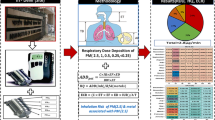
Abstract
Inhalation of particulate matter (PM) has been extensively associated with the worsening and onset of cardiorespiratory diseases, being responsible for millions of deaths annually. Assessment of PM deposition in the human respiratory tract is critical to better understand the health risks from environmental exposure of vulnerable age groups. In this study, PM2.5 and PM10 day-night monitoring campaigns during the cold season were carried out in Bragança, Portugal. The multiple-path particle dosimetry (MPPD) model was used to quantify total and regional depositions in the human respiratory tract for four different age groups: infant (3 months), child (9 years), adult (21 years) and elderly (65 years). The results showed that concentrations for both PM fractions were higher during the night, a period marked by the burning of biomass for residential heating. Regional deposition fractions (DF) for PM2.5 were in the ranges 17–38% (head), 4–14% (tracheobronchial) and 20–28% (pulmonary), while for PM10 were 24–67% (head), 4–27% (tracheobronchial) and 12–22% (pulmonary). Children and the elderly were found to be the most vulnerable groups to PM deposition, especially for the TB and H regions, respectively. The lifetime cancer and non-cancer risks associated with exposure to PM2.5 exceeded the recommended limits, especially for children <10 years old. These findings provide useful information to alert authorities to the need to take action to reduce the pollution burden and protect the health, in particular of those most susceptible.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Urban air pollution has grown attention over the past decades due to the clear deleterious human health effects resulting from exposure to various contaminants, among which particulate matter (PM) with aerodynamic diameters below 10 and 2.5 μm (PM10 and PM2.5, respectively) stands out as the most dangerous (Alemayehu et al. 2020; Duan et al. 2021; Huang et al. 2021), especially due to its significant relationship with cardiorespiratory diseases (Bateson and Schwartz 2007; Khosravipour et al. 2022; Zhang et al. 2018). Plenty of studies support the hypothesis that the health effects of inhaling air particles are governed by more than just particle mass, since the chemical composition and the size fraction are highly variable in space and time (Alves et al. 2021; Cesari et al. 2018; Lucarelli et al. 2018; Phuc and Oanh 2022; Pio et al. 2020; Stojić et al. 2016). The complexity of urban aerosols comes from the different emission sources, which can produce different size-segregated PM as function of the strength of the sources in specific periods (Cipoli et al. 2023; Li et al. 2023; Rogula-Kozłowska 2016; Rönkkö et al. 2018; Theodosi et al. 2011; Yang et al. 2023).
Apart from particle size, the deposition of PM in the human respiratory tract (HRT) also depends on lung morphometry and respiratory physiology. Ultrafine particles are prone to penetrate into the lung alveoli and bloodstream, while coarse particles are mainly settled in the upper respiratory tract (Hofmann 2011; Rissler et al. 2012; Sá et al. 2015; Deng et al. 2019), demonstrating the pivotal role of size in PM deposition. It has been documented that PM deposition depends on age, with children and the elderly identified as the groups most susceptible to exposure to air pollution (Sacks et al. 2011). The amounts deposited in the HRT are a relevant potential causative factor for investigating the health effects of PM pollution (Voliotis et al. 2021). Different models have been developed to estimate the total and regional deposition fractions: the ExDoM (Exposure Dose Model) (Aleksandropoulou and Lazaridis 2013), the ICRP model (International Commission on Radiological Protection), the NCRP model (National Council on Radiation Protection and Measurement) and the MPPD model (Multiple-Path Particle Dosimetry) (Asgharian et al. 2001; ICRP and Protection 1994; Koblinger and Hofmann 1990; National Council on Radiation Protection and Measurements 1997). Due to its user-friendly interface, the MPPD model has had a wide application in the literature (Behera et al. 2015; Oller and Oberdörster 2016; Avino et al. 2016; Lv et al. 2021; Amoatey et al. 2022; Khan et al. 2022). Most previous studies on PM exposure in Portugal are based on daily data (Almeida et al. 2014; Alves et al. 2023; Garrett and Casimiro 2011; Vicente et al. 2017). However, temporal variation in air pollutant concentrations and human activities may be crucial factors in health outcomes (Cheng et al. 2021; Lin et al. 2018). The concentration of pollutants in ambient air shows a temporary pattern, with higher levels at night compared to daytime, especially in winter (Barmpadimos et al. 2012; Gama et al. 2018). On the other hand, human behaviour, PM sources and meteorological conditions in urban areas show significant variations throughout the day, affecting the formation, removal and transport of atmospheric aerosols (Freiman et al. 2006; Galindo and Yubero 2017). In addition, day-night variations end up being reflected in different exposure patterns and, consequently, in distinctive health outcomes. Recent studies have pointed out that exposure to air pollution can vary dramatically between periods, with significant differences in the toxicological properties of PM between day and night periods (Galindo and Yubero 2017; Jalava et al. 2015).
Adverse health effects from exposure to PM result from its inhalation through the mouth or nose and subsequent deposition in the HRT. Particle deposition in HRT is governed and influenced especially by respiratory parameters, characteristics of lung morphology, and particle properties (Heyder et al. 1986; Heyder et al. 1980). Depositions in specific regions, including the head (H), tracheobronchial (TB) and pulmonary (P), of the respiratory system, are important to determine the adverse effects to human health (Goel et al. 2018). Currently, there are few studies focused on PM deposition for different periods of the day (Cipoli et al. 2022; Lv et al. 2021). In addition, most of the existing PM data of lung deposition target adult males (Subramaniam et al. 2003; Kameda et al. 2005; Avino et al. 2016; Menon and Nagendra 2018; Rajput et al. 2019). Only a few works have focused on PM deposition in children (Protano et al. 2017; Rissler et al. 2017; Patterson et al. 2014), and less attention has been paid to elderly groups (Kim and Jaques 2005; Manojkumar et al. 2019a; Manojkumar and Srimuruganandam 2022). As far as we know, there are no previous studies addressing PM2.5 and PM10 deposition for different age groups using daytime and nighttime measurements in urban environments in Portugal. Furthermore, there is a lack of data on deposition in the HRT of vulnerable groups such as children and the elderly. For a clearer understanding of the PM2.5 and PM10 deposition in the HRT, this study considers the concentrations obtained from different periods in an urban background site, aiming at (1) investigating the diurnal and nocturnal variability of PM2.5 and PM10 concentrations during the cold season, (2) identifying potential risks, both carcinogenic and non-carcinogenic, due to PM exposure and (3) evaluating the total and regional deposition fractions, mass rate depositions and inhaled doses in the HRT of four age groups, by applying the widely accepted MPPD model. To establish a comparative scenario between day and night for different age groups, doses per minute were estimated assuming very light activities. The estimates produced by the model for the night and day periods constitute essential information to define the doses to be tested in vitro in PM toxicity assays to be carried out in the near future.
Material and methods
Sampling site
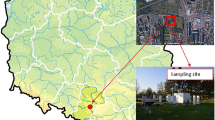
The small urban area of Bragança (10 km2) has approximately 35,000 inhabitants (PORDATA 2021). The city is located in the northeast of Portugal in a mountainous region (700 m altitude). Bragança is in a Csa climate region (in the Köppen-Geiger classification, Peel et al. 2007), with rainy periods centred in the cold season (December to February), while the summer and spring are drier. Deterioration of air quality is more evident during the cold season because of vehicular traffic emissions and residential heating activities associated with more stable atmospheric conditions. In addition, as other regions of the Iberian Peninsula, Bragança is sometimes affected by Saharan dust intrusions, which are more persistent in the warm period and with some less frequent occurrences in the cold period (Pereira et al. 2008; Russo et al. 2020). The city has been the subject of just a few climate and air quality studies (Dantas et al. 2019; Cipoli et al. 2022; Cipoli et al. 2023).
Particulate matter data (PM2.5 and PM10) were collected on the roof of the Agricultural High School, Polytechnic University of Bragança (41°48′20″ N, 6°45′42″ W) at about 15 m above ground level (Fig. 1). The selected site can be considered representative of the average altitude of the city. The school is located in the southwest part of the city and is surrounded by cafes, bars, supermarkets, small companies and residential buildings. In addition, the school is in the vicinity of busy avenues, which are adjacent to bike path often used by the population that runs around the entire outer perimeter of the Polytechnic (3 km long). Adults with infants and the elderly generally use this pathway for short walks during the midday, especially on sunny days in the cold season. However, it is observed that most adults who practice outdoor activities in this time of the year chose the beginning of the night period after the workday.
Particulate matter monitoring
The monitoring campaign took place over 55 days in the 2021 cold season (January 14th to March 17th), covering weekdays and weekends. Continuous monitoring of PM2.5 was performed with a VEREWA Beta Attenuation Monitor (BAM model F701-20, Durag group, Germany). The BAM was operated with a flow rate of 1 m3 h−1 and a temporal resolution of 2 h. Simultaneously, collection of PM10 was performed by a low-volume sampler (Echo PM TECORA, Italy) operated at a flow rate of 2.3 m3 h−1, equipped with pre-weighed 47 mm quartz filter, covering two different periods (08:00 a.m – 06:00 p.m and 06:30 p.m – 07:30 a.m, local time) in order to determine intra-day concentrations. The selection of sampling periods was based on the hours of sunlight and the predominant activities in each period. The daytime period was monitored during the hours of sunlight, during which there is a high probability of the elderly and children frequenting the outdoor environment. The night period covered the return home after the working day, during which there is an intensification of biomass burning. Due to partial or complete loss of data caused by power outages, equipment failures and/or heavy rains, only 40 days with valid data for both size fractions were selected for model application.
Both the Beta monitor and the gravimetric sampler were factory calibrated before the monitoring campaign, and flow/zero checks are made during the whole period of experiments. Additionally, weather variables (air temperature, relative humidity, precipitation, wind direction and speed) were obtained at 1-h resolution from a portable weather station (Campbell Scientific Inc., USA), installed next to the other equipment on the roof.
As gravimetric measurements of PM2.5 were not performed due to the unavailability of a gravimetric equipment with this cutoff size, and since BAM values were used in the model without any correction, some discrepancies between estimated and actual PM2.5 doses may occur. However, several intercomparisons between gravimetric and BAM measurements have shown very good correlations with slopes close to 1, although in winter conditions the continuous monitor tends to slightly underestimate the concentrations of the gravimetric reference method (Chung et al. 2001; Hauck et al. 2004; Shukla and Aggarwal 2022; Takahashi et al. 2008).
Multiple-path particle dosimetry model
The mass rate and deposition fractions of PM2.5 and PM10 were estimated for each region of the human respiratory tract (Head (H), Tracheobronquial (TB) and Pulmonary (P)) using the Multiple-path particle dosimetry model (MPPD). The Age-Specific 5-Lobe model was selected to simulate the deposition of lung particles, which has 10 different geometric configurations of the lung structure for different age groups from 3 months to 21 years. Four different age groups were considered in this study: infants (3 months), children (9 years), adults (21 years) and the elderly (>65 years). The lung structure for the elderly was not available, so the lung geometry of adults was used, and specific respiratory parameters were changed to reflect the characteristics of the elderly population. The physiological parameters of specific age groups (functional residual capacity, upper respiratory tract volume, breathing frequency and tidal volume) used in the software are given in Table 1, according to the recommendations of the International Radiological Protection Commission (ICRP 1994) and the model itself. For the infant, child and adult groups, the default values provided by the model were used. As the values for the elderly were not available, these parameters were adopted from the literature (Manojkumar and Srimuruganandam 2022). In addition, to establish a comparative scenario of short-term outdoor exposure between age groups, the level of activity was considered to be very light.
The properties of inhaled PM play an essential role in lung deposition. Due to the unavailability of specific information during the study period, the values found by Cipoli et al. (2022) for the mass median aerodynamic diameter (MMAD) and for the geometric standard deviation (GSD) during the cold season for the city of Bragança were used. These values can be consulted in more detail in Table S1.
In order to estimate the deposition fraction (DF) in the lung airways, several assumptions were made. Particles have a density of 1 g cm−3, all particles enter the respiratory tract through the nose and body orientation is assumed to be vertical, except for infants, who were considered to be lying on their backs. Additional information about the model and its capabilities can be found at Anjilvel (1995) and Miller et al. (2016). Version 3.04 of the model can also be downloaded for free at https://www.ara.com/products/multiple-path-particle-dosimetry-model-mppd-v-304.
Inhalation dose calculations
Based on the mean concentrations obtained for both PM2.5 and PM10 and the deposition fractions calculated using MPPD, the individual short-term exposure dose was estimated for each period and age group using the following equation (Goel et al. 2018):
where PMi is the mean PM concentration of fraction i (μg m−3), DF is the deposition fraction and VE represents ventilation per minute (m3 min−1). VE values for infant, child, adult and the elderly were 2.4 × 10−3, 8.4 × 10−3, 10.9 × 10−3 and 9.9 × 10−3 USEPA (2011) respectively.
Health risk assessment
The methodology proposed by the United States Environmental Protection Agency (USEPA 2009) was applied for estimating the health risk from inhalation for all age groups. This methodology is commonly used to estimate the risks of specific PM-bound compounds such as metals and polycyclic aromatic hydrocarbons (e.g. Alves et al. 2021; Alves et al. 2020; Liu et al. 2019; Slezakova et al. 2014) but has also been successfully applied to PM concentrations in other studies (Heydari et al. 2019; Pavel et al. 2020; Yunesian et al. 2019). The lifetime excess cancer risk (ELCR) and the non-cancer risk were calculated for PM inhalation based on the mean concentrations obtained in each period. It has been considered that ambient levels are representative of the total population exposure given the lack of time-activity patterns for distinct microenvironments (Kazakos et al. 2020). According to the World Health Organisation (WHO), acceptable ELCR limit values range from 1 × 10−5 to 1 × 10−6 (USEPA 2007). The ELCR was calculated using the following equation:
where LADD refers to the lifetime average daily dose and SF is the slope carcinogenic potency factor. LADD was calculated as follows:
where C is the PM concentration, IR is the inhalation rate, ET refers to the exposure time (hours/day), EF is the exposure frequency, ED is the duration of exposure, BW is body weight for each age group and AT is the average lifetime. Given that the risk analysis was based on a winter campaign, a period during which concentrations in Bragança are higher than those recorded in summer (Cipoli et al. 2022), the results obtained must be seen as the ‘worst’ scenario. All input parameters segregated by age and period are described in detail in Table S2. To determine the SF value, Eq. (4) provided by the USEPA was used:
where UR is the unit risk (μg m−3), BW is body weight (kg) and IR is the inhalation rate (m3 day−1). No studies were found that reported UR for PM10. The UR value for PM2.5 (0.008 μg m−3) was obtained from the study by Greene and Morris (2006). Thus, ELCR was estimated only for PM2.5. Since there are no government or health agencies (e.g. USEPA and WHO) reporting unit risks for PM10 and PM2.5, the carcinogenic risk results should be regarded as indicative. The non-carcinogenic risk of PM2.5 and PM10 was assessed using Eq. (5), based on the ratio between the LADD and the reference dose (RfD):
To calculate the RfD (Eq. 6), the inhalation reference concentration (RfC) of 5 μg m−3 for PM2.5 and 50 μg m−3 for PM10 were used (De Oliveira et al. 2012; Li et al. 2017), in order to assess the harmful impacts on human health.
In general, if HQ is greater than 1, the potential non−carcinogenic chronic risks are considered to be of concern and require action to be taken, while values lower than 1 are considered as acceptable risk (USEPA 2007).
General statistical analysis
PM concentration data were statistically analysed using SPSS software (IBM Statistics software v. 24). The nonparametric method of Mann-Whitney was used to evaluate the differences between daytime and nighttime. Pearson’s correlation test was utilised to analyse linear correlations between PM2.5 and PM10 and obtain significant differences at a confidence level of 95% (p < 0.05).
Results and discussion
General overview
During the sampling campaign, the mean air temperature and relative humidity varied between 1.2 and 15.2 °C and 39.8 and 98.3% respectively. Accumulated precipitation was 12.8 mm, falling on 15 days out of the 55 days of sampling. The average wind speed was 2.7 m s−1 with a predominantly southeasterly direction.
The in situ intercomparison between the gravimetric sampler and the BAM produced a high degree of agreement with significant correlations (r2 = 0.93, slope of 1.72 and y-intercept of 12.3 μg m−3 for daytime with a p < 0.05 and r2 = 0.91, slope of 1.76 and y-intercept of 9.74 μg m−3 for nighttime with a p < 0.05) for the entire period. Overall, the mean concentrations of PM2.5 and PM10 (Fig. 2) were 38% and 17% higher at night than during the day, respectively. In the daytime, mean concentrations of PM2.5 ranged between 4.78 and 46.9 μg m−3, while PM10 varied from 13.6 to 92.3 μg m−3. At night, values from 3.77 to 44.9 μg m−3 were found for PM2.5 and from 12.4 to 96.7 μg m−3 for PM10.
The mean PM2.5/PM10 ratio exhibited significant differences between nighttime (0.46) and daytime (0.37), which can be explained by the presence of sources that contribute to the emission of fine particles in the nocturnal period (e.g. biomass burning for residential heating) and lower dispersion conditions. Results of a previous source apportionment study in the city for the same monitoring campaign (Cipoli et al. 2023) indicated that, during the nocturnal period, the main contributions to the PM10 levels were as follows: biomass burning (30%) > traffic (26%) > dust (22%) > secondary inorganic aerosol (12%) > sea salt (10%). It has been shown that the PM2.5/PM10 ratio varies widely between regions in Europe. Ratios in the range 0.6–0.8 have been reported for regional background sites, while at urban background sites the values varied from 0.4 to 0.5 (Southern Spain and the Canary Islands) to 0.8 in Germany, The Netherlands and Northern and Central regions of Spain. At kerbside sites, the ratios are typically 0.6–0.7, but lower values (0.4) have been obtained in Sweden and in the Canary Islands, pointing to stronger inputs from road dust emissions and intrusions of PM10 plumes from the Sahara desert, respectively (Querol et al. 2004).
The difference between daytime and nighttime periods in the present study may, at least in part, be related to the possible underestimation of PM2.5 concentrations by the Beta monitor under typical winter meteorological conditions (Le et al. 2020). The mean daily concentrations of PM2.5 exceeded the value of 15 μg m−3 recommended by the World Health Organisation in 45% of the time, while for PM10 the exceedances of the daily value of 50 μg m−3 (Directive 2008/50/EC) corresponded to 30% of the monitoring period. In the previous source apportionment study it was demonstrated that biomass burning during nighttime is the main responsible for those exceedances (Cipoli et al. 2023).
Total and regional PM deposition fractions
The deposition fractions segregated by PM size, age (Infant (I), Child (C), Adult (A) and Elderly (E)) and period of the day are shown in Table 2. PM10 showed the highest DF (0.58 – 0.86) in the respiratory tract, followed by PM2.5 (0.43 – 0.64). Furthermore, for both fractions, higher DFs were found during the day than at night, with more pronounced increments (1.38 times for PM2.5 and 1.46 times for PM10) for the elderly category. When considering the PM2.5 fraction, the following order of vulnerability was found for the daytime period: child > elderly > infant > adult. For the night period, the order was as follows: child > infant > elderly > adult. For PM10, the next sequence was observed for the daytime period: elderly > adults > child > infant. The corresponding order for the night period was: child > infant > elderly > adult. It is worth noting that the estimated DF for the daytime period for both fractions pointed to the elderly as the most vulnerable age group, while for the nighttime period, the child category proved to be the most susceptible. Similar results for total DFs have been reported elsewhere (Madureira et al. 2020; Manojkumar et al. 2019; Manojkumar and Srimuruganandam 2022).
In relation to the regional DF, the deposition in the H, TB and P regions varied in a considerable range. During the day, PM10 and PM2.5 showed deposition percentages in the H region for both size fractions (PM10: I (33%), C (40%), A (63%), E (67%) and PM2.5: I (24%), C (29%), A (34%) E (38%)) higher than those of the night period. However, during the night, the deposition of PM2.5 was dominant in the P region (PM2.5: I (24%), C(28%), A (21%), E (21%)). Head and pulmonary depositions for specific periods agree with the results of other studies (Cipoli et al. 2022; Lv et al. 2021). The deposition percentages in the TB region for both PM fractions were generally lower than in the other regions of the respiratory tract, except for PM10 in the infant category, where TB (27% daytime and 23% nighttime) exceeded the percentages found in the P region (15% daytime and 13% nighttime).
In general, considering both periods, the percentages of PM2.5 deposition in the P region were more pronounced for children, followed by infants, while for the H region, the most affected groups were the elderly, followed by adults. For the TB region, the infant category had the highest DFs. PM10 deposition was dominant in the H region for all age categories, with substantial differences in the regional deposition in P. For PM10, the more persistent deposition in the upper respiratory tract may be related to impaction and sedimentation mechanisms, while PM2.5 is governed by the Brownian diffusion mechanism, penetrating deeper into the airways. It should be noted that it was estimated that, during the day, the contribution of dust represented up to 25% of PM10 concentrations, while traffic (exhaust and non-exhaust emissions) accounted for 38% (Cipoli et al. 2023). In summary, the main factors that induce these variations are linked to the properties of the particles (MMAD and GSD), which depend on the emission sources, secondary reactions in the atmosphere and deposition mechanisms in the HRT (as a function of age).
Deposited mass rate
The deposited mass rate by age, period and particle size (Fig. 3) were also estimated for this study. PM2.5 showed more evident deposition in the lung region compared to PM10, especially for infants. The highest deposition rates of PM2.5 were found in the elderly category (daytime 2.54 × 10−5 μg min−1 and nighttime 4.66 × 10−5 μg min−1), while for PM10, children were subject to a higher deposition rate (daytime 2.70 × 10−3 μg min−1 and nighttime 1.80 × 10−3 μg min−1). In general, mass deposition for PM2.5 decreased with age, being lower in infants (4.65 × 10−5 μg min−1) and higher in the elderly (3.60 × 10−4 μg min−1). These results are in line with those reported by Madureira et al. (2020) and Manojkumar and Srimuruganandam (2022), who found similar relationships for fine particles as a function of age.
Considering all age groups, the deposited mass rate of PM2.5 and PM10 was higher for the night period. Although this can be explained by the higher concentrations and specific particle properties in this period (e.g. smaller aerodynamic diameter due to biomass combustion for residential heating), the mass deposition rates are greatly influenced by the GSD values. In fact, changes in particle properties and distribution can affect the region and deposition rate in the respiratory tract.
Lobar deposition
The deposition fractions in the lobar region were estimated up to the 23rd generation and can be seen in Fig. 4. The first generation begins in the bronchi, and the last corresponds to the alveolar sacs. In addition, deposition was evaluated for the entire lung (EL), and for the 5 lobes: right upper (RU), right middle (RM), right lower (RL), left upper (LU) and left lower (LL). Both PM fractions have high deposition in the LL (PM2.5: 31–37% and PM10: 30–37%) and RL (PM2.5: 26–39% and PM10:26–38%) regions, whereas the smallest depositions are found in RM (PM2.5: 5–8% and PM10: 5–8%). Infants and children presented lobar depositions according to the following order: RL > LL > LU> RU > RM, while for adults and the elderly the sequence was as follows: LL > RL > LU > RU > RM. Regardless of age groups, the lowest deposition rates were found for the upper and middle lobes. This difference can be attributed to lobar lengths and volumes, since LU, RU and RM have about 3 to 4 times less volumes than the lower lobes, and therefore, lower lobar deposition (Islam et al. 2017).
The child and infant age groups presented the highest total lobar deposition fractions for PM2.5 and PM10 in both periods, respectively. For exposure to PM10, infants showed a bimodal behaviour, with PD peaks related to the 8th and 23rd generations. For the other age groups, the highest PDs were centred on generations 21–23. The 23rd generation (alveolar) received about 4–19 and 1–14 times more deposition of PM2.5 and PM10 than the bronchial region (5th generation), except for the PM10 exposure of the infant age group, whose deposition in the 5th generation was 1.3 times higher than that of the 23rd generation. The peaks of PM10 lobar deposition in infants can be explained by the smaller diameter of the airways and higher respiratory rate in this age group (book toxicology of the lung). Although the deposition fraction between the number of generations varied in all age groups, the trend towards an increase in the deposition fraction after the 20th generation was similar.
In general, lung anatomical structure, inhalation rates and particle inertia play a key role in understanding deposition in specific regions of the lung (Islam et al. 2017). Deposition in lobar regions is linked to decreased lung function and an increase in chronic diseases (Guo et al. 2018; Zhao et al. 2017), as well as to the development of carcinomas in specific regions (Hofmann 2011).
Inhaled PM doses
The inhalation doses associated with specific deposition by region of the HRT can be seen in Fig. 5. Regardless of the period analysed, the elderly age group experienced the highest average PM10 inhaled doses by region: H (267.6 × 10−3 μg min−1), TB (15.2 × 10−3 μg min−1) and P (57.5 × 10−3 μg min−1). Detailed information on inhaled doses by sampling period and day period can be found in the supplementary material (Tables S3-S6). The doses found for the daytime period by PM2.5 inhalation were only higher than those for the nighttime period for the TB region of the infant age group (5.4 × 10−3 μg min−1 in daytime and 3.7 × 10−3 μg min−1 in nighttime) and for the H region of adults and the elderly (57.2 × 10−3 μg min−1 in daytime and 36.4 × 10−3 μg min−1 in nighttime, and 57.9 × 10−3 μg min−1 in daytime and 37.6 × 10−3 μg min−1 in nighttime, respectively). The higher nighttime dose of fine particles is in agreement with the results reported by Lv et al. (2021). These findings show that the deposited dose is dependent not only on the ambient concentration but also on the particle size. Day–night regional doses of PM2.5 showed statistically significant differences (p < 0.05) for all groups, due to specific sources in different periods. In summary, the vulnerability of the age groups followed the following order: Head—E > A > C > I, Tracheobronchial—E > C > A > I and Pulmonary—E > A > C > I.
Inhaled doses of PM10, regardless of age groups, were high for all regions during the day compared to the night. Children revealed the highest doses of PM10 (57.4 × 10−3 μg min−1 in daytime and 53.4 × 10−3 μg min−1 in nighttime) for the TB region, being higher than those estimated for infants, adults and the elderly by about 2.5, 2.3 and 2.1 times, respectively. The H and P regions presented the highest deposited doses of PM10 for the elderly age group, being up to 15 and 7 times higher than those of infants. With regard to PM10 inhalation, in general, the following order was observed for each region: Head—E > A > C > I, Tracheobronchial—C > E > I > A and Pulmonary—E > C > A > I. For both PM fractions significant differences were found in the H region for adults and the elderly depending on the period analysed (p < 0.05). This suggests that specific sources act in certain periods, contributing to the emission of different PM sizes. According to the results obtained, some kind of long-term health effect is to be expected, especially for the elderly and children, who are the most vulnerable groups to PM exposure.
Health risk assessment of PM
Carcinogenic (ELCR) and non-carcinogenic (HQ) risks were calculated for all age groups and are shown in Table 3. All the ELCR values exceeded the recommended limits (1 × 10−5 to 1 × 10−6) for all age groups. The higher levels for children aged 9 and under indicate that this age group is at risk due to exposure to PM2.5, and therefore measures to mitigate and control the levels of this pollutant must be applied to reduce potential carcinogenic risks. In agreement with the present results, similar findings were previously reported by Yunesian et al. (2019), who also observed that children and young people in Teheran are at high risk of adverse health effects.
The non-carcinogenic risk pointed out that exposure to both PM fractions by all age groups can trigger or aggravate the development of non-malignant chronic diseases (HQ > 1). Higher ELCR and HQ values for PM2.5 exposure in the night period reinforce the strength of specific sources, such as biomass burning, contributing to fine particles that are potentially harmful to human health.
Implications and limitations
Exposure to PM2.5 and PM10 in the cold season was associated with high risks of chronic effects on human health, with the elderly and children being especially susceptible. In the city of Bragança, 30% of the population is elderly (PORDATA 2021), which reinforces the importance of adopting mitigation measures by the authorities. As biomass burning for residential heating has been shown to be the main source of PM in winter, greater attention should be given to the heating systems, since most devices are old and out of date (unpublished results from the group), not complying with the emissions of the eco-design directive (EU Regulation 2015/1185). Because of the usual lack of time-activity patterns for different microenvironments, inhalation exposure assessments are generally based on ambient concentrations, which as considered representative of the total population exposure (Kazakos et al. 2020). However, outdoor levels may not always be surrogates of inhaled doses in the indoor air. In fact, outdoor measurements may underestimate concentrations in dwellings, as reported by studies that simultaneously evaluated the indoor and outdoor air quality in residences equipped with fireplaces and woodstoves (e.g. Vicente et al. 2021).
Some additional limitations can be identified in the present study. The monitoring campaign did not cover other seasons, and the results were considered representative of the worst exposure scenario. Furthermore, as there was no equipment available to monitor the particle size distribution, specific values of MMAD and GSD obtained in a previous campaign to characterise the properties of the local aerosol were assumed. However, these properties can change depending on the local emission sources, atmospheric conditions, monitoring distance from the sources and secondary reactions. Changes in particle properties can lead to different depositions and inhaled doses. Therefore, the results are important indicators for the identification of potentially susceptible groups, but should be analysed with caution, requiring the determination of specific factors for understanding the properties of the particles in future studies. In addition, to more accurately estimate exposure of different age groups to PM, monitoring in different seasonal periods should be carried out.
Conclusions
PM2.5 and PM10 showed higher concentrations at night, which can be explained by greater atmospheric stability, accumulation of pollutants due to lower wind speed (when compared to daytime) and specific emission sources, such as residential wood combustion for heating during winter. Furthermore, the highest PM2.5/PM10 ratios were found at night, reinforcing the presence of fine particles in the atmosphere due to incomplete combustion processes. The highest deposition values in the upper respiratory tract (head and tracheobronchial regions) were mostly related to PM10 for all age groups, while depositions in the pulmonary region were dominated by PM2.5. In addition, higher deposited doses for both PM fractions were found as a function of age: elderly > adults > child > infant. Regarding the deposited dose, the elderly group was identified as the most vulnerable. However, the assessment of potential carcinogenic and non-carcinogenic risks indicated values above the safety limits for the infant and child groups, highlighting the adverse impact on the health of these vulnerable groups from exposure to fine particles resulting from intense biomass burning. The results of this study show the urgency and importance of controlling air pollution, reinforcing the need to update the systems used for residential heating in order to reduce personal exposure (particularly of risk groups) to anthropogenic emissions. Given that exposure varies depending on PM concentrations, which in turn may show seasonal variations, to complement the present study, a new experimental campaign should be designed with samplings during the summer.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Aleksandropoulou V, Lazaridis M (2013) Development and application of a model (ExDoM) for calculating the respiratory tract dose and retention of particles under variable exposure conditions. Air Qual Atmos Heal 6:13–26. https://doi.org/10.1007/s11869-010-0126-z
Alemayehu YA, Asfaw SL, Terfie TA (2020) Exposure to urban particulate matter and its association with human health risks. Environ Sci Pollut Res 27:27491–27506. https://doi.org/10.1007/S11356-020-09132-1
Almeida SM, Silva AV, Sarmento S (2014) Effects of exposure to particles and ozone on hospital admissions for cardiorespiratory diseases in SetúBal, Portugal. J Toxicol Environ Health Part A 77(14-16):837–848. https://doi.org/10.1080/15287394.2014.887399
Alves C, Evtyugina M, Vicente E et al (2023) PM2.5 chemical composition and health risks by inhalation near a chemical complex. J Environ Sci 124:860–874. https://doi.org/10.1016/J.JES.2022.02.013
Alves C, Rienda IC, Vicente A et al (2021) Morphological properties, chemical composition, cancer risks and toxicological potential of airborne particles from traffic and urban background sites. Atmos Res 264:105837. https://doi.org/10.1016/J.ATMOSRES.2021.105837
Alves CA, Vicente ED, Evtyugina M et al (2020) Indoor and outdoor air quality: a university cafeteria as a case study. Atmos Pollut Res 11:531–544. https://doi.org/10.1016/J.APR.2019.12.002
Amoatey P, Omidvarborna H, Al-Jabri K et al (2022) Deposition modeling of airborne particulate matter on human respiratory tract during winter seasons in arid-urban environment. Aerosol Sci Eng 6:71–85. https://doi.org/10.1007/s41810-021-00125-2
Anjilvel S (1995) A multiple-path model of particle deposition in the rat lung. Fundam Appl Toxicol 28:41–50. https://doi.org/10.1006/FAAT.1995.1144
Asgharian B, Hofmann W, Bergmann R (2001) Particle deposition in a multiple-path model of the human lung. Aerosol Sci Technol 34:332–339. https://doi.org/10.1080/02786820119122
Avino P, Protano C, Vitali M, Manigrasso M (2016) Benchmark study on fine-mode aerosol in a big urban area and relevant doses deposited in the human respiratory tract. Environ Pollut 216:530–537. https://doi.org/10.1016/J.ENVPOL.2016.06.005
Barmpadimos I, Keller J, Oderbolz D et al (2012) One decade of parallel fine (PM2.5) and coarse (PM10-PM2.5) particulate matter measurements in Europe: trends and variability. Atmos Chem Phys 12:3189–3203. https://doi.org/10.5194/ACP-12-3189-2012
Bateson TF, Schwartz J (2007) Children’s response to air pollutants. J Toxicol Environ Part A 71(3):238–243. https://doi.org/10.1080/15287390701598234
Behera SN, Betha R, Huang X, Balasubramanian R (2015) Characterization and estimation of human airway deposition of size-resolved particulate-bound trace elements during a recent haze episode in Southeast Asia. Environ Sci Pollut Res 22:4265–4280. https://doi.org/10.1007/s11356-014-3645-6
Cesari D, De Benedetto GE, Bonasoni P et al (2018) Seasonal variability of PM2.5 and PM10 composition and sources in an urban background site in Southern Italy. Sci Total Environ 612:202–213. https://doi.org/10.1016/J.SCITOTENV.2017.08.230
Cheng J, Su H, Xu Z (2021) Intraday effects of outdoor air pollution on acute upper and lower respiratory infections in Australian children. Environ Pollut 268:115698. https://doi.org/10.1016/J.ENVPOL.2020.115698
Chung A, Chang DPY, Kleeman MJ et al (2001) Comparison of real-time instruments used to monitor airborne particulate matter. J Air Waste Manag Assoc 51(1):109–120. https://doi.org/10.1080/10473289.2001.10464254
Cipoli YA, Alves C, Rapuano M et al (2023) Nighttime–daytime PM10 source apportionment and toxicity in a remoteness inland city of the Iberian Peninsula. Atmos Environ 303:119771. https://doi.org/10.1016/j.atmosenv.2023.119771
Cipoli YA, Targino AC, Krecl P et al (2022) Ambient concentrations and dosimetry of inhaled size-segregated particulate matter during periods of low urban mobility in Bragança, Portugal. Atmos Pollut Res 13:101512. https://doi.org/10.1016/J.APR.2022.101512
Dantas L, Azevedo JC, Feliciano M (2019) O impacto da queima doméstica de biomassa lenhosa nos níveis de PM2.5 na cidade de Bragança, Portugal. Rev Ciências Agrárias 40:282–290. https://doi.org/10.19084/RCA16191
Duan R, Niu H, Yu T et al (2021) Adverse effects of short-term personal exposure to fine particulate matter on the lung function of patients with chronic obstructive pulmonary disease and asthma: a longitudinal panel study in Beijing, China. Environ Sci Pollut Res 28:47463–47473. https://doi.org/10.1007/S11356-021-13811-Y
De Oliveira BFA, Ignotti E, Artaxo P et al (2012) Risk assessment of PM2.5 to child residents in Brazilian Amazon region with biofuel production. Environ Health 11(1):1–11. https://doi.org/10.1186/1476-069X-11-64
Deng Q, Deng L, Miao Y et al (2019) Particle deposition in the human lung: health implications of particulate matter from different sources. Environ Res 169:237–245. https://doi.org/10.1016/j.envres.2018.11.014
Freiman MT, Hirshel N, Broday DM (2006) Urban-scale variability of ambient particulate matter attributes. Atmos Environ 40:5670–5684. https://doi.org/10.1016/J.ATMOSENV.2006.04.060
Galindo N, Yubero E (2017) Day-night variability of water-soluble ions in PM10 samples collected at a traffic site in southeastern Spain. Environ Sci Pollut Res 24:805–812. https://doi.org/10.1007/S11356-016-7836-1
Gama C, Monteiro A, Pio C et al (2018) Temporal patterns and trends of particulate matter over Portugal: a long-term analysis of background concentrations. Air Qual Atmos Heal 11:397–407. https://doi.org/10.1007/S11869-018-0546-8
Garrett P, Casimiro E (2011) Short-term effect of fine particulate matter (PM2.5) and ozone on daily mortality in Lisbon, Portugal. Environ Sci Pollut Res 18:1585–1592. https://doi.org/10.1007/S11356-011-0519-Z
Goel A, Izhar S, Gupta T (2018) Study of environmental particle levels, its effects on lung deposition and relationship with human behaviour. Energy, Environ Sustain 77–91. https://doi.org/10.1007/978-981-10-7332-8_4
Greene NA, Morris VR (2006) Assessment of public health risks associated with atmospheric exposure to PM2.5 in Washington, DC, USA. Int J Environ Res Public Health 3:86. https://doi.org/10.3390/IJERPH2006030010
Guo C, Zhang Z, Lau AKH et al (2018) Effect of long-term exposure to fine particulate matter on lung function decline and risk of chronic obstructive pulmonary disease in Taiwan: a longitudinal, cohort study. Lancet Planet Heal 2:e114–e125. https://doi.org/10.1016/S2542-5196(18)30028-7
Hauck H, Berner A, Gomiscek B et al (2004) On the equivalence of gravimetric PM data with TEOM and beta-attenuation measurements. J Aerosol Sci 35:1135–1149. https://doi.org/10.1016/J.JAEROSCI.2004.04.004
Heydari G, Taghizdeh F, Fazlzadeh M et al (2019) Levels and health risk assessments of particulate matters (PM2.5 and PM10 ) in indoor/outdoor air of waterpipe cafés in Tehran, Iran. Environ Sci Pollut Res 26:7205–7215. https://doi.org/10.1007/S11356-019-04202-5
Heyder J, Gebhart J, Rudolf G et al (1986) Deposition of particles in the human respiratory tract in the size range 0.005–15 μm. J Aerosol Sci 17:811–825. https://doi.org/10.1016/0021-8502(86)90035-2
Heyder J, Gebhart J, Rudolf G, Stahlhofen W (1980) Physical factors determining particle deposition in the human respiratory tract. J Aerosol Sci 11:505–515. https://doi.org/10.1016/0021-8502(80)90123-8
Hofmann W (2011) Modelling inhaled particle deposition in the human lung—a review. J Aerosol Sci 42:693–724. https://doi.org/10.1016/J.JAEROSCI.2011.05.007
Huang F, Zhao Y, Wang P et al (2021) Short-term exposure to particulate matter on heart rate variability in humans: a systematic review of crossover and controlled studies. Environ Sci Pollut Res 28:35528–35536. https://doi.org/10.1007/S11356-021-14494-1
ICRP (1994) Human respiratory tract model for radiological protection. ICRP Publication 66((1-3))
Islam MS, Saha SC, Sauret E et al (2017) Pulmonary aerosol transport and deposition analysis in upper 17 generations of the human respiratory tract. J Aerosol Sci 108:29–43. https://doi.org/10.1016/J.JAEROSCI.2017.03.004
Jalava PI, Wang Q, Kuuspalo K et al (2015) Day and night variation in chemical composition and toxicological responses of size segregated urban air PM samples in a high air pollution situation. Atmos Environ 120:427–437. https://doi.org/10.1016/J.ATMOSENV.2015.08.089
Kameda Y, Shirai J, Komai T (2005) Atmospheric polycyclic aromatic hydrocarbons : size distribution , estimation of their risk and their depositions to the human respiratory tract. Sci Total Environ 340:71–80. https://doi.org/10.1016/j.scitotenv.2004.08.009
Khan S, Gurjar BR, Sahu V (2022) Deposition modeling of ambient particulate matter in the human respiratory tract. Atmos Pollut Res 13:101565. https://doi.org/10.1016/J.APR.2022.101565
Kazakos V, Luo Z, Ewart I (2020) Quantifying the health burden misclassification from the use of different PM2.5 exposure tier models: a case study of London. Int J Environ Res Public Health 17:1099. https://doi.org/10.3390/ijerph17031099
Khosravipour M, Safari-Faramani R, Rajati F, Omidi F (2022) The long-term effect of exposure to respirable particulate matter on the incidence of myocardial infarction: a systematic review and meta-analysis study. Environ Sci Pollut Res 29:42347–42371. https://doi.org/10.1007/S11356-022-18986-6
Kim CS, Jaques PA (2005) Total lung deposition of ultrafine particles in elderly subjects during controlled breathing. Inhal Toxicol:387–399. https://doi.org/10.1080/08958370590929493
Koblinger L, Hofmann W (1990) Monte Carlo modeling of aerosol deposition in human lungs. Part I: Simulation of particle transport in a stochastic lung structure. J Aerosol Sci 21:661–674. https://doi.org/10.1016/0021-8502(90)90121-D
Le TC, Shukla KK, Chen YT et al (2020) On the concentration differences between PM2.5 FEM monitors and FRM samplers. Atmos Environ 222:117138. https://doi.org/10.1016/J.ATMOSENV.2019.117138
Li F, Xiao M, Zhang J et al (2017) Health risk assessment on tunnel workers’ exposure to PM10 based on triangular fuzzy numbers. AIP Conf Proc 1820:040011. https://doi.org/10.1063/1.4977283
Lin H, Wang X, Qian Z et al (2018) Daily exceedance concentration hours: a novel indicator to measure acute cardiovascular effects of PM2.5 in six Chinese subtropical cities. Environ Int 111:117–123. https://doi.org/10.1016/J.ENVINT.2017.11.022
Li J, Yao J, Zhou H et al (2023) Size Distribution of chemical components of particulate matter in Lhasa. Atmosphere (Basel) 14:1–16. https://doi.org/10.3390/atmos14020339
Liu X, Ouyang W, Shu Y et al (2019) Incorporating bioaccessibility into health risk assessment of heavy metals in particulate matter originated from different sources of atmospheric pollution. Environ Pollut 254:113113. https://doi.org/10.1016/J.ENVPOL.2019.113113
Lucarelli F, Calzolai G, Chiari M et al (2018) Study of atmospheric aerosols by IBA techniques: the LABEC experience. Nucl Instruments Methods Phys Res Sect B Beam Interact with Mater Atoms 417:121–127. https://doi.org/10.1016/J.NIMB.2017.07.034
Lv H, Li H, Qiu Z et al (2021) Assessment of pedestrian exposure and deposition of PM10, PM2.5 and ultrafine particles at an urban roadside: a case study of Xi’an, China. Atmos Pollut Res 12:112–121. https://doi.org/10.1016/J.APR.2021.02.018
Madureira J, Slezakova K, Silva AI et al (2020) Assessment of indoor air exposure at residential homes: Inhalation dose and lung deposition of PM10, PM2.5 and ultrafine particles among newborn children and their mothers. Sci Total Environ 717:137293. https://doi.org/10.1016/J.SCITOTENV.2020.137293
Manojkumar N, Srimuruganandam B (2022) Age-specific and seasonal deposition of outdoor and indoor particulate matter in human respiratory tract. Atmos Pollut Res 13:101298. https://doi.org/10.1016/J.APR.2021.101298
Manojkumar N, Srimuruganandam B, Shiva Nagendra SM (2019) Application of multiple-path particle dosimetry model for quantifying age specified deposition of particulate matter in human airway. Ecotoxicol Environ Saf 168:241–248. https://doi.org/10.1016/J.ECOENV.2018.10.091
Menon JS, Nagendra SMS (2018) Personal exposure to fine particulate matter concentrations in central business district of a tropical coastal city. J Air Waste Manag Assoc 68:415–429. https://doi.org/10.1080/10962247.2017.1407837
Miller FJ, Asgharian B, Schroeter JD, Price O (2016) Improvements and additions to the Multiple Path Particle Dosimetry model. J Aerosol Sci 99:14–26. https://doi.org/10.1016/J.JAEROSCI.2016.01.018
National Commission of Radiological Protection (NCRP) (1997) Deposition, retention and dosimetry of inhaled radioactive substances. NCRP Report No. 125. National Council on Radioprotection and Measurements. NCRP, Bethesda, MD
Oller AR, Oberdörster G (2016) Incorporation of dosimetry in the derivation of reference concentrations for ambient or workplace air: a conceptual approach. J Aerosol Sci 99:40–45. https://doi.org/10.1016/j.jaerosci.2016.01.015
Patterson RF, Zhang Q, Zheng M, Zhu Y (2014) Particle deposition in respiratory tracts of school-aged children. Aerosol Air Qual Res 14:64–73. https://doi.org/10.4209/aaqr.2013.04.0113
Peel MC, Finlayson BL, Mcmahon TA (2007) Hydrology and earth system sciences updated world map of the Köppen-Geiger climate classification. Hydrol Earth Syst Sci 11:1633–1644
Pereira S, Wagner F, Silva AM (2008) Scattering properties and mass concentration of local and long-range transported aerosols over the South Western Iberia Peninsula. Atmos Environ 42:7623–7631. https://doi.org/10.1016/J.ATMOSENV.2008.06.008
Phuc NH, Oanh NTK (2022) Large spatio-temporal variations of size-resolved particulate matter and volatile organic compounds in urban area with heavy traffic. Environ Sci Pollut Res 29:21491–21507. https://doi.org/10.1007/S11356-021-16921-9
Pio C, Alves C, Nunes T et al (2020) Source apportionment of PM2.5 and PM10 by Ionic and Mass Balance (IMB) in a traffic-influenced urban atmosphere, in Portugal. Atmos Environ 223:117217. https://doi.org/10.1016/J.ATMOSENV.2019.117217
PORDATA (2021) Municipality database. https://www.pordata.pt/censos/quadro-resumo-municipios-e-regioes/braganca-446. Accessed 26 January 2023.
Protano C, Manigrasso M, Avino P, Vitali M (2017) Second-hand smoke generated by combustion and electronic smoking devices used in real scenarios: Ultrafine particle pollution and age-related dose assessment. Environ Int 107:190–195. https://doi.org/10.1016/j.envint.2017.07.014
Querol X, Alastuey A, Ruiz CR et al (2004) Speciation and origin of PM10 and PM2.5 in selected European cities. Atmos Environ 38:6547–6555. https://doi.org/10.1016/J.ATMOSENV.2004.08.037
Rajput P, Izhar S, Gupta T (2019) Deposition modeling of ambient aerosols in human respiratory system: health implication of fine particles penetration into pulmonary region. Atmos Pollut Res 10:334–343. https://doi.org/10.1016/J.APR.2018.08.013
Rissler J, Swietlicki E, Bengtsson A et al (2012) Experimental determination of deposition of diesel exhaust particles in the human respiratory tract. J Aerosol Sci 48:18–33. https://doi.org/10.1016/j.jaerosci.2012.01.005
Rissler J, Gudmundsson A, Nicklasson H et al (2017) Deposition efficiency of inhaled particles (15-5000 nm) related to breathing pattern and lung function: an experimental study in healthy children and adults. Part Fibre Toxicol 14:1–12. https://doi.org/10.1186/s12989-017-0190-8
Rogula-Kozłowska W (2016) Size-segregated urban particulate matter: mass closure, chemical composition, and primary and secondary matter content. Air Qual Atmos Heal 9:533–550. https://doi.org/10.1007/s11869-015-0359-y
Rönkkö TJ, Jalava PI, Happo MS et al (2018) Emissions and atmospheric processes influence the chemical composition and toxicological properties of urban air particulate matter in Nanjing, China. Sci Total Environ 639:1290–1310. https://doi.org/10.1016/j.scitotenv.2018.05.260
Russo A, Sousa PM, Durão RM et al (2020) Saharan dust intrusions in the Iberian Peninsula: predominant synoptic conditions. Sci Total Environ 717:137041. https://doi.org/10.1016/J.SCITOTENV.2020.137041
Pavel MRS, Tarannum M, Zaman SU et al (2020) Health risk assessment of high-level particulate matter exposure in different environments in Mega City Dhaka, Bangladesh. Bang J Sci Res 2:78–87
Sá RC, Zeman KL, Bennett WD et al (2015) Effect of posture on regional deposition of coarse particles in the healthy human lung. J Aerosol Med Pulm Drug Deliv 28:423–431. https://doi.org/10.1089/jamp.2014.1189
Sacks JD, Stanek LW, Luben TJ et al (2011) Particulate matter-induced health effects: who is susceptible? Environ Health Perspect 119:446–454. https://doi.org/10.1289/ehp.1002255
Shukla K, Aggarwal SG (2022) A technical overview on beta-attenuation method for the monitoring of particulate matter in ambient air. Aerosol Air Qual Res 22:220195. https://doi.org/10.4209/AAQR.220195
Slezakova K, Morais S, Pereira M (2014) Trace metals in size-fractionated particulate matter in a Portuguese hospital: exposure risks assessment and comparisons with other countries. Environ Sci Pollut Res 21:3604–3620. https://doi.org/10.1007/S11356-013-2316-3
Stojić A, Stojić SS, Reljin I et al (2016) Comprehensive analysis of PM10 in Belgrade urban area on the basis of long-term measurements. Environ Sci Pollut Res 23:10722–10732. https://doi.org/10.1007/S11356-016-6266-4
Subramaniam B, Asgharian JI, Freijer FJ, Miller S, Anjilvel S (2003) Analysis of lobar differences in particle deposition in the human lung Inhalation. Toxicology 15:1–21. https://doi.org/10.1080/08958370304451
Takahashi K, Minoura H, Sakamoto K (2008) Examination of discrepancies between beta-attenuation and gravimetric methods for the monitoring of particulate matter. Atmos Environ 42:5232–5240. https://doi.org/10.1016/J.ATMOSENV.2008.02.057
Theodosi C, Grivas G, Zarmpas P et al (2011) Mass and chemical composition of size-segregated aerosols (PM1, PM2.5, PM10) over Athens, Greece: local versus regional sources. Atmos Chem Phys 11:11895–11911. https://doi.org/10.5194/acp-11-11895-2011
USEPA (2007) Reference concentration for chronic inhalation exposure (RfC). IRIS Summary. Integrated Risk Information System
USEPA (2009) Risk assessment guidance for superfund volume 1: human health evaluation manual (Part F, Supplemental guidance for inhalation risk assessment). Office of Superfund Remediation and Technology Innovation, Environmental Protection Agency, Washington, D.C.
USEPA (2011) Exposure factors handbook: chapter 6—inhalation rates
Vicente ED, Figueiredo D, Gonçalves C et al (2021) In vitro toxicity of indoor and outdoor PM10 from residential wood combustion. Sci Total Environ 782:146820. https://doi.org/10.1016/J.SCITOTENV.2021.146820
Vicente ED, Ribeiro JP, Custódio D, Alves CA (2017) Assessment of the indoor air quality in copy centres at Aveiro, Portugal. Air Qual Atmos Heal 10:117–127. https://doi.org/10.1007/S11869-016-0401-8
Voliotis A, Bezantakos S, Besis A et al (2021) Mass dose rates of particle-bound organic pollutants in the human respiratory tract: implications for inhalation exposure and risk estimations. Int J Hyg Environ Health 234:113710. https://doi.org/10.1016/j.ijheh.2021.113710
Yang M, Zeng HX, Wang XF et al (2023) Sources, chemical components, and toxicological responses of size segregated urban air PM samples in high air pollution season in Guangzhou, China. Sci Total Environ 865:161092. https://doi.org/10.1016/j.scitotenv.2022.161092
Yunesian M, Rostami R, Zarei A et al (2019) Exposure to high levels of PM2.5 and PM10 in the metropolis of Tehran and the associated health risks during 2016–2017. Microchem J 150:104174. https://doi.org/10.1016/J.MICROC.2019.104174
Zhang Y, Cui L, Xu D et al (2018) The association of ambient PM2.5 with school absence and symptoms in schoolchildren: a panel study. Pediatr Res 84:28–33. https://doi.org/10.1038/s41390-018-0004-1
Zhao Y, Wang S, Lang L et al (2017) Ambient fine and coarse particulate matter pollution and respiratory morbidity in Dongguan, China. Environ Pollut 222:126–131. https://doi.org/10.1016/J.ENVPOL.2016.12.070
Funding
Open access funding provided by FCT|FCCN (b-on). Yago A. Cipoli and Leonardo C. Furst has a PhD scholarship (SFRH/BD/04992/2021 and SRFH/BD/08461/2020, respectively) supported by the Portuguese Foundation of Science and Technology (FCT). This work was supported through national funds FCT/MCTES (PIDDAC) to CIMO (UIDB/00690/2020 and UIDP/00690/2020), SusTEC (LA/P/0007/2020) and CESAM (UIDP/50017/2020, UIDB/50017/2020 and LAP/0094/2020).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. YC: investigation, methodology, conceptualisation, writing original draft and editing; CA: writing original draft and review, conceptualisation, methodology, supervision, funding acquisition; LF: investigation, methodology; MF: supervision, conceptualisation, funding acquisition. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• MPPD was applied to PM2.5 and PM10 for night/day periods and different age groups
• Regional doses of PM2.5 showed significant differences between day and night periods
• PM2.5 deposits mainly in the lung and PM10 in the head and tracheobronchial regions
• Children and infants presented higher percentages of deposition in the lung regions
• Non-carcinogenic and carcinogenic risks were found to be higher at night
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cipoli, Y.A., Furst, L., Feliciano, M. et al. Respiratory deposition dose of PM2.5 and PM10 during night and day periods at an urban environment. Air Qual Atmos Health 16, 2269–2283 (2023). https://doi.org/10.1007/s11869-023-01405-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11869-023-01405-1