/outdoor environments and methodological approaches for exposure estimates -the European paradigm. J Hazard Mater Adv 8. https://doi.org/10.1016/j.hazadv.2022.100197
Kumar P, Singh AB, Arora T, Singh S, Singh R (2023) Critical review on emerging health effects associated with the indoor air quality and its sustainable management. Sci Total Environ 872:162163. https://doi.org/10.1016/j.scitotenv.2023.162163
Latif MT, Abd Hamid HH, Ahamad F, Khan MF, Mohd Nadzir MS, Othman M, Sahani M, Abdul Wahab MI, Mohamad N, Uning R, Poh SC, Fadzil MF, Sentian J, Tahir NM (2019) BTEX compositions and its potential health impacts in Malaysia. Chemosphere 237:124451. https://doi.org/10.1016/j.chemosphere.2019.124451
López-Pingarrón L, Almeida H, Soria-Aznar M, Reyes-Gonzales MC, Terrón MP, García JJ (2023) Role of oxidative stress on the etiology and pathophysiology of amyotrophic lateral sclerosis (ALS) and its relation with the enteric nervous system. Curr Issues Mol Biol 45(4):3315–3332. https://doi.org/10.3390/cimb45040217
Malek AM, Barchowsky A, Bowser R, Heiman-Patterson T, Lacomis D, Rana S, Youk A, Talbott EO (2015) Exposure to hazardous air pollutants and the risk of amyotrophic lateral sclerosis. Environ Pollut 197:181–186. https://doi.org/10.1016/j.envpol.2014.12.010
Masrori P, Van Damme P (2020) Amyotrophic lateral sclerosis: a clinical review. Eur J Neurol 27(10):1918–1929. https://doi.org/10.1111/ene.14393
Mata S, Bussotti M, Del Mastio M, Barilaro A, Piersanti P, Lombardi M, Cincotta M, Torricelli S, Leccese D, Sperti M, Rodolico GR, Nacmias B, Sorbi S (2023) Epidemiology of amyotrophic lateral sclerosis in the north east Tuscany in the 2018–2021 period. eNeurologicalSci 31:100457. https://doi.org/10.1016/j.ensci.2023.100457
Moridzadeh M, Dehghani S, Rafiee A, Hassanvand MS, Dehghani M, Hoseini M (2020) Assessing BTEX exposure among workers of the second largest natural gas reserve in the world: a biomonitoring approach. Environ Sci Pollut Res Int 27(35):44519–44527. https://doi.org/10.1007/s11356-020-10379-x
Motataianu A, Serban G, Barcutean L, Balasa R (2022) Oxidative stress in amyotrophic lateral sclerosis: synergy of genetic and environmental factors. Int J Mol Sci 23(16):9339. https://doi.org/10.3390/ijms23169339
Murata H, Barnhill LM, Bronstein JM (2022) Air Pollution and the risk of Parkinson’s disease: a review. Mov Disord 37(5):894–904. https://doi.org/10.1002/mds.28922
Myung W, Lee H, Kim H (2019) Short-term air pollution exposure and emergency department visits for amyotrophic lateral sclerosis: a time-stratified case-crossover analysis. Environ Int 123:467–475. https://doi.org/10.1016/j.envint.2018.12.042
Nunez Y, Boehme AK, Weisskopf MG, Re DB, Navas-Acien A, van Donkelaar A, Martin RV, Kioumourtzoglou MA (2021) Fine particle exposure and clinical aggravation in neurodegenerative diseases in New York State. Environ Health Perspect 129(2):27003. https://doi.org/10.1289/EHP7425
Opie-Martin S, [a], Wootton RE, Budu-Aggrey A, Shatunov A, Jones AR, Iacoangeli A, Al Khleifat A, Davey-Smith G, Al-Chalabi A (2020) Relationship between smoking and ALS: mendelian randomisation interrogation of causality. J Neurol Neurosurg Psychiatry 91(12):1312–1315. https://doi.org/10.1136/jnnp-2020-323316
Opie-Martin S, [b], Jones A, Iacoangeli A, Al-Khleifat A, Oumar M, Shaw PJ, Shaw CE, Morrison KE, Wootton RE, Davey-Smith G, Pearce N, Al-Chalabi A (2020b) UK case control study of smoking and risk of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2020;21(3–4):222–227. https://doi.org/10.1080/21678421.2019.1706580
Pamphlett R, Rikard-Bell A (2013) Different occupations associated with amyotrophic lateral sclerosis: is diesel exhaust the link? PLoS ONE 8(11):e80993. https://doi.org/10.1371/journal.pone.0080993
Park HR, Yang EJ (2021) Oxidative stress as a therapeutic target in amyotrophic lateral sclerosis: opportunities and limitations. Diagnostics (Basel) 11(9):1546. https://doi.org/10.3390/diagnostics11091546
Parks RM, Nunez Y, Balalian AA, Gibson EA, Hansen J, Raaschou-Nielsen O, Ketzel M, Khan J, Brandt J, Vermeulen R, Peters S, Goldsmith J, Re DB, Weisskopf MG, Kioumourtzoglou MA (2022) Long-term traffic-related Air Pollutant exposure and amyotrophic lateral sclerosis diagnosis in Denmark: a bayesian hierarchical analysis. Epidemiology 33(6):757–766. https://doi.org/10.1097/EDE.0000000000001536
Ratner MH, Jabre JF, Ewing WM, Abou-Donia M, Oliver LC (2018) Amyotrophic lateral sclerosis-A case report and mechanistic review of the association with toluene and other volatile organic compounds. Am J Ind Med 61(3):251–260. https://doi.org/10.1002/ajim.22791
Re DB, Yan B, Calderón-Garcidueñas L, Andrew AS, Tischbein M, Stommel EW (2022) A perspective on persistent toxicants in veterans and amyotrophic lateral sclerosis: identifying exposures determining higher ALS risk. J Neurol 269(5):2359–2377. https://doi.org/10.1007/s00415-021-10928-5
Riancho J, Lozano-Cuesta P, Santurtún A, Sánchez-Juan P, López-Vega JM, Berciano J, Polo JM (2016) Amyotrophic lateral sclerosis in Northern Spain 40 years later: what has changed? Neurodegener Dis 16(5–6):337–341. https://doi.org/10.1159/000445750
Riancho J, Gonzalo I, Ruiz-Soto M, Berciano J (2019) Why do motor neurons degenerate? Actualization in the pathogenesis of amyotrophic lateral sclerosis. Neurologia (Engl Ed) 34(1):27–37. https://doi.org/10.1016/j.nrl.2015.12.001
Riancho J, de la Sanchez JR, Paz-Fajardo L, Limia C, Santurtun A, Cifra M, Kourtidis K, Fdez-Arroyabe P (2021) The role of magnetic fields in neurodegenerative diseases. Int J Biometeorol 65(1):107–117. https://doi.org/10.1007/s00484-020-01896-y
Royé D, Zarrabeitia MT, Riancho J, Santurtún A A time series analysis of the relationship between apparent temperature, air pollutants and ischemic stroke in Madrid, Spain. Environ Res 173:349–358. https://doi.org/10.1016/j.envres.2019.03.065
Ruiz-Soto M, Riancho J, Tapia O, Lafarga M, Berciano MT (2020) Satellite glial cells of the dorsal Root Ganglion: a New Guest/Physiopathological Target in ALS. Front Aging Neurosci 12:595751. https://doi.org/10.3389/fnagi.2020.595751
Sainio MA, Sr (2015) Neurotoxicity of solvents. Handb Clin Neurol 131:93–110. https://doi.org/10.1016/B978-0-444-62627-1.00007-X
Sakowski SA, Koubek EJ, Chen KS, Goutman SA, Feldman EL (2024) Role of the Exposome in Neurodegenerative Disease: recent insights and future directions. Ann Neurol. https://doi.org/10.1002/ana.26897
Saucier D, Registe PPW, Bélanger M, O’Connell C (2023) Urbanization, air pollution, and water pollution: identification of potential environmental risk factors associated with amyotrophic lateral sclerosis using systematic reviews. Front Neurol 14:1108383. https://doi.org/10.3389/fneur.2023.1108383
Scarian E, Viola C, Dragoni F, Di Gerlando R, Rizzo B, Diamanti L, Gagliardi S, Bordoni M, Pansarasa O (2024) New insights into oxidative stress and inflammatory response in neurodegenerative diseases. Int J Mol Sci 25(5):2698. https://doi.org/10.3390/ijms25052698
SCHER (The Scientific Committee on Health and Environmental Risks of the European Commission) (2007) Opinion on risk assessment on indoor air quality. https://ec.europa.eu/health/opinions/en/indoor-air-pollution/l-2/5-pollutant-mixtures.htm
Seelen M, Toro Campos RA, Veldink JH, Visser AE, Hoek G, Brunekreef B, van der Kooi AJ, de Visser M, Raaphorst J, van den Berg LH, Vermeulen RCH (2017) Long-Term Air Pollution exposure and amyotrophic lateral sclerosis in Netherlands: a Population-based case-control study. Environ Health Perspect 125(9):097023. https://doi.org/10.1289/EHP1115
Shefner JM, Al-Chalabi A, Baker MR, Cui LY, de Carvalho M, Eisen A, Grosskreutz J, Hardiman O, Henderson R, Matamala JM, Mitsumoto H, Paulus W, Simon N, Swash M, Talbot K, Turner MR, Ugawa Y, van den Berg LH, Verdugo R, Vucic S, Kaji R, Burke D, Kiernan MC (2020) A proposal for new diagnostic criteria for ALS. Clin Neurophysiol 131(8):1975–1978. https://doi.org/10.1016/j.clinph.2020.04.005
Shrubsole C, Dimitroulopoulou S, Foxall K, Gadeberg B, Doutsi A (2019) IAQ guidelines for selected volatile organic compounds (VOCs) in the UK. Build Environ 165. https://doi.org/10.1016/j.buildenv.2019.106382
Sienes Bailo P, Llorente Martín E, Calmarza P, Montolio Breva S, Bravo Gómez A, Pozo Giráldez A, Sánchez-Pascuala Callau JJ, Vaquer Santamaría JM, Dayaldasani Khialani A, Cerdá Micó C, Camps Andreu J, Sáez Tormo G, Fort Gallifa I (2022) The role of oxidative stress in neurodegenerative diseases and potential antioxidant therapies. Adv Lab Med 3(4):342–360. https://doi.org/10.1515/almed-2022-0111
Słomińka M, Konieczka P, Namieśnik J (2014) The fate of BTEX compounds in Ambient Air. Crit Rev Environ Sci Technol 44(5):455–472. https://doi.org/10.1080/10643389.2012.728808
Vázquez-Costa JF, Borrego-Hernández D, Paradas C, Gómez-Caravaca MT, Rojas-Garcia R, Varona L, Povedano M, García-Sobrino T, Jericó Pascual I, Gutiérrez A, Riancho J, Turon-Sans J, Assialioui A, Pérez-Tur J, Sevilla T, Esteban Pérez J, García-Redondo A, ALSGESCO (2022) Characterizing SOD1 mutations in Spain. The impact of genotype, age, and sex in the natural history of the disease. Eur J Neurol. https://doi.org/10.1111/ene.15661
Viegas S, Zare Jeddi M, Hopf B, Bessems N, Palmen J, Galea NS, Jones K, Kujath K, Duca P, Verhagen RC, Santonen H, Pasanen-Kase T R (2020) Biomonitoring as an underused exposure Assessment Tool in Occupational Safety and Health Context-Challenges and Way Forward. Int J Environ Res Public Health 17(16):5884. https://doi.org/10.3390/ijerph17165884
Wang H, O’Reilly ÉJ, Weisskopf MG, Logroscino G, McCullough ML, Thun MJ, Schatzkin A, Kolonel LN, Ascherio A (2011) Smoking and risk of amyotrophic lateral sclerosis: a pooled analysis of 5 prospective cohorts. Arch Neurol 68(2):207–213. https://doi.org/10.1001/archneurol.2010.367
Werder EJ, Engel LS, Blair A, Kwok RK, McGrath JA, Sandler DP (2019) Blood BTEX levels and neurologic symptoms in Gulf states residents. Environ Res 175:100–107. https://doi.org/10.1016/j.envres.2019.05.004
WHO (World Health Organization) (2014) Air pollution: Indoor air pollution. https://www.who.int/news-room/questions-and-answers/item/air-pollution-indoor-air-pollution
Wu F, Malek AM, Buchanich JM, Arena VC, Rager JR, Sharma RK, Vena JE, Bear T, Talbott EO (2024) Exposure to ambient air toxicants and the risk of amyotrophic lateral sclerosis (ALS): a matched case control study. Environ Res 242. https://doi.org/10.1016/j.envres.2023.117719
Yedavalli VS, Patil A, Shah P (2018) Amyotrophic Lateral Sclerosis and its Mimics/Variants: A Comprehensive Review. J Clin Imaging Sci. 2018;8:53. https://doi.org/10.4103/jcis.JCIS_40_18
Zhan Y, Fang F (2019) Smoking and amyotrophic lateral sclerosis: a mendelian randomization study. Ann Neurol 85(4):482–484. https://doi.org/10.1002/ana.25443
Zufiría M, Gil-Bea FJ, Fernández-Torrón R, Poza JJ, Muñoz-Blanco JL, Rojas-García R, Riancho J, López de Munain A (2016) ALS: a bucket of genes, environment, metabolism and unknown ingredients. Prog Neurobiol 142:104–129. https://doi.org/10.1016/j.pneurobio.2016.05.004
Funding
This work was supported by the Instituto de Salud Carlos III, Madrid, Spain (PI23/00905), and by the Instituto de Investigación Valdecilla, IDIVAL, Santander, Spain (TRANSVAL22/03 and INT/A23/07)
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All the author have approved the publication in the Journal.
Competing interests
The authors do not have any competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Santurtún, A., Fdez-Arroyabe, P., Sedano, M.J. et al. Are BTEX (Benzene, Toluene, Ethylbenzene and Xylenes) involved in the development of amyotrophic lateral sclerosis?. Air Qual Atmos Health (2024). https://doi.org/10.1007/s11869-024-01612-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11869-024-01612-4