Abstract
The introduction of Tier 3 light-duty vehicles with reduced emissions began in New York State (NYS) in 2017, with required compliance by 2025. We hypothesized that improved air quality during the early implementation of Tier 3 (2017–2019) would result in reduced rates of hospitalizations and emergency department (ED) visits for respiratory infection associated with increased PM2.5 compared to 2014–2016 (pre-Tier 3). Using data on adult patients hospitalized or having an ED visit for influenza, upper respiratory infection, culture-negative pneumonia, or respiratory bacterial infection, living within 15 miles of six air quality monitoring sites in NY, and a case-crossover design and conditional logistic regression, we estimated the rates of respiratory infection hospitalizations and ED visits associated with increased ambient PM2.5 concentrations in the previous 0–6 days and each week thereafter up to 1 month. Interquartile range (IQR) increases in PM2.5 in the previous 6 days were associated with 4.6% (95% CI: − 0.5, 10.1) and 11.9% (95% CI = 6.1, 18.0) increased rates of influenza hospitalizations in 2014–2016 and 2017–2019, respectively. This pattern of larger relative rates in 2017–2019 observed at all lag times was only present in males hospitalized for influenza but not other infections or in females. The rates of respiratory infection visits associated with increased PM2.5 were generally not reduced in this early Tier 3 implementation period compared to 2014–2016. Limited fleet penetration of Tier 3 vehicles and differences in particle deposition, infection type, and sex by period may all have contributed to this lack of improvement.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Influenza and bacterial pneumonia together are the ninth leading cause of death in the USA (Santo et al. 2021), and ambient air pollution has been identified as a risk factor for both respiratory viral and bacterial infections worldwide (Kirwa et al. 2021). While pandemic viruses such as SARS-CoV-2 have recently dominated current international attention, seasonal respiratory viral infections (RVIs), ranging from severe influenza to less severe upper respiratory tract infections (URIs), represent an ongoing threat to health. Individuals with RVI may require hospitalization and can even suffer from bacterial superinfection leading to bacterial pneumonia (Rynda-Apple et al. 2015). Thus, understanding the risk factors for hospitalization from acute upper and lower respiratory tract infections is a crucial step in reducing the worldwide burden of disease from both respiratory viral and bacterial infections.
We and others have reported increased rates of respiratory infections associated with short-term increases in ambient air pollution (Croft et al. 2019; Horne et al. 2018; Renzi et al. 2022; Zhang et al. 2023). Previously in New York State, the rate of respiratory infection hospitalizations/emergency department (ED) visits associated with interquartile range (IQR) increases in PM2.5 concentration were generally largest in the 6 or 7 days before the hospitalization/ED visit (e.g., influenza: excess rate = 3.9%; 95% CI: 2.1, 5.6) compared to earlier time periods (1 day prior to influenza diagnosis: excess rate = 0.5%; 95% CI: − 0.8, 1.8) (Croft et al. 2019). In contrast to a 1 week lag window, Horne et al. examined pollutant concentrations up to 4 weeks before the hospitalization/ED visit, and often reported the largest effect estimates for PM2.5 concentrations 4 weeks before hospitalization (e.g., acute lower respiratory infection: odds ratio = 1.2; 95% CI: 1.1, 1.3) (Horne et al. 2018). More recently, the Multiethnic Study of Atherosclerosis (MESA) study in six US regions reported a 4% higher risk of outpatient respiratory infection (95% CI = 0, 9) associated with each 5.7 µg/m3 increase in PM2.5 concentration in the preceding 2–6 weeks (composite lag time) and a 21% increase in the risk of respiratory infection (95% CI = 10, 33) associated with each 14.4 ppb increase in NO2 concentration in the preceding 2–6 weeks (Kirwa et al. 2021). A study in China, with laboratory-confirmed influenza infection, reported a 3.8% increased risk of influenza (95% CI = 1.6, 6.0) associated with each 50 µg/m3 increase in PM2.5 in the previous 5 days (Lu et al. 2020). Although our prior work focused only on lower respiratory infections (i.e., influenza and pneumonia), other studies reported associations between increased concentrations of ambient air pollutants and upper respiratory tract infections (Chen et al. 2018; Horne et al. 2018).
Over the past decade, the state of New York has undergone changes in air quality as a result of upwind coal-fired power plant closures, changes from coal to natural gas for energy generation, reduction in sulfur concentrations in distillate fuels, the North American Emissions Control Area (NA-ECA), and the phase-out of residual oil for space heating in New York City (Chen et al. 2023). In addition, economic drivers such as the 2007–2009 recession and the change in the relative costs of natural gas and coal drove changes in the mixture of electricity-generating unit technologies and downwind air quality. While these changes resulted in reduced PM concentrations across New York State, a change in PM composition also occurred (i.e., the proportion of PM from secondary organic carbon and spark-ignition vehicle pollution increased from 2006 to 2016 (Masiol et al. 2019)). Thus, despite this reduction in PM2.5 concentration, the risk for hospitalization and ED visits for respiratory infection (e.g., influenza ED visits) associated with each 5.7 µg/m3 increase in PM2.5 concentration was higher in 2014–2016 (excess rate = 6.4%, 95% CI: 2.8, 10.1) than in 2009–2013 (excess rate = 2.1%, 95% CI 4.2, 20.1). This suggested that the toxicity of the PM per unit mass may have increased in New York from 2005–2016. In 2017, new regulations improving emissions of Tier 3 light-duty vehicles began in NY State, with all vehicles mandated to meet this standard by 2025 (Hopke and Hidy 2022). The expected reduction in tailpipe and evaporative emissions from the Tier 3 program provided an opportunity to determine if the rate of hospitalization or ED visits for respiratory infection associated with increased PM concentrations was reduced after these Tier 3 vehicle regulations were implemented in 2017.
Using a New York statewide database of respiratory infection healthcare encounters paired with ambient air pollution concentrations at monitoring stations in Buffalo, Rochester, Albany, Manhattan, Queens, and the Bronx from 2014 to 2019, we estimated the rate of healthcare encounters for upper and lower respiratory infection associated with increased air pollution concentrations in the previous few days and weeks for adult residents of New York State. Our study examined the association between PM2.5 and respiratory infection healthcare visits and whether these relative rates were reduced during the early implementation of the Tier 3 emissions controls. Specifically, we compared the 2014–2016 period to the 2017–2019 period, hypothesizing that the introduction of Tier 3 vehicles into the light-duty fleet would result in a lower rate of hospitalization and ED visits from respiratory infection associated with increased PM in 2017–2019 than 2014–2016.
Methods
Study population
Respiratory infection hospital admissions and ED visits for adult New York residents were obtained from the Statewide Planning and Research Cooperative System (SPARCS) database. In total, N = 135,236 hospitalizations and N = 549,528 ED visits of adults living within 15 miles of the Buffalo, Rochester, Albany, Bronx, Manhattan, or Queens PM2.5 monitoring sites from January 1, 2014. to December 31, 2019. were retained. The 15-mile radius was selected to be consistent with our prior study in New York State (Croft et al. 2019). This distance balances the tradeoff between minimizing exposure misclassification and optimizing sample size (Zikova et al. 2017). We included participants with a primary diagnosis (at time of healthcare encounter) of influenza (ICD9 = 4870, 4871, 48811, 48812, 48881, 48882; ICD10 = J09X1, J09X2, J1000, J1001, J1008, J101, J1100, J1108 and J111, bacterial pneumonia (ICD9 = 481, 482, 4830, 4831; ICD10 = J13, J14, J15, J16, A48.1) or culture-negative pneumonia (ICD9 = 485, 486; ICD10 = J18). Culture-negative pneumonia is a common diagnosis, as modern culture techniques identify a causative pathogen in < 50% of the patients diagnosed with pneumonia (Purcaro et al. 2018). It is best viewed as an undifferentiated infection that can be bacterial or viral in origin. This study was approved by the Institutional Review Board at the University at Albany, State University of New York.
Air pollution and weather
From 2014 to 2019, hourly PM2.5 concentrations at the Buffalo, Rochester, Albany, Bronx, Manhattan, and Queens monitoring stations were retrieved from the US Environmental Protection Agency (https://aqs.epa.gov/api). Additional details on the monitor types used for PM2.5 measurements and the measurements of temperature and relative humidity have been described previously (Chen et al. 2023). Each participant was assigned daily PM2.5, temperature, and relative humidity values from the monitoring station closest to their residence.
Statistical analysis
We used a time-stratified, case-crossover design (Maclure 1991; Levy et al. 2001) to estimate the rates of respiratory infection hospital admissions and emergency department (ED) visits associated with each interquartile range increase in PM2.5 concentration on the same day (lag day 0). For influenza hospital admissions from all six urban sites, assuming a common slope across sites, we fit a conditional logistic regression model stratified on each respiratory infection hospital admission matched set (1 case and 3–4 control periods per subject). This conditional logistic regression model regressed case-control status (i.e., case = 1, control = 0) against the mean PM2.5 concentration on case and control days. The case-crossover approach controls for non-time-varying confounders, such as underlying medical conditions, patient insurance status, long-term time trends, and season, by design. However, we included natural splines for temperature and relative humidity (4 degrees of freedom determined using the Akaike information criterion) (Aho et al. 2014). This same model was run for the mean PM2.5 concentration on lag days 0–1, 0–2, 0–3, 0–4, 0–5, 0–6, 7–13, 14–20, and 21–27, and then separately for ED visits and hospitalizations for URI, bacterial pneumonia, and culture-negative pneumonia. From each model, we estimated the rate of hospitalizations or ED visits associated with each interquartile range increase in PM2.5 concentration. Since we examined ten lag times for each disease subgroup, statistical significance was defined as p < 0.005 (0.05/10).
Next, we examined whether the rates of respiratory infection admission associated with each interquartile range increase in PM2.5 concentration differed by period (2014–2016 and 2017–2019) by adding an interaction term (period * PM2.5) to the model. Last, we stratified by reported sex (males versus females) and re-ran the above models separately for adult males and females. All analyses were done using R version 3.0.1 (https://www.r-project.org/).
Results
The majority of participants requiring hospitalization or ED visits were female (54% and 62%, respectively), with hospitalized patients generally being older (mean age = 68 years old) than patients treated in the ED only (mean age 40 years old) (Table 1). Of the participants hospitalized, 48% were white and 25% were black, while 27% and 39% of participants treated in the ED were white and black, respectively. The majority of hospitalized participants had culture-negative pneumonia (66%), while the majority of participants treated and released from the ED suffered from upper respiratory tract infections (79%). Hospitalized participants stayed in the hospital for approximately 5 days on average. Predictably, more infections occurred in the winter and spring (60%) in both care settings than in the summer and fall (40%). Among control days, Manhattan had the highest median PM2.5 concentration (8.2 µg/m3), while Albany had the highest maximum PM2.5 concentration (45.4 µg/m3) (Table 2). Rochester had both the lowest median and lowest maximum PM2.5 concentration (5.9 µg/m3 and 24.8 µg/m3 respectively). There were similar patterns among case periods.
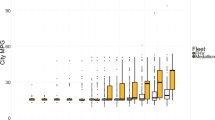
Rates of influenza hospitalizations and ED visits associated with interquartile range (IQR) increases in PM2.5 concentrations were largest in the previous 4, 5, and 6 days prior to presentation, throughout the entire study period 2014–2019 (Table 3). Although not significantly different, influenza hospitalization rate ratios were generally larger in the 2017–2019 period compared to the 2014–2016 period (Fig. 1). For example, each 4.1 µg/m3 increase in PM2.5 in the previous 6 days was associated with an 11.9% increased rate of influenza hospitalization (95% CI = 6.1, 18.0) in the 2017–2019 period, but only a 4.6% increased rate (95% CI: − 0.5, 10.1) during the 2014–2016 period. However, in the 2014–2016 period, each interquartile range increase in PM2.5 concentration in the previous 7–13 days was associated with an 8.0% decrease in the rate of influenza hospitalizations (95% CI = − 12.5, − 3.3) and a 6.3% decrease in the rate of influenza ED visits (95% CI = − 9.5, − 2.9). In contrast, in 2017–2019, each interquartile range increase in PM2.5 was associated with a 12.6% increase in the rate of influenza hospitalization (95% CI = 6.4, 19.1) (Fig. 1) and a 5.7% increase in the rate of influenza ED visits (95% CI = − 2.6, 14.6) (Fig. 2).
There were not substantial differences in influenza admissions and ED visit excess rates between males and females. Both males and females generally had the highest excess rates of influenza hospitalization associated with interquartile range increases in PM2.5 concentration in the previous 4, 5, and 6 days in both the 2014–2016 and 2017–2019 periods (Table 4)(Fig. 3). However, for males, the 2017–2019 influenza excess rates were substantially larger than in the 2014–2016 period. For females, excess rates were not consistently different between the 2014–2016 and 2017–2019 periods. Specifically, for males in 2014–2016, each IQR increase in PM2.5 concentration in the previous 5 days was associated with a 0.6% decrease in the rate of hospitalization for influenza (95% CI = − 7.4, 6.6) compared to an 8.6% increase in the rate of hospitalization (95% CI = 0.9, 16.8) in the 2017–2019 period. For females, excess rates of hospitalization associated with interquartile range increases in PM2.5 concentration for the 2014–2016 and 2017–2019 periods were similar (lag days 0–4: 10.6 and 10.2, respectively). For influenza ED visits in males, the excess rates appeared smaller in the 2017–2019 period than in 2014–2016, while the excess rates for females between periods were not consistently different.
Rates of upper respiratory infection ED visits associated with IQR increases in PM2.5 concentrations in the previous few days and weeks were larger in the 2017–2019 period than in the 2014–2016 period (Table 3). For example, each IQR increase in PM2.5 concentration in the previous 5 days was associated with a 2.4% increase in the rate of ED visits for upper respiratory infection (95% CI = 0.9, 3.8) in the 2017–2019 period, but not the rate of upper respiratory infection ED visits (excess rate = 0.0%; 95% CI: − 0.6, 0.6) in the 2014–2016 period. Upper respiratory infection hospitalization excess rates were not different between the 2017–2019 period and the 2014–2016 period, and there were no differences in upper respiratory infection excess rates between males and females (Table S1).
There were no associations between bacterial pneumonia hospitalizations and increased PM2.5 concentrations in the previous few days and weeks, in either the 2014–2016 period or 2017–2019 period, with excess rates ranging from − 3.0 to 3.8% (Table 3 and Fig. 1). However, there were similarly sized to slightly decreased excess rates of bacterial pneumonia ED visits associated with IQR increases in PM2.5 concentrations in the 2017–2019 period compared to the 2014–2016 period. For example, each 4.0 µg/m3 increase in PM2.5 concentration in the previous day was associated with a 7.3% increase in the rate of bacterial pneumonia ED visits (95% CI: 2.2, 12.6) in the 2014–2016 period, with a smaller 2.6% excess rate in the 2017–2019 period (95% CI: − 3.2, 8.8). In separate analyses for males and females, there were generally no associations between the rate of bacterial pneumonia associated with IQR increases in PM2.5 concentration in the previous few days and weeks in either the 2014–2016 or 2017–2019 period. There were also no consistent differences between period-specific excess rates for males and females (Table S2).
Interquartile range increases in PM2.5 concentration in the previous 1–7 days were generally associated with increased rates of culture-negative pneumonia hospitalizations in both the 2014–2016 and 2017–2019 periods, although the excess rates were slightly larger in the 2017–2019 period (Fig. 1, Table 3). For example, each 4.2 µg/m3 increase in PM2.5 concentration in the previous 3 days was associated with a 2.5% (95% CI: 1.1, 3.9) increased rate of culture-negative pneumonia hospitalization in the 2014–2016 period, and a 3.1% increased rate (95% CI: 1.2, 5.0) in the 2017–2019 period. For culture-negative pneumonia ED visits, excess rate estimates were generally larger in the 2017–2019 period (Fig. 2). For example, each 3.2 µg/m3 increase in PM2.5 concentration in the previous 7 days was associated with a 7.8% excess rate in the 2017–2019 period (95% CI = 5.7, 9.9), but not the 2014–2016 period (excess rate = 0.8%; 95% CI: − 3.5, 0.0). There were no sex differences in excess rates of culture-negative pneumonia hospitalizations and ED visits across periods (Table S3).
Discussion
As expected, increased ambient PM2.5 concentrations in the previous 7 days were associated with increased hospitalizations and ED visits for influenza and culture-negative pneumonia and ED visits for upper respiratory infection among adults living in Buffalo, Rochester, Albany, and New York City, New York, from 2014 to 2019. These associations were independent of concurrent changes in temperature and relative humidity, as well as any subject-specific demographic and clinical characteristics that did not vary over the study period. Inconsistent with our a priori hypothesis, rates of influenza hospitalizations, upper respiratory infection ED visits, and culture-negative pneumonia hospitalizations and ED visits associated with increased PM2.5 concentrations were larger in the 2017–2019 period (i.e., after the early implementation period of Tier 3 vehicle introduction) than in the 2014–2016 period. However, for other infection hospitalization and ED visit categories, we did not see this pattern. Finally, outside of differences in influenza infections, there were no consistent differences in cause-specific excess rates by sex.
Our finding of increased rates of respiratory infection (i.e., influenza, upper respiratory infection, bacterial pneumonia, and culture-negative pneumonia) hospitalization and ED visits associated with increased ambient PM2.5 concentrations in the previous few days and weeks is consistent with our and others previous work (Croft et al. 2019; Horne et al. 2018; Pirozzi et al. 2018; Kirwa et al. 2021; Zhang et al. 2023). However, our study also compared the size of any PM2.5/hospitalization-ED visit associations between the 2014–2016 period and the 2017–2019 period. Despite air pollution concentration reductions from an average PM2.5 concentration of 7.9 µg/m3 in the 2014–2016 period to an average PM2.5 concentration of 7.0 µg/m3 in the 2017–2019 period (Chen et al. 2023), the excess rate of healthcare contacts for respiratory infection associated with PM2.5 concentrations in our study did not decrease. In our prior study among New York State adults from 2005 to 2016, we found that each 5.7 µg/m3 increase in PM2.5 concentration in the previous 6 days was associated with a 6.4% increase in influenza ED visits in the 2014–2016 period, but only a 2.1% increase from 2008 to 2013. Since we observed a decrease in PM2.5 and other pollutant concentrations from 2005 to 2016 (Squizzato et al. 2018), we hypothesized that an increase in PM toxicity per unit mass concentration (i.e., the change in the chemical composition of PM may have made the same mass concentration more toxic) may have occurred following the series of air quality regulations and economic drivers occurring from 2008 to 2013 (Croft et al. 2019). A reason for the increased toxicity would be an increase in oxidative potential (reactivity) of PM2.5. An increase in oxidative potential may contribute to a decrease in macrophage-mediated phagocytosis and clearance of pathogens, possibly increasing the severity of infection (Wu et al. 2018). Similar to this prior study (Croft et al. 2019), our current findings when comparing respiratory infection relative rates associated with PM2.5 between 2014–2016 and 2017–2019 are not consistent with our a priori hypothesis and suggest that the current market penetration of the Tier 3 vehicle introductions in 2017 may not have a measurable respiratory infection health benefit in New York State. Specifically, despite the decreases in PM concentrations from 2014 to 2019 (Chen et al. 2023), we did not observe a decrease in the rate of healthcare encounters for respiratory infection associated with PM. While the exact percentage of market penetration of Tier 3 vehicles into the New York fleet is not yet known, a repeated assessment of this association will be helpful in understanding the full impact of the Tier 3 policy.
In addition to our prior concern of increased toxicity per unit mass of PM2.5, there are several other possible explanations for the trends we observed in the excess rate of respiratory infection. First, the increased excess rate of influenza hospitalizations (but no change in influenza ED visit relative rates) in the 2017–2019 period compared to the 2014–2016 period may indicate that the severity of health care encounters for influenza associated with PM increased between periods. Nationally, there were 22 million (19%) more symptomatic influenza illnesses in the USA, 260,000 (15%) more influenza hospitalizations, and also 7000 (5%) fewer influenza deaths than in the 2014–2016 period (CDC 2023). While there were more illnesses in the 2017–2019 period, fewer deaths would support a potentially more infectious but less lethal strain of influenza being present. Overall, while more influenza infections occurred in 2017–2019 than in 2014–2016, this is unlikely to fully explain the observed increased rate of influenza hospitalization and ED visits associated with increased ambient PM2.5 concentrations in the previous few days among New York adults during this time period.
The proportion of particles smaller than 500 nm comprised of ultrafine particles (UFP) increased from the 2014–2016 period to the 2017–2019 period, particularly in the 10–20 nm and 20–50 nm particle sizes (Chen et al. 2023). The increase in smaller particle formation (< 50 nm) may be due to reductions in the concentration of accumulation mode particles (AMP; 100–500 nm), which serve as a condensation sink for condensable substances such as UFP (Chen et al. 2023). It appears unlikely that the change in Tier 3 standards or even prior gasoline direct injection (GDI) would result in a decrease in AMP concentration, although prior GDI standard changes to meet fuel efficiency standards may have contributed to an increase in small particle generation (Yi et al. 2022). The smaller UFP particles (< 50 nm) are known to predominately deposit by diffusion (Brownian motion) in the upper airways (upper trachea/posterior pharynx). However, larger UFP particles (≥ 50 nm) have a weaker deposition (diffusional or inertial) in the upper or lower airways (Oberdörster et al. 2005). The deposition of the smaller UFP particles (< 50 nm) in the upper respiratory tract may lead to a greater risk of influenza and upper respiratory infection associated with increased PM2.5, compared to lower respiratory tract bacterial infection, given the disruption of the mucosa in the upper airway.
Influenza is a particularly dangerous infection, as it can spread from the initial site of infection in the upper airway down to the lower airway to cause pneumonia (whether influenza alone or a bacterial superinfection of influenza). While upper respiratory infections also have an initial site of infection in the upper airway, upper respiratory infections typically lead to less severe infections due to the lower likelihood of spreading to the lower airways. As expected, we observed null associations between PM and upper respiratory infection hospitalizations in both periods. However, we observed larger ED visit excess rates for upper respiratory infections in the 2017–2019 period than in the 2014–1016 period. This pattern may also be related to the increased UFP component of PM2.5, increasing the susceptibility to URIs.
The lack of clear differences in the association between bacterial pneumonia and PM2.5 between periods may reflect the change in deposition location (i.e., being in the upper airway rather than in the lower airway where bacterial pneumonia typically originates). The increased magnitude of excess rates for culture-negative pneumonia ED visits in the 2017–2019 period compared to 2014–2016, and no clear change in hospitalization excess rates was different from all other types of infections. This may be related to the fact that culture-negative pneumonia are comprised of both undiagnosed bacterial and viral infections that are likely unique from influenza or typical bacterial pneumonia organisms. While the type of infection is likely an important factor in whether an association between PM and respiratory infection is observed, the PM deposition location (related to its composition) may be another important factor to consider.
While distinct sex-specific immune responses to respiratory viral infection have been observed previously (Ghosh and Klein 2017; Silveyra et al. 2021), a recent study focused on the sex-specific response to respiratory infection in the setting of a controlled wood smoke exposure (Rebuli et al. 2019). Specifically, in this randomized controlled trial of a 2-h chamber exposure to 500 µg/m3 of wood smoke particles followed by exposure to a live attenuated influenza virus, the authors observed a greater nasal mucosal inflammatory gene response in adult males compared to females. In our study, females had the highest excess rates of influenza and bacterial pneumonia in both periods, though males had the larger increase in influenza hospitalization excess rates between the two periods. One possible explanation for this could be that the composition of PM in 2017–2019 led to a greater inflammatory effect in males with influenza, compared to the exposure in 2014–2016, while not changing the risk of respiratory viral infection admission in females. In addition to the need to study sex-specific effects, determining the effect of air pollution on specific types of infections rather than general groups is another important focus area for future research. Secondly, we did not have access to viral or bacterial respiratory infection diagnoses in outpatient clinics and physician offices. This may have led to an underestimation of the counts of healthcare encounters with a comparable severity to ED visits for upper respiratory infections and to a lesser degree, influenza ED visits during each of the respective time periods.
Although this study had several important strengths, including large sample size and its resulting enhanced statistical power, use of the case-crossover study and its control of non-time varying subject characteristics/confounders and any interactions between them by design, and a statewide comprehensive dataset of health care encounters for respiratory infection, there are several weaknesses that should be considered when making inference. First, study subjects were assigned PM2.5 concentrations from the monitoring station closest to their home, no matter if they lived near the monitor or as far as 15 miles from the station (or spent most of the day at work in a different community). However, this non-differential exposure misclassification should just result in the underestimation of excess rates. Second, in our period analyses, we were not able to control for the potential differences in influenza or Streptococcus pneumoniae vaccine status of participants, influenza strain variation, or the number of illnesses from influenza between the two periods.
Last, the use of the case-crossover design and its time-referent selection strategy (i.e. control periods matched to case periods by weekday within the same calendar month and year) (Levy et al. 2001)) allows estimation of the rate of infection associated with increased PM2.5 concentrations in the previous 1 to 7 days, keeping case and control period dates and PM2.5 concentrations separate (i.e., the dates of the case period are completely separate from the dates of the control periods for each participant). However, when this design is applied to estimate the rate of infection associated with increased PM2.5 concentrations in the previous weeks (e.g., lag days 7–13, 14–21), as we did in this study and others have done previously (Horne et al. 2018), calendar dates that were included as “case periods” in one lagged analysis (e.g., lag days 0–6, 0–5, 0–4) may now be included as “control periods” in a second lagged analysis (e.g., lag days 7–13, 14–21). For example, assume we are interested in estimating the rate of influenza hospitalization associated with increased PM2.5 concentrations in the previous 7 days (lag days 0–6). If the influenza hospitalization for a hypothetical subject occurred on Thursday, November 15, 2018, we would contrast the PM2.5 from the case period (November 9–15) with PM2.5 concentrations from 4 control periods (November 23–29, November 16–22, November 2–8, and October 25–November 1). Now, as part of the same study, assume we also want to estimate the rate of influenza hospitalization associated with increased PM2.5 concentrations in the prior week (e.g., lag days 7–13). For that same subject influenza hospitalization, we would contrast the PM2.5 from the case period (November 2–8) with PM2.5 concentrations from 4 control periods (November 16–22, November 9–15, October 25–November 1, and October 19–25). However, what was a case period in the lag day 0–6 analysis (November 9–15) is now a control period in the lag day 7–13 analysis, and what was a control period in the lag days 0–6 analysis (November 2–8) is now a case period in the lag days 7–13 analysis. Thus, if there is any association between PM2.5 and the rate of influenza hospitalization, effect estimates for these two different lagged analyses would be expected to be quite different. If one effect estimate is > 1.0, you may expect the other effect estimate to be < 1.0, and thus, making inferences on the triggering of influenza by short-term increases in PM2.5 in these later weeks may be problematic. In our analyses in the 2014–2016 period, we found that each IQR increase in PM2.5 concentration in lag days 0–6 was associated with a 4.6% increase in the rate of influenza hospitalizations, but an IQR increase in PM2.5 concentration in lag days 7–13 was associated with an 8.0% decrease in the rate of influenza hospitalization. The large difference in effect size between these two lagged influenza hospitalization analyses (positive association to negative association) may, in part, be a result of this referent selection problem for lag times longer than 7 days. Thus, we suggest future analyses use a modified case-crossover referent selection algorithm without such sharing of dates and PM2.5 concentrations or preferably use a different study design to examine lag times longer than 7 days.
Conclusions
Despite the introduction of Tier 3 vehicles with lower pollutant emissions in 2017, the excess rates of influenza, URI, bacterial pneumonia, and culture-negative pneumonia hospitalizations and ED visits associated with increased PM2.5 concentrations in the previous 7 days in 2017–2019 were not reduced in this early Tier 3 implementation period, compared to 2014–2016. It is possible that an increase in the proportion of the ultrafine component of PM2.5 may have led to an increase in toxicity per unit mass of PM2.5. Due to deposition in the upper airway, it is possible that increases in ultrafine particles contributed to an increased rate of influenza hospitalizations but did not contribute to the rate of bacterial pneumonia hospitalizations. The increase in the influenza hospitalization excess rate associated with increased PM2.5 in the 2017–2019 period compared to the 2014–2016 period appeared to be primarily driven by an increase in hospitalization in males. In summary, limited penetration of Tier 3 vehicles to the New York State vehicle fleet, pollution deposition location in the lung, type of infection, and differences in relative rates by sex may all have contributed to the lack of improvement in respiratory infection rates associated with increased PM after Tier 3 emissions regulations were implemented.
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available due to the training and registration requirements of the Statewide Planning and Research Cooperative System (SPARCS)database. Queries can be addressed via https://www.health.ny.gov/statistics/sparcs/
References
Aho K, Derryberry D, Peterson T (2014) Model selection for ecologists: the worldviews of AIC and BIC. Ecology 95:631–636
CDC (2023) Flu Vaccination Coverage, United States, 2021–22 Influenza Season. https://www.cdc.gov/flu/fluvaxview/coverage-2022estimates.htm. Accessed 12 Mar
Chen M-J, Yang P-H, Hsieh M-T, Yeh C-H, Huang C-H, Yang C-M, Lin G-M (2018) Machine learning to relate PM2. 5 and PM10 concentrations to outpatient visits for upper respiratory tract infections in Taiwan: a nationwide analysis. World J Clin Cases 6:200
Chen Y, Rich DQ, Masiol M, Hopke PK (2023) Changes in ambient air pollutants in New York State from 2005–2019: Effects of policy implementations and economic and technological changes. Atmos Environ 311:119996. https://doi.org/10.1016/j.atmosenv.2023.119996
Croft DP, Zhang WJ, Lin S, Thurston SW, Hopke PK, Masiol M, Squizzato S, van Wijngaarden E, Utell MJ, Rich DQ (2019) The association between respiratory infection and air pollution in the setting of air quality policy and economic change. Ann Amer Thoracic soC 16:321–330
Ghosh S, Klein RS (2017) Sex drives dimorphic immune responses to viral infections. J Immunology 198:1782–1790
Hopke PK, Hidy G (2022) Changing emissions results in changed PM2. 5 composition and health impacts. Atmosphere 13:193
Horne BD, Joy EA, Hofmann MG, Gesteland PH, Cannon JB, Lefler JS, Blagev DP, Korgenski EK, Torosyan N, Hansen GI, Kartchner D, Pope CA 3rd (2018) Short-term elevation of fine particulate matter air pollution and acute lower respiratory infection. Am J Respir Crit Care Med 198:759–766
Kirwa K, Eckert CM, Vedal S, Hajat A, Kaufman JD (2021) Ambient air pollution and risk of respiratory infection among adults: evidence from the multiethnic study of atherosclerosis (MESA). BMJ Open Respir Res 8:e000866
Levy D, Lumley T, Sheppard L, Kaufman J, Checkoway H (2001) Referent selection in case-crossover analyses of acute health effects of air pollution. Epidemiology 12:186–192
Lu B, Wang Y, Zhu Z, Zhang Z, Dong T, Li F, Gao Y, Du X, Qu Z (2020) Epidemiological and genetic characteristics of influenza virus and the effects of air pollution on laboratory-confirmed influenza cases in Hulunbuir, China, from 2010 to 2019. Epidemiol Infect 148:e159. https://doi.org/10.1017/S0950268820001387
Maclure M (1991) The case-crossover design: a method for studying transient effects on the risk of acute events. Am J Epidemiol 133:144–153
Masiol M, Squizzato S, Rich DQ, Hopke PK (2019) Long-term trends (2005–2016) of source apportioned PM2.5 across New York State. Atmos Environ 201:110–20
Oberdörster G, Oberdörster E, Oberdörster J (2005) Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect 113:823–839
Pirozzi CS, Jones BE, VanDerslice JA, Zhang Y, Paine R 3rd, Dean NC (2018) Short-term air pollution and incident pneumonia. A case-crossover study. Ann Am Thorac Soc 15:449–459
Purcaro G, Rees CA, Wieland-Alter WF, Schneider MJ, Wang X, Stefanuto PH, Wright PF, Enelow RI, Hill JE (2018) Volatile fingerprinting of human respiratory viruses from cell culture. J Breath Res 12:026015
Rebuli ME, Speen AM, Martin EM, Addo KA, Pawlak EA, Glista-Baker E, Robinette C, Zhou H, Noah TL, Jaspers I (2019) Wood smoke exposure alters human inflammatory responses to viral infection in a sex-specific manner. A randomized, placebo-controlled study. Am J Respir Crit Care Med 199:996–1007
Renzi M, Scortichini M, Forastiere F, DeDonato F, Michelozzi P, Davoli M, Gariazzo C, Viegi G, Stafoggia M, Ancona C (2022) A nationwide study of air pollution from particulate matter and daily hospitalizations for respiratory diseases in Italy. Sci Total Environ 807:151034
Rynda-Apple A, Robinson KM, Alcorn JF (2015) Influenza and bacterial superinfection: illuminating the immunologic mechanisms of disease. Infect Immun 83:3764–3770
Santo Loredana, Schappert Susan M, Ashman Jill J (2021) Emergency Department Visits for Influenza and Pneumonia: United States, 2016–2018. US Department of Health and Human Services, Centers for Disease Control
Silveyra P, Al Housseiny H, Rebuli ME (2021) Sex and gender differences in the susceptibility to environmental exposures. In: Silveyra P, Tigno XT (eds) Sex-Based Differences in Lung Physiology. Physiology in Health and Disease. Springer, Cham, pp 251–90
Squizzato S, Masiol M, Rich DQ, Hopke PK (2018) PM2.5 and gaseous pollutants in New York State during 2005–2016: spatial variability, temporal trends, and economic influences. Atmos Environ 183:209–224
Wu W, Jin Y, Carlsten C (2018) Inflammatory health effects of indoor and outdoor particulate matter. J Allergy Clin Immunol 141:833–844
Yi H, Seo J, Young Soo Yu, Lim Y, Lee S, Lee J, Song H, Park S (2022) Effects of lubricant-fuel mixing on particle emissions in a single cylinder direct injection spark ignition engine. Sci Rep 12:18
Zhang R, Lai KY, Liu W, Liu Y, Ma X, Webster C, Luo L, Sarkar C (2023) Associations between short-term exposure to ambient air pollution and influenza: an individual-level case-crossover study in Guangzhou, China. Environ Health Perspect 131:127009
Zikova N, Masiol M, Chalupa DC, Rich DQ, Ferro AR, Hopke PK (2017) Estimating hourly concentrations of PM2.5 across a metropolitan area using low-cost particle monitors. Sensors 17:1922
Funding
This work was supported by Contract #s 156226 and 125993 from the New York State Energy Research and Development Authority and National Institutes of Health (NIH) Grant # P30 ES001247. Daniel Croft was supported by NIH/National Institute of Environmental Health Sciences Grant # K23 ES032459.
Author information
Authors and Affiliations
Contributions
Conceptualization: DPC, MJU, SL, SWT, PKH, DQR; data curation: HL, SL, YC, PKH, DQR; formal analysis: DPC, HL, SL, SWT, DQR; funding acquisition: DQR, PKH, SL, SWT, MJU; methodology: DQR, SWT, DPC, SL, PKH; supervision: DQR, PKH, SL; roles/writing—original draft: DPC, DQR, PKH, MJU; writing—review an editing: all authors.
Corresponding author
Ethics declarations
Ethics approval
This is an observational study. The University at Albany Research Ethics Committee has confirmed that no ethical approval is required.
Consent to participate and publish
Given the statewide database is managed by the New York State Department of Health, informed consent for participation or publication was not obtained by the study authors.
Competing interests
David Q Rich is an associate editor at Air Quality, Atmosphere and Health. No other competing interests for authors exist.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Croft, D.P., Utell, M.J., Liu, H. et al. Change in rate of healthcare encounters for respiratory infection from air pollution exposure after improved vehicle emissions standards in New York State. Air Qual Atmos Health (2024). https://doi.org/10.1007/s11869-024-01505-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11869-024-01505-6