Abstract
This study was commenced to evaluate the indoor and outdoor air quality concentrations of PM2.5, sub-micron particles (PM>2.5, PM1.0–2.5, PM0.50 –1.0, PM0.25–0.50, and PM<0.25), heavy metals, and microbial contaminants along with their identification in three different hospitals of Lucknow City. The study was conducted from February 2022 to April 2022 in hospitals situated in the commercial, residential, and industrial belts of the city. The indoor concentration trend of particulate matter as observed during the study suggested that most of the highest concentrations belonged to the hospital situated in an industrial area. The highest obtained indoor and outdoor concentrations for PM1.0–2.5, PM0.50–1.0, PM0.25–0.50, and PM<0.25 are 40.44 µg/m3, 56.08 µg/m3, 67.20 µg/m3, 74.50 µg/m3, 61.9 µg/m3, 79.3 µg/m3, 82.0 µg/m3, and 93.9 µg/m3, respectively, which belonged to hospital C situated in the industrial belt. However, for PM>2.5, the highest indoor concentration obtained belonged to hospital B, i.e., 30.7 µg/m3, which is situated in the residential belt of the city. Regarding PM2.5, the highest indoor and outdoor concentrations obtained are 149.41 µg/m3 and 227.45 µg/m3, which were recorded at hospital A and hospital C, respectively. The present study also observed that a high bacterial load of 1389.21 CFU/m3 is recorded in hospital B, and the fungi load was highest in hospital C with 786.34 CFU/m3. Henceforth, the present study offers thorough information on the various air pollutants in a crucial indoor setting, which will further aid the researchers in the field to identify and mitigate the same more precisely.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Globally air pollution has been proven to be a major health risk responsible for 3.7 million premature deaths and fourth-ranked in terms of risk factors in premature mortality in 2019 according to the most recent Global Burden of Diseases Study (Jin et al. 2022) and affecting human health at multiple levels (Ding et al. 2019). Just like ambient air quality, indoor air quality is equally important; however, it is often neglected. The indoor air quality relies not only on outdoor sources but also on indoor activities like fossil fuel burning, e.g., wood, coal, etc., and waste burning such as animal dung, waste crop, etc. According to estimates from the World Health Organization (WHO), 2.6 billion people utilize these sources for heating and various types of cooking (Lee et al. 2020). Air pollution rose to the position of second-most significant health risk in 2022 (September 15, 2022) (https://www.republicworld.com/india-news/general-news/23-03-2022). Furthermore, indoor air pollution is one of the leading hazard issues associated with India’s national burden of disease (Lim et al. 2012). A greater body of work on indoor air quality assessment is based on households, whereas indoor air quality assessment in vulnerable settings like hospitals needs attention and is crucial at the same time. Microbial contaminants and their assessment and identification are essential in hospitals to avoid any hospital-acquired infections [HAI] (Weiss et al. 2017). Molds and dust can cause acquaintance with pathogenic bacteria, viruses, and fungi leading to aspergillosis and pneumocystosis (Gangneux et al. 2016). People working together in offices, schools, buildings, hospitals, etc., can also cause serious infections by sneezing, coughing, and direct as well as indirect contact (Verde et al. 2015). Air quality maintenance is even more important in hospitals as the population present in the setting has comparatively lower immunity and, hence, is more prone to infections. Also, patients have a lower metabolic rate and need a higher operative temperature, which affects their metabolism. Because of this, the indoor environment in hospitals is complex and unique from that in household settings. Because patients are sensitive to chemical and biological contaminants, hospitals and healthcare facilities should be closely scrutinized for contamination. The continuous outside airflow also degrades the air quality inside hospitals, which is crucial for patients and also has a negative effect on hospital staff. Furthermore, particles in confined settings transport pathogenic bacteria and microbial metabolites, both of which are common in hospitals (Slezakova et al. 2014), posing health dangers such as airborne infectious disease transmission (Hogrefe et al. 2011). The inhalable microorganisms, i.e., Cladosporium, Penicillium, and Aspergillus spores, and Staphylococcus species in closed environments may lead to a number of health issues (Fekadu and Getachewu 2015), particularly in hospital setups. Although this microenvironment is particularly important as there are a number of potential sources such as disinfectants, sterilizers, and limonene-based products that lead to the emission of noxious pollutants like ethylene oxide, formaldehyde, alcohols, etc. (Bessonneau et al. 2013), yet very scarce literature is available on the quantification of chemical and physical pollutants along with microbial assessments in hospitals and clinics. Various deadly diseases in association with HAI are common and need imperative attention (Singh et al. 2020). The commutation of visitors, workers, staff members, and patients also contribute to HAI (hospital-acquired infections). To protect the health and well-being of patients, workers, physicians, and others from such infectious illnesses, it is necessary to use different air quality standards (Andualem et al. 2019). CO, carbon dioxide (CO2), ozone (O3), particulate matter (PM2.5 and PM10), sulfur dioxide (SO2), airborne bacteria, fungi, and TVOCs (total volatile organic compounds) are some of the common air pollutants spotted so far. Particles can also be carriers of pathogens increasing the risk of respiratory infections (Gralton et al. 2011). Apart from the above, microorganisms can be transferred through improper functioning of HVAC (heating, ventilation, and air conditioning) systems (Mousavi et al. 2019). Studies conducted in countries like Pakistan, Iran, and Turkey have shown that bioaerosols can enter the body through ingestion and consumption, influencing human well-being (Nasir et al. 2012). Another study to evaluate indoor air quality issues that was carried out in hospitals of Finland found that employees are particularly impacted by poor indoor air quality although it did not mention the specific pollutants responsible for the symptoms experienced by the same (Kumar et al 2021a, b, c; Dey et al. 2019). Bioaerosols may exacerbate infectious disorders (Bolookat et al. 2018; Faridi et al. 2017; Montazer et al. 2021; Mbareche et al. 2019) including asthma, allergies, and neurological conditions, according to research conducted in a hematology unit (Brilhante et al. 2010). According to Sri Lankan research, the Kandy General Hospital is one of the sites in Kandy City with the highest degree of indoor air pollution (Sivagnanasundaram et al. 2019). Penicillium, Mucor, Aspergillus, and Fusarium were among the fungal isolates found in the hospital’s interior air in a different investigation carried out in Benin City, Nigeria (Ekhaise and Ogboghodo 2011). According to another study, Aspergillus sp., Candida sp., Fusarium sp., and Mucorales sp. are the most common pathogens in Sari, Iran’s hospitals (Sorkheh et al. 2022). According to studies from the World Health Organization (WHO), the overall microbial load in living and working spaces should not be more than 1000 CFU/m3 (Ikhtiar et al. 2017). The level of particulate contamination may be influenced by human movement in indoor spaces (Meng et al. 2010). Numerous studies have also shown the negative impact of high ambient PM concentrations on hospital admission, especially for cardiac and respirational conditions (Atkinson et al. 2014). Furthermore, conferring to the World Health Organization (WHO), 92% of the world’s population resides in regions with annual mean PM2.5 levels greater than 10 g/m3, which is above their air quality recommendation for PM2.5 exposure. Recent research has looked into the impacts of PM on health from a wider range of sources, such as biomass burning, and in other geographical locations. Consistent evidence of respiratory morbidity was found in a valuation of the health impacts of wildfire smoke, while the effects on cardiovascular health were less obvious (Reid et al. 2016). According to many researchers, exposure to HCHO (formaldehyde) and TVOC (total volatile organic compounds) may potentially raise the chance of developing allergy disorders or cancer (Smith et al. 2012; DeLeon-Rodriguez et al. 2013). Metal compounds and particulate matter in the ambient air especially allied with sub-micron constituent parts have lately been a major factor harmfully affecting human well-being because of their smaller size and higher number. Metals such as lead, arsenic, mercury, chromium, cadmium, aluminum, copper, iron, manganese, lead, nickel, mercury, and zinc are abundantly present and associated with fine particulate matter (PM2.5) in the air (Lin et al. 2020). They may cause a lot of adverse effects like asthma, cough, brain/kidney damage, etc. Many metals produce reactive oxygen species (ROS) in biological tissues and may lead to the generation of free radicals (Kermani et al. 2021; Domingo et al. 2017). Also, Ni, Cr, As, and Pb were found profusely in fine particles [PM2.5] than in the course [PM10] fraction (Slezakova et al. 2012). Moreover, the COVID-19 outbreak has placed unprecedented challenges on hospital environmental hygiene and indoor air quality. The second wave of the SARS-CoV-2 (COVID-19) infection has hit India with full force and affected the whole country. Uttar Pradesh stands in the second position with a high viral load. Air pollution has emerged as a potent factor related to the transmission of the coronavirus. Long-lived microorganisms in the hospital environment are directly connected to the incidence of associated illnesses (Beggs et al. 2015). Thus, it is very crucial to assess the hospital’s indoor air quality. It is also pertinent to monitor the thermal comfort, humidity, chemical contamination, ventilation, and air distribution in hospitals as the detrimental effects of indoor air quality would not just reduce comfort levels of the occupants but also lead to increased occurrence of symptoms including, headache, dry throat, fatigue, anxiety, etc. (Zuo et al. 2019).
The present study was conducted in hospitals situated in three belts, i.e., commercial, residential, and industrial belts of Lucknow City. Lucknow is the second-largest city in northern and central India and the capital of Uttar Pradesh, which is the most polluted and populated state in the world. The study deals with major air pollutants, comprising fine and sub-micron particulate matter, i.e., PM2.5, PM>2.5, PM1.0–2.5, PM0.50–1.0, PM0.25–0.50, and PM<0.25, together with associated heavy metals and biological contaminants. Apart from biological contaminants, all the other pollutants were assessed in both indoor and outdoor environments for appropriate evaluation of the analogy between the two. The study illustrates the mass concentrations of the pollutants in consort with indoor/outdoor [I/O] ratios. This study was conducted in the city hospitals, and the study projects an overview of the air quality of the hospitals and healthcare facilities of the region to regulate and govern the standards, particularly for sensitive environments. The findings may help policymakers with the implementation of guidelines for confined spaces guided by substantial data.
Materials and methods
Area of study
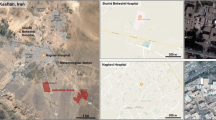
The present study was conducted in three hospitals in Lucknow City from February to April 2022 (shown in Fig. 1). Lucknow is the second-largest city in northern and central India and the capital of Uttar Pradesh. Additionally, it serves as the administrative center for the division and district with the same name. The city is located at a height of around 123 m (404 feet) above sea level. Lucknow is located on the Gomti River's western coast. There were 110 wards in the city as of 2008 (Khan et al. 2022). The city’s population is expected to surpass 7 million in 2021, representing an increase of over 90% in 10 years (Dwivedi et al. 2022). The study was conducted in three different hospitals, one each situated in (a) commercial, (b) residential, and (c) industrial microenvironments. The microenvironments were selected on the basis of population and traffic density (depicted in Fig. 2). Written consent was sought from the management of the hospitals for the study period. During the study period, PM>2.5, PM1.0–2.5, PM0.50–1.0, PM0.25–0.50, and PM<0.25 were monitored in the indoor air. Microbial contaminants were also monitored in the indoor air of different wards. Since the identification of the hospitals is kept confidential, therefore, the hospitals located in the commercial, residential, and industrial area have been marked as A, B, and C, respectively. The general characteristics of the selected hospitals are given in Table 1. Hospital A, located in the commercial area, had a capacity of 60 beds with an occupancy of 350 people, including paramedic staff and attendants. According to the presiding authority, approximately 50 patients were either admitted or availed of the outpatient facility daily, on an average of 950 patients per month. Hospital B, located in the residential colony, had a capacity of 35 beds, including an operation theater, a general ward, and an outpatient facility with an intake of 15–20 patients daily and 450 patients per month. Hospital C, located in an industrial area, had a capacity of 15 beds with a footfall of 10–12 patients daily and on an average 300 patients monthly. The description of the respective hospital is displayed in Table 2.
Sampling and analyses of microbiological contaminants
The Petri plate gravitational settling (passive) method of sampling was chosen due to its straightforward approach and practical viability, which makes use of existing resources. The technique is based on the media’s ability to adhere, which traps airborne particles onto their surface when plates containing the media are exposed face up to the atmosphere to gather particles falling by gravity. This method has been used by various researchers around the world in past decades (Sudharsanam et al. 2008; Verdier et al. 2014; Kumar et al 2021a, b, c; Kumar et al. 2022). The Petri plates were exposed (9-cm diameter) in general wards and OT of the hospitals for 60 min at a breathing height of approximately 70 cm with media of MacConkey agar (MCA) nutrient agar (NA), (Salmonella Shigella) SS agar, and (E. coli-Coliform Selective Agar Chromo Select) ECC agar for bacteria and Sabouraud dextrose agar (SDA) and RBA (rose Bengal agar) for fungal sampling (shown in Fig. 3). After exposure, all the agar plates were sealed and transferred to the laboratory for incubation; the bacterial plates were incubated for 24–48 h at 37 °C and for fungi at room temperature (25–27 °C) for 5 to 6 days. The sample was processed and identified for various bacteria, gram-positive and gram-negative. After being heat fixed on the slide, the microbial culture was stained for 1 min with a crystal violet staining solution. Slides were cleaned with water before being flooded with the mordant (3% water/iodine gram’s mixture). Slides were then treated with a decolorizing chemical (95% ethanol) after being cleaned with water. Slides were counter stained with safranin for 30 s to 1 min after decolorization. Slides were afterward rinsed with water until no more colored effluent was visible, and then they were blotted dry with absorbent paper. Slides were then examined using a brightfield microscope (100X) while submerged in oil, and pictures were taken with a Nikon Optiphot microscope fitted with an Amscope MU1000 Camera. And, for the determination of morphological structures of fungi, the lactophenol cotton blue staining process was used. Direct counting is used for quantitative measurement of microbial communities (bacteria and fungi load) on samples. Bergey’s manual of systematic microbiology was also used to identify and categorize all bacteria into groupings like gram-negative and gram-positive (Kumar et al 2021a, b, c). A fungal colony’s appearance, color, and other characteristics were initially used to categorize it. To get the right surface density for counting and to figure out the load in proportion to the exposure period, the sample intervals were set at 60 min during the day. After the incubation period, the number of bacteria and fungi was counted as colony-forming units (CFU), and the formula for CFU/m3 was used to calculate it (Omeliansky 1940; Fleischer et al. 2006; Toivola et al. 2002).
- N:
-
microbial CFU/m3 of indoor air
- A:
-
number of colonies per Petri dish
- B:
-
dish surface (cm2)
- t:
-
exposure time (minutes)
Sampling of particulate matter
The Leland Legacy sample pump (SKC Cat. No. 100-3002; Inc. Eighty-Four, PA, USA) with a five-stage Sioutas cascade impactor was used to collect PM in the size range of PM>2.5, PM1.0–2.5, PM0.50–1.0, PM0.25– 0.50, PM<0.25 on 25-mm Millipore filter paper and 37-mm GF/A filter paper (for PM<0.25). The instrument was adjusted to 9 l min−1 airflow rate. PM2.5 was measured using GF/A 47-mm filter paper (pore size, 0.5 μm) through Envirotech APM 550 sampler, which was set at a flow rate of 17.57 l pm for 8 h. All of the filter papers were conditioned by being placed in a desiccator for 24 h at a temperature of 20 to 30 °C, and then being weighed three times on a SHIMADZU ATX 224 weighing scale with a sensitivity of ± 0.2 mg and a capacity of 230 g. The instruments were placed in the general wards of the three selected hospitals at approximately 2 m away from the doors and walls of the room as depicted in Fig. 4. For outdoor monitoring, the instruments were placed in the hospital yard. The instruments were placed 1 m away from any potential source of pollution and 1.5 m above the ground. The monitoring was done during the daytime (9:00 am to 4:00 pm). In each of the selected hospitals, monitoring was done for 14 consecutive days. In total, monitoring was done for 42 days. Also, seven common heavy metals viz. Pb, Mn, Cr, Fe, Ni, Cu, and Zn were estimated using inductively coupled plasma optical emission spectroscopy (ICP-OES).
Quality check and quality assurance
The quality of the instruments was examined each and every time before and after the relocation of the instruments from one place to another. All the instruments were calibrated at least once a month.
Metrological parameters
Metrological parameters such as relative humidity, temperature, wind speed, and wind direction were recorded during the monitoring period. The average temperature was found to be 30.83 °C, average relative humidity was found to be 38.38 [%], and the wind speed was 11.79 km/h, respectively. The prominent wind direction was west to the northwest, and no rainfall was observed during monitoring days.
Results and discussion
Concentration of particulate matter
Particulate exposure has been associated with adverse health outcomes. The exposure is particularly risky for those with compromised immunity and people with pre-existing health conditions (Mohammadyan et al. 2017a; b). The indoor-to-outdoor (I/O) ratio of PM2.5 concentrations has been explored, and they can differ, owing to a number of features like the location of a building, its design, or human activities (López-Villarrubia et al. 2021). For the hospital situated in commercial microenvironment, the I/O (indoor/outdoor) ratio for average values of PM2.5 was found to be 0.69 (130.9 µgm−3/189.9 µgm−3), whereas I/O ratios for PM>2.5, PM1.0–2.5, PM0.50–1.0, PM0.25–0.5, and PM<0.25 were found to be 0.72 (22.4 µgm−3/31.5 µgm−3), 0.82 (28.7 µgm−3/34.6 µgm−3), 0.75 (36.4 µgm−3/48.8 µgm−3, 0.72 (55.1 µgm−3/75.3 µgm−3), and 0.79 (59.0 µgm−3/74.5 µgm−3). For hospital situated in residential microenvironment, the I/O ratio of PM2.5, PM>2.5, PM1.0–2.5, PM0.50–1.0, PM0.25–0.5, and PM<0.25 was found to be 0.47 (74.5 µgm−3/158.4 µgm−3), 0.56 (20.1 µgm−3/36.1 µgm−3), 0.80 (25.6 µgm−3/32.8 µgm−3), 0.68 (33.8 µgm−3/49.0 µgm−3), 0.66 (37.7 µgm−3/55.2 µgm−3), and 0.56 (27.2 µgm−3/49.3 µgm−3), respectively. For hospital situated in industrial microenvironment, the I/O ratio of PM2.5, PM>2.5, PM1.0–2.5, PM0.50–1.0, PM0.25–0.5, and PM<0.25 was found to be 0.53 (117.8 µgm−3/220.8 µgm−3), 0.59 (26.8 µgm−3/44.9 µgm−3), 0.63 (37.1 µgm−3/58.4 µgm−3), 0.72 (52.9 µgm−3/73.3 µgm−3), 0.77 (61.9 µgm−3/79.8 µgm−3), and 0.70 (59.4 µgm−3/84.2 µgm−3), respectively. The concentration and composition of these particles depend upon different parameters and factors like temperature, humidity, ventilation mechanism, and rate of exchange of air (Faridi S et al. 2018). Their health effects are also dependent on their composition like black carbon, toxic minerals (metals), and carbon-based components (Krajewska-Kułak et al. 2007). Indoor air studies of fungal contamination at the Neonatal Department and Intensive Care Unit have shown that health care workers are more likely to get cancer as a result of exposure of more than 8 h based on the I/O ratio, which is quite alarming (Gillum et al. 2011). Since the hospitals were nonsmoking environments, with no unvented combustion sources, hence, the indoor concentration of ultrafine particulate may be attributed to indoor activities like the use of vacuum cleaners, electrical equipment, centrifuge machines, automated blood pressure monitors, etc. (Riesenfeld et al. 2000). Indoor PM2.5 concentrations at each hospital were positively related to the outdoor PM2.5. Variables such as the number of occupants, ambient temperature, areas of the room, windows, and doors may have an effect on the concentrations as well. Wind speed may also have a role to play in the indoor and outdoor concentrations of particulate contamination. The PM2.5 concentrations were compared with the USEPA standards (35 μg/m3) (Esworthy and Bearden 2015). In the conducted study, the indoor fractions were found to be lower than the outdoor fractions. Re-suspension of particulate matter due to cleaning and human movement may have also contributed to the concentrations of PM2.5 (Mohammadyan et al. 2017a, b). Indoor PM concentrations have also been evaluated in hospital settings of cities like Lahore (Pakistan), Istanbul (Turkey), and Guangzhou (China), which have climatic and economic similarities to the selected city for the study. All these cities reported higher PM levels, exceeding the 24-h, PM2.5 standards recommended by USEPA and WHO (Yurtseven et al. 2012). Additional studies conducted in the People Hospital of Shijing, People Hospital of Liwan, Phthisic Hospital, and Pediatric Hospital in Guangzhou, China (Wang et al. 2020) have shown high PM2.5 concentrations indoors as these hospitals were situated next to busy roads in heavily populated regions. The elevated concentrations may be attributed to outdoor sources as well.
Sudden rises in the concentrations were also observed on particular days. From the hospital located in a commercial area, a sudden rise with respect to PM2.5 (149.41 µg/m3) given in Fig. 5 and PM0.25–0.50 (67.12 µg/m3) was obtained, whereas from the hospital in an industrial area, a sudden raise in the concentration of PM<0.25 fraction (86.32 µg/m3) was obtained on the second day of monitoring. Episodic increases in the concentrations may be due to the increase in the number of inhabitants on a particular day.
The average concentration of sub-micron particles has been for hospital A, hospital B, and hospital C as depicted in Figs. 6, 7, and 8, respectively.
Chronic daily intake [CDI] and total cancer risk [TCR] associated with heavy metals
A study conducted by Qin et al. 2022. reported that the order of concentration of metals in the total suspended particulate matter was Cu > Pb > Cr > Ni > Cd and claimed that usage of plastics in electronic gadgets has the highest and lowest proportion of Cu and Cd, respectively. A study conducted by Bisht et al. 2022 on the concentration of heavy metals in road dust, reported a high concentration in commercial whereas, a considerable level in residential areas. Also, the PCA analysis done during the study suggested the source of Fe contamination as building construction activities, rolling and sliding of tires, etc. Zn sources as suggested were brakes and tires of vehicles followed by Cu as metallic materials, corrosion of alloys, etc. (Pant and Harrison 2013; Roy et al. 2019; Bhattacharya et al. 2013; Bisht, L. et al. 2022). Heavy metals in indoor dust require extensive research due to their non-biodegradability, high toxicity, and negative effects on humans (Darus et al. 2012). Metals considered during the present study have been reported to be vital content of cigarette and tobacco smoke (Ghoma et al. 2022). These metals are reported to be carcinogenic, causing urinary, prostate, breast, pancreas, and endometrium cancers (Adams et al. 2012). Heavy metals in dust can also enter the human body via ingestion, inhalation, and absorption through the skin (Latif et al. 2014).
During the current study, seven heavy metals, namely chromium (Cr), iron (Fe), manganese (Mn), nickel (Ni), copper (Cu), zinc (Zn), lead (Pb), were estimated. Chronic daily intake [CDI] and total cancer risk [TCR] associated with heavy metals were calculated.
The total cancer risk (TCR) of all the heavy metals was in the range of 7.21 × 10−13 1.25 × 10−10. The TCR range was 1 × 10−6 to 1 × 10−4, while TNCR (total noncancer risk) for heavy metals was below 1; the values established by US EPA.
where CS depicts exposure point concentration: mg/kg, IR is ingestion rate: 100 mg/day,
EF is exposure frequency: 350 days/year,
ED is exposure duration: 30 years (EPA/540/1-89/002 1989; US EPA 1996) BW is body weight: 65 kg,
AT that is averaging time for carcinogens is 365 × 70 days, CF is units conversion factor (10−6 kg mg−.1) (USEPA 2002)
The indoor and outdoor concentrations of the metals in respective hospitals have been given individually in Tables 3, 4, and 5.
Microbial contamination
Bacterial concentration in an indoor environment
In case of bacterial load, the highest concentration [1389 CFU/m3] is recorded in hospital B, situated in the commercial area in a general ward at NA [nutrient agar] during daytime (2:00 p.m.) at 60-min exposure, while in the same context, the concentration of bacteria in all general wards was high at nutrient agar. On the other hand, the lower concentration is in SSA agar media in the same microenvironment (microbial loads and their levels of contamination are depicted in Fig. 9). Table 6 shows the concentration of bacteria in all the selected microenvironments at different agar media.
In addition to this, the high bacterial load is mostly due to problems in temperature, humidity, insufficient ventilation, etc. (Hayleeyesus and Manaye 2014). Biological contaminants may find their way indoors through both natural and artificial sources. Also, there are several factors responsible for presence and transmission of microbial contaminants (depicted in Fig. 10). Meteorological parameters such as air pollutants (PM2.5), temperature, moisture content, air currency fluctuations, etc., have a significant correlation with microbiological concentrations (Kumar et al 2021a, b, c).
Fungal concentration (load) in an indoor environment
The result revealed that the fungal concentration on SDA (Sabouraud dextrose agar) media is highest in hospital C (786.34 CFU/m3) during the daytime, at 60 min exposure, and the lowest concentration (131.05 CFU/m3) on RBA (rose Bengal agar) was recorded at hospital A in OT (operation theater). Table 7 shows the fungal load in each hospital and details of microbiological profile through the passive method (gram-positive and gram-negative) bacteria and fungi are enumerated in Table 8
Limitations of the study
Several communicable ailments, such as whooping cough, tuberculosis, and other fungal infections, human and avian influenza, chickenpox, and some of the emerging viruses, such as Middle East respiratory syndrome coronavirus (MERS-CoV), can possibly escalate via aerosol transmission. However, deep-sequencing technologies can be applied to environmental air samples to characterize the presence and variety of airborne pathogens in the air we breathe in different environments such as hospitals, clinics, offices, entertainment venues, public transport, etc. The present study investigated air-borne particulate concentration in indoors for 24 h. However, time-bound study with proper bifurcation of particulate size is required. Future study based on size-segregated PM-targeting specific indoor sources will be prolific for policy makers and researchers in the field. Moreover, simultaneous evaluation of pollutants at multiple places over longer period of time is essential to provide a thorough understanding of the airborne microbiome and the various factors that influence its ecology. Our future study will employ active methods for microbial sampling to give a better insight of the variation in the species of bacteria and fungi targeting a larger surface area.
Conclusion
This study is the first to analyze particles, heavy metals, and microbiological pollutants in three sample locations in an integrated manner. Hospitals are microenvironments with people who can be more sensitive to air pollution. It might be difficult to achieve adequate indoor air quality in hospitals. Among the numerous air contaminants within due to their fatal effects on people, particulate matter of various sizes, and the heavy metals linked with it, along with microbiological contaminants are some of the key pollutants researched throughout the work. Three hospitals were selected based on their locations and areas nearby. It was observed that the outdoor vehicular load, greenery, industries, etc., played a crucial role in the maintenance of indoor air quality. It was found during the study that the general ward of hospital B, situated in a semi-urban, moderately polluted area, was highly contaminated with high bacterial load, i.e., 1389.21 CFU/m3, whereas fungi concentrations were high in hospital C (786.34 CFU/m3) in the general ward. A low bacterial (183.48 CFU/m3) and fungal load (131.05 CFU/m3) was found in hospital A, which is situated at a commercial belt out of three hospitals. At the end, it is worth mentioning that the variation in the concentration of pollutants in the different hospitals directs toward the fact that outdoor situations play a vital role in upkeeping indoor air quality; therefore, to uphold sustainable air quality inside the hospital, proper nearby location of the same should be managed along with apposite greenery inside as well as outside to tackle air pollution.
Data availability
The datasets generated during and/or analyzed during this study are not publicly available as they were monitored, analyzed, and calculated but are not publicly available as they were monitored, analyzed, and calculated but are available from the corresponding author on reasonable request.
References
Adams SV, Passarelli MN, Newcomb PA (2012) Cadmium exposure and cancer mortality in the Third National Health and Nutrition Examination Survey cohort. Occup Environ Med 69(2):153–156. https://doi.org/10.1136/oemed-2011-100111
Andualem Z, Gizaw Z, Bogale L, Dagne H (2019) Indoor bacterial load and its correlation to physical indoor air quality parameters in public primary schools. Multidiscip Respir Med 14(1):1–7. https://doi.org/10.1186/s40248-018-0167-y
Atkinson RW, Kang S, Anderson HR, Mills IC, Walton HA (2014) Epidemiological time series studies of PM2. 5 and daily mortality and hospital admissions: a systematic review and meta-analysis. Thorax 69(7):660–665. https://doi.org/10.1136/thoraxjnl-2013-204492
Beggs C, Knibbs LD, Johnson GR, Morawska L (2015) Environmental contamination and hospital-acquired infection: factors that are easily overlooked. Indoor Air 25(5):462–474. https://doi.org/10.1111/ina.12170
Bessonneau V, Mosqueron L, Berrubé A, Mukensturm G, Buffet-Bataillon S, Gangneux JP, Thomas O (2013) VOC contamination in hospital, from stationary sampling of a large panel of compounds, in view of healthcare workers and patients exposure assessment. PloS One 8(2):e55535. https://doi.org/10.1371/journal.pone.0055535
Bhattacharya T, Chakraborty S, Tuteja D, Patel M (2013) Zinc and chromium load in road dust, suspended particulate matter and foliar dust deposits of Anand City. Gujarat Open J Metal 3(02):42. https://doi.org/10.4236/ojmetal.2013.32A1006
Bisht L, Gupta V, Singh A, Gautam AS, Gautam S (2022) Heavy metal concentration and its distribution analysis in urban road dust: A case study from most populated city of Indian state of Uttarakhand. Spat Spatio-tempor Epidemiol 40:100470. https://doi.org/10.1016/j.sste.2021.100470
Bolookat F, Hassanvand MS, Faridi S, Hadei M, Rahmatinia M, Alimohammadi M (2018) Assessment of bioaerosol particle characteristics at different hospital wards and operating theaters: a case study in Tehran. MethodsX 5:1588–1596. https://doi.org/10.1016/j.mex.2018.11.021
Brilhante RSN, Castelo-Branco DDSCM, Soares GDP, Astete-Medrano DJ, Monteiro AJ, Cordeiro RDA, Rocha MFG (2010) Characterization of the gastrointestinal yeast microbiota of cockatiels (Nymphicus hollandicus): a potential hazard to human health. J Med Microbiol 59(6):718–723. https://doi.org/10.1099/jmm.0.017426-0
Darus FM, Nasir RA, Sumari SM, Ismail ZS, Omar NA (2012) Heavy metals composition of indoor dust in nursery schools building. Procedia Soc Behav Sci 38:169–175. https://doi.org/10.1016/j.sbspro.2012.03.337
DeLeon-Rodriguez N, Lathem TL, Rodriguez-R LM, Barazesh JM, Anderson BE, Beyersdorf AJ, Konstantinidis KT (2013) Microbiome of the upper troposphere: species composition and prevalence, effects of tropical storms, and atmospheric implications. Proc Natl Acad Sci 110(7):2575–2580. https://doi.org/10.1073/pnas.1212089110
Dey D, Ghosal K, Bhattacharya SG (2019) Aerial fungal spectrum of Kolkata, India, along with their allergenic impact on the public health: a quantitative and qualitative evaluation. Aerobiologia 35:15–25. https://doi.org/10.1007/s10453-018-9534-6
Ding D, Xing J, Wang S, Liu K, Hao J (2019) Estimated contributions of emissions controls, meteorological factors, population growth, and changes in baseline mortality to reductions in ambient PM2.5 and PM2.5-related mortality in China, 2013–2017. Environ Health Perspect 127:67009–67009. https://doi.org/10.1289/EHP4157
Domingo JL, García F, Nadal M, Schuhmacher M (2017) Autopsy tissues as biological monitors of human exposure to environmental pollutants. A case study: concentrations of metals and PCDD/Fs in subjects living near a hazardous waste incinerator. Environ Res 154:269–274. https://doi.org/10.1016/j.envres.2017.01.014
Dwivedi S, Tewari-Singh N, Masih J, Taushiba A, Lawrence A (2022) Evaluation of indoor particulate matter and associated PAHs during the winter season in Northern India: A comprehensive impact of regional appearances. Journal of Hazardous Materials Advances 8:100195. https://doi.org/10.1016/j.hazadv.2022.100195
Ekhaise FO, Ogboghodo BI (2011) Microbiological indoor and outdoor air quality of two major hospitals in Benin City, Nigeria. Sierra Leone J Biomed Res 3(3):169–174
EPA/540/1-89/002 (1989) Risk assessment guidance for superfund volume 1. Human Health Evaluation Manual (Part A)
Esworthy R, Bearden DM (2015) Environmental Protection Agency (EPA): FY2016 appropriations. Library of Congress, Congressional Research Service
Faridi S, Naddafi K, Kashani H, Nabizadeh R, Alimohammadi M, Momeniha F, Yunesian M (2017) Bioaerosol exposure and circulating biomarkers in a panel of elderly subjects and healthy young adults. Sci Total Environ 593:380–389. https://doi.org/10.1016/j.scitotenv.2017.03.186
Faridi S, Shamsipour M, Krzyzanowski M, Künzli N, Amini H, Azimi F, Naddafi K (2018) Long-term trends and health impact of PM2. 5 and O3 in Tehran, Iran, 2006–2015. Environ Int 114:37–49. https://doi.org/10.1016/j.envint.2018.02.026
Fekadu S, Getachewu B (2015) Microbiological assessment of indoor air of teaching hospital wards: a case of Jimma University specialized hospital. Ethiop J Health Sci 25(2):117–122. https://doi.org/10.4314/ejhs.v25i2.3
Fleischer M, Bober-Gheek B, Bortkiewicz O, Rusiecka-Ziolkowskaa ´ J. (2006) Microbiological control of airborne contamination in hospitals. Indoor Built Environ 15(1):53–56. https://doi.org/10.1177/1420326X06062230
Gangneux JP, Bougnoux ME, Hennequin C, Godet C, Chandenier J, Denning DW, Dupont B (2016) An estimation of burden of serious fungal infections in France. J Mycol Med 26(4):385–390. https://doi.org/10.1016/j.mycmed.2016.11.001
Ghoma WEO, Sevik H, Isinkaralar K (2022) Using indoor plants as biomonitors for detection of toxic metals by tobacco smoke. Air Qual Atmos Health 15(3):415–424. https://doi.org/10.1007/s11869-021-01146-z
Gillum LA, Gouveia C, Dorsey ER, Pletcher M, Mathers CD, McCulloch CE, Johnston SC (2011) NIH disease funding levels and burden of disease. PloS One 6(2):e16837. https://doi.org/10.1371/journal.pone.0016837
Gralton J, Tovey E, McLaws ML, Rawlinson WD (2011) The role of particle size in aerosolised pathogen transmission: a review. J Infect 62(1):1–13. https://doi.org/10.1016/j.jinf.2010.11.010
Hayleeyesus SF, Manaye AM (2014) Microbiological quality of indoor air in University libraries. Asian Pac J Trop Biomed 4:S312–S317. https://doi.org/10.12980/APJTB.4.2014C807
Hogrefe C, Isukapalli SS, Tang X, Georgopoulos PG, He S, Zalewsky EE, Sistla G (2011) Impact of biogenic emission uncertainties on the simulated response of ozone and fine particulate matter to anthropogenic emission reductions. J Air Waste Manag Assoc 61(1):92–108. https://doi.org/10.3155/1047-3289.61.1.92
Ikhtiar M, Alzad H, Paramita S (2017) Microbiological assessment of indoor air of Takalar County Hospital wards in south Sulawesi. Indonesia Sci J Public Heal 5:172–177. https://doi.org/10.11648/j.sjph.20170503.13
Jin L, Apte JS, Miller SL, Tao S, Wang S, Jiang G, Li X (2022) Global endeavors to address the health effects of urban air pollution. Environ Sci Technol 56(11):6793–6798. https://doi.org/10.1021/acs.est.2c02627
Kermani M, Jonidi Jafari A, Gholami M, Arfaeinia H, Shahsavani A, Fanaei F (2021) Characterization, possible sources and health risk assessment of PM2. 5-bound heavy metals in the most industrial city of Iran. J Environ Health Sci Eng 19:151–163. https://doi.org/10.1007/s40201-020-00589-3
Khan T, Lawrence A, Dwivedi S, Arif S, Dwivedi S, Upadhyay A, Abraham A, Roberts V (2022) Air pollution trend and variation during a mega festival of firecrackers (Diwali) in context to COVID-19 pandemic. Asian Journal of Atmospheric Environment (AJAE) 16(3). https://doi.org/10.5572/ajae.2022.016
Krajewska-Kułak E, Łukaszuk C, Tsokantaridis C, Hatzopoulu A, Theodosopoyloy E, Hatzmanasi D, Kosmois D (2007) Indoor air studies of fungi contamination at the Neonatal Department and Intensive Care Unit an Palliative Care in Kavala Hospital in Greece. Advances in Medical Sciences (De Gruyter Open) 52
Kumar P, Kausar MA, Singh AB, Singh R (2021a) Biological contaminants in the indoor air environment and their impacts on human health. Air Qual Atmos Health 14(11):1723–1736. https://doi.org/10.1007/s11869-021-00978-z
Kumar P, Singh AB, Singh R (2021b) Seasonal variation and size distribution in the airborne indoor microbial concentration of residential houses in Delhi and its impact on health. Aerobiologia 37(4):719–732. https://doi.org/10.1007/s10453-021-09718-3
Kumar P, Singh AB, Singh R (2021c) Spatial variation and comprehensive health risk assessment of microbial indoor air quality in microenvironments of North Delhi. https://doi.org/10.21203/rs.3.rs-445730/v1
Kumar P, Singh AB, Singh R (2022) Comprehensive health risk assessment of microbial indoor air quality in microenvironments. Plos one 17(2):e0264226. https://doi.org/10.1371/journal.pone.0264226
Latif MT, Yong SM, Saad A, Mohamad N, Baharudin NH, Mokhtar MB, Tahir NM (2014) Composition of heavy metals in indoor dust and their possible exposure: a case study of preschool children in Malaysia. Air Qual Atmos Health 7(2):181–193. https://doi.org/10.1007/s11869-013-0224-9
Lee KK, Bing R, Kiang J, Bashir S, Spath N, Stelzle D, Shah AS (2020) Adverse health effects associated with household air pollution: a systematic review, meta-analysis, and burden estimation study. Lancet Glob Health 8(11):e1427–e1434. https://doi.org/10.1016/S2214-109X(20)30343-0
Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, Pelizzari PM (2012) A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380(9859):2224–2260
Lin YC, Li YC, Amesho KT, Shangdiar S, Chou FC, Cheng PC (2020) Chemical characterization of PM2.5 emissions and atmospheric metallic element concentrations in PM2.5 emitted from mobile source gasoline-fueled vehicles. Sci Total Environ 739:139942. https://doi.org/10.1016/j.scitotenv.2020.139942
López-Villarrubia E, Estirado OC, Hernández CÍ, Díez FB (2021) Do Saharan dust days carry a risk of hospitalization from respiratory diseases for citizens of the Canary Islands (Spain)? Archivos de Bronconeumología (English Edition) 57(7):464–470
Mbareche H, Morawska L, Duchaine C (2019) On the interpretation of bioaerosol exposure measurements and impacts on health. J Air Waste Manag Assoc 69(7):789–804. https://doi.org/10.1080/10962247.2019.1587552
Meng ZY, Xu XB, Wang T, Zhang XY, Yu XL, Wang SF, An XQ (2010) Ambient sulfur dioxide, nitrogen dioxide, and ammonia at ten background and rural sites in China during 2007–2008. Atmos Environ 44(21–22):2625–2631. https://doi.org/10.1016/j.atmosenv.2010.04.008
Mohammadyan M, Alizadeh-Larimi A, Etemadinejad S, Latif MT, Heibati B, Yetilmezsoy K, Abdul-Wahab SA, Dadvand P (2017) Particulate air pollution at schools: indoor-outdoor relationship and determinants of indoor concentrations. Aerosol Air Qual Res 17:857–864. https://doi.org/10.4209/aaqr.2016.03.0128
Mohammadyan M, Ghoochani M, Kloog I, Abdul-Wahab SA, Yetilmezsoy K, Heibati B, Godri Pollitt KJ (2017) Assessment of indoor and outdoor particulate air pollution at an urban background site in Iran. Environ Monit Assess 189:1–9. https://doi.org/10.1007/s10661-017-5951-1
Montazer M, Soleimani N, Vahabi M, Abtahi M, Etemad K, Zendehdel R (2021) Assessment of bacterial pathogens and their antibiotic resistance in the air of different wards of selected teaching hospitals in Tehran. Indian J Occup Environ Med 25(2):78. https://doi.org/10.4103/ijoem.IJOEM_234_19
Mousavi MS, Hadei M, Majlesi M, Hopke PK, Yarahmadi M, Emam B, Shahsavani A (2019) Investigating the effect of several factors on concentrations of bioaerosols in a well-ventilated hospital environment. Environ Monit Assess 191(7):1–11. https://doi.org/10.1007/s10661-019-7559-0
Nasir ZA, Colbeck I, Sultan S, Ahmed S (2012) Bioaerosols in residential micro-environments in low-income countries: a case study from Pakistan. Environ Pollut 168:15–22. https://doi.org/10.1016/j.envpol.2012.03.047
Omeliansky VL (1940) Manual in microbiology. USSR academy of sciences, Moscow
Pant P, Harrison RM (2013) Estimation of the contribution of road traffic emissions to particulate matter concentrations from field measurements: a review. Atmos Environ 77:78–97. https://doi.org/10.1016/j.atmosenv.2013.04.028
Qin Y, Liu Y, Wang J, Lu Y, Xu Z (2022) Emission of PAHs, PCBs, PBDEs and heavy metals in air, water and soil around a waste plastic recycling factory in an industrial park, Eastern China. Chemosphere 294:133734. https://doi.org/10.1016/j.chemosphere.2022.133734
Reid CE, Brauer M, Johnston FH, Jerrett M, Balmes JR, Elliott CT (2016) Critical review of health impacts of wildfire smoke exposure. Environ Health Perspect 124(9):1334–1343. https://doi.org/10.1289/ehp.1409277
Riesenfeld E, Chalupa D, Gibb FR, Oberdörster G, Gelein R, Morrow PE, Frampton MW (2000) Ultrafine particle concentrations in a hospital. Inhalation Toxicol 12(sup2):83–94. https://doi.org/10.1080/08958378.2000.11463201
Roy S, Gupta SK, Prakash J, Habib G, Baudh K, Nasr M (2019) Ecological and human health risk assessment of heavy metal contamination in road dust in the National Capital Territory (NCT) of Delhi India. Environ Sci Pollut Res 26:30413–30425. https://doi.org/10.1007/s11356-019-06216-5.10.1007/s11356-019-06216-5
Singh J, Gupta P, Gupta D, Verma S, Prakash D, Payra S (2020) Fine particulate pollution and ambient air quality: a case study over an urban site in Delhi. India J Earth Syst Sci 129(1):1–15. https://doi.org/10.1007/s12040-020-01495-w
Sivagnanasundaram P, Amarasekara RWK, Madegedara RMD, Ekanayake A, Magana-Arachchi DN (2019) Assessment of airborne bacterial and fungal communities in selected areas of teaching hospital, Kandy, Sri Lanka. BioMed Res Int 2019. https://doi.org/10.1155/2019/7393926
Slezakova K, da Conceição Alvim-Ferraz M, do Carmo Pereira, M. (2012) Elemental characterization of indoor breathable particles at a Portuguese urban hospital. J Toxicol Environ Health A 75(13–15):909–919. https://doi.org/10.1080/15287394.2012.690707
Slezakova K, Morais S, Pereira MDC (2014) Trace metals in size-fractionated particulate matter in a Portuguese hospital: exposure risks assessment and comparisons with other countries. Environ Sci Pollut Res 21(5):3604–3620. https://doi.org/10.1007/s11356-013-2316-3
Smith DJ, Jaffe DA, Birmele MN, Griffin DW, Schuerger AC, Hee J, Roberts MS (2012) Free tropospheric transport of microorganisms from Asia to North America. Microb Ecol 64(4):973–985. https://doi.org/10.1007/s00248-012-0088-9
Sorkheh M, Asgari HM, Zamani I, Ghanbari F (2022) The relationship between dust sources and airborne bacteria in the southwest of Iran. Environ Sci Pollut Res 29(54):82045–82063. https://doi.org/10.1007/s11356-022-21563-6
Sudharsanam S, Srikanth P, Sheela M, Steinberg R (2008) Study of the indoor air quality in hospitals in South Chennai, India—microbial profile. Indoor Built Environ 17(5):435–441. https://doi.org/10.1177/1420326X08095568
Toivola M, Alm S, Reponen T, Kolari S, Nevalainen A (2002) Personal exposures and microenvironmental concentrations of particles and bioaerosols. J Environ Monit 4(1):166–174. https://doi.org/10.1039/B108682K
US EPA (1996) Soil screening guidance: technical background document. Office of Emergency and Remedial Response, Washington, DC. EPA/540/R95/128
USEPA D (2002) Calculating upper confidence limits for exposure point concentrations at hazardous waste sites. OAWER9285(2002):6–10
Verde SC, Almeida SM, Matos J, Guerreiro D, Meneses M, Faria T, Viegas C (2015) Microbiological assessment of indoor air quality at different hospital sites. Res Microbiol 166(7):557–563. https://doi.org/10.1016/j.resmic.2015.03.004
Verdier T, Coutand M, Bertron A, Roques C (2014) A review of indoor microbial growth across building materials and sampling and analysis methods. Build Environ 80:136–149. https://doi.org/10.1016/j.buildenv.2014.05.030
Wang R, Yang B, Yao Y, Bloom MS, Feng Z, Yuan Y, Dong G (2020) Residential greenness, air pollution and psychological well-being among urban residents in Guangzhou, China. Sci Total Environ 711:134843. https://doi.org/10.1016/j.scitotenv.2019.134843
Weiss E, Essaied W, Adrie C, Zahar JR, Timsit JF (2017) Treatment of severe hospital-acquired and ventilator-associated pneumonia: a systematic review of inclusion and judgment criteria used in randomized controlled trials. Crit Care 21(1):1–12. https://doi.org/10.1186/s13054-017-1755-5
Yurtseven E, Erdogan MS, Ulus T, Alver Sahin U, Onat B, Erinoz E, Vehid S, Koksal S (2012) An assessment of indoor PM2.5 concentrations at a medical faculty in Istanbul. Turkey Environ Prot Eng 38:115–127
Zuo B, Liu C, Chen R, Kan H, Sun J, Zhao J, Bai H (2019) Associations between short-term exposure to fine particulate matter and acute exacerbation of asthma in Yancheng, China. Chemosphere 237:124497. https://doi.org/10.1016/j.chemosphere.2019.124497
Acknowledgements
The authors are thankful to Dr. (Mrs.) V. Prakash, principal, of Isabella Thoburn College, Lucknow, for her valuable support. The authors also extend their thanks to Dr. Jamson Masih, Wilson College, Mumbai, India, for lending the Leland Legacy sample pump (SKC Cat. No. 100-3002; Inc. Eighty-Four, PA, USA).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Taushiba, A., Dwivedi, S., Zehra, F. et al. Assessment of indoor air quality and their inter-association in hospitals of northern India—a cross-sectional study. Air Qual Atmos Health 16, 1023–1036 (2023). https://doi.org/10.1007/s11869-023-01321-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11869-023-01321-4