Abstract
Fireworks pollute the local atmosphere with various air pollutants, which can pose a health hazard for the local population. Mass and number concentrations of aerosols were measured before, during, and after the 2016/2017 New Year event in Ljubljana, Slovenia. Our findings highlight the negative impact of fireworks on the environment. First, both the mass concentration of black carbon and the number of concentrations of nanoparticles between 80 and 150 nm increased shortly after midnight. Second, on Jan 1, 2017, there was an increase in the average daily mass concentrations of PM10 and PM2.5. Third, on this day, our devices also detected increased air pollution by Al, Ba, Sr, and Cu, that is, heavy metals usually associated with fireworks. Their Jan 1 mass concentrations were more than 10 times (and Sr more than 140 times) higher than their average daily mass concentrations from Jan 3 (when their mass concentrations returned to more normal levels) to Jan 31. We also found that pairwise correlations between nanoparticles, PM10, and black carbon are positive, strong, and statistically significant. Besides carbon, the chemical analysis of the collected particles revealed the presence of typical elements used in pyrotechnic devices and their significant positive correlation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
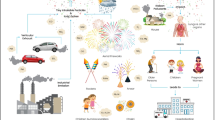
Fireworks are popular due to spectacular explosions and light effects, which depend on the chemical elements present in pyrotechnic devices. They are often used to celebrate important milestones, like the New Year, at the opening and closing ceremonies of various sport events, and frequently at important national or cultural ceremonies. In addition to major public events, smaller but numerous firework activities are often carried out locally by individuals. In contrast to aesthetic enjoyment and sound effects, frequent physical injuries such as lacerations, burns, hearing loss, and eye injuries are reported due to the use of pyrotechnic devices (Berger et al. 1985; Wisse et al. 2010; Van Yperen et al. 2021). Fireworks undoubtedly pollute the local atmosphere with particulate matter mainly composed of carbon used as fuel in pyrotechnic devices, sulphur, and various compounds used as colouring agents (Patrón et al. 2017; Cao et al. 2018; Pirker et al. 2020). Usually, metal halide salts, metal oxides, and carbonates form during the exothermic reaction in the pyrotechnic device where the oxidants (oxygen, potassium nitrate, potassium perchlorate, barium nitrate) react with fuels (charcoal, sulphur, resins), colouring agents (sodium nitrate (yellow), strontium carbonate (red), copper oxide (blue), barium nitrate (green), calcium carbonate (orange)), and colour-enhancing agents (PVC, Saran) (Cao et al. 2018). Elements such as silicon and iron can also be detected in minor concentrations after firework displays (Pirker et al. 2020). The source of these elements could be some additives to produce sparks (iron) and additives to enhance the pyrotechnic device performance (silicon) (Cao et al. 2018). Because fireworks are an acute air pollution problem only for up to a few days after the firework event, their effect on health cannot be directly addressed. Several studies have reported on particulate matter posing a health risk (Brook et al. 2002; Pöschl 2005; Curtis et al. 2006; Gauderman et al. 2015; Hamad et al. 2016; Fu et al. 2021). Hirai et al. (2000) reported a case of acute eosinophilic pneumonia, following inhalation of smoke from fireworks for three consecutive nights. Asthma exacerbation was diagnosed in two patients in a hospital in Philadelphia during the week of July 4, following exposure to elevated particulate matter (PM) concentrations from fireworks (Becker et al. 2000). The case-crossover analysis showed some positive associations between New Year’s firework events, PM10 concentrations, and daily mortality in the Netherlands (Greven et al. 2019). Recently, in vitro experiments demonstrated that the PM10 samples, which contained copper and lead, produced the greatest inflammatory response in mammalian cells and lungs (Hickey et al. 2020).
The frequent temperature inversion in winter time contributes to lasting air pollution in many urban areas. Temperature inversions stop atmospheric convection and in combination with windless conditions can lead to high concentrations of atmospheric pollutants especially when fireworks are used. Both acute and chronic exposures to the pollutants released by fireworks contribute to the formation of reactive oxygen species in the lungs, which induce a cellular and mediator inflammatory response (Kelly 2003; Organization 2016). Particles directly released or formed during explosions of pyrotechnic devices in the atmosphere range from 10 nm to a few µm (Li et al. 2013). Local geographic-meteorological conditions and use of different types of pyrotechnic devices make each firework and its pollution effects unique.
The monitoring of air pollution caused by fireworks is important for increasing public awareness that this popular entertainment might harm the environment and could also pose a hazard to public health. Due to the relatively short time of intensive pollution, one could assume that a New Year firework or other single-day events where pyrotechnic devices are used, do not contribute substantially to a long-term pollution to a degree, which would represent a health risk. In addition, the main pollutant released by fireworks is carbon, which originates also from wood and fossil fuel combustion (Ning et al. 2013; Zhang et al. 2020). Therefore, to clarify the effect of fireworks on air quality, the monitoring data are necessary. For a successful measurement, the monitoring ought to be performed at an appropriate distance from the fireworks, where the concentration of the pollution caused by fireworks is still substantial in comparison with pollutants of another origin. Besides location, wind direction and its speed, absence of rain or heavy fog, and low temperatures contribute to reliable results. Namely, the intensified combustion of carbon-based fuels for heating purposes and increased traffic mask the contributions of fireworks to air pollution. Nevertheless, from a comparison of results obtained by different techniques at different locations with respect to the firework platform, it is possible to estimate the environmental burden caused by fireworks.
Here, we report on comparative monitoring data on air pollution obtained from Dec 28, 2016, to Jan 3, 2017, during the New Year festivities in Ljubljana. Ljubljana is the capital of Slovenia with 288,179 inhabitants (in 2016) (Statistical Office of the Republic of Slovenia) and is situated in the Ljubljana Basin, which is frequently covered with a long-lasting fog in winter time. In this study, we analysed mass and number concentrations of nanoparticles (NPs), PM2.5, PM10, and black carbon (BC), their chemical composition, and evaluated the effect of fireworks on the air quality during New Year.
Materials and methods
Mass and number concentrations of nano- and micro-particles and carbonaceous aerosols in the air were measured at four locations in Ljubljana Basin before, during, and after the New Year fireworks. The measurements were obtained by a low-pressure cascade impactor, a scanning mobility particle sizer, and an aerosol absorption photometer. Chemical analysis was performed by inductively coupled plasma mass spectrometry. Public data measurements (meteorological parameters, hourly and daily mass concentrations of PM2.5 and PM10, and daily concentrations of heavy metals in PM10) were also analysed and compared to our measurements.
Sampling site
Positions of monitoring stations of air pollution caused by New Year firework in Ljubljana are schematically shown in Fig. 1. Public data provided by the Slovenian Environment Agency (ARSO) was used to evaluate meteorological parameters and the influence of fireworks on particulate matter concentrations in the air, namely PM10 and PM2.5, as well as the presence of heavy metals (ARSO). The official firework platform was situated in the city centre on top of the Castle hill, i.e. approx. 70 m above the level of Ljubljana Basin.
Map of Ljubljana showing the locations of the monitoring stations (ARSO). Measured parameters at each location: JSI, nanoparticles, black carbon, mass distribution of aerosols, and mass concentrations of various heavy metals; FMF, meteorological parameters; ARSO 1, PM10; ARSO 2, PM10 and meteorological parameters; ARSO 3, PM10, PM2.5, and mass concentrations of heavy metals
Meteorological parameters were measured at the Faculty of Mathematics and Physics (FMF), University of Ljubljana, approx. 1.7 km west-southwest of the firework platform, and at the ARSO 2 station situated approx. 1.9 km north-northeast of the firework platform. Relative humidity, temperature, wind speed, and its direction were recorded.
At ARSO 1 station, situated approx. 1 km north-northwest of the firework platform, and at ARSO 2 station, hourly mass concentrations of PM10 were measured. The ARSO 3 station was located at the Biotechnical Faculty, University of Ljubljana, approx. 2.8 km west of the firework platform where daily mass concentrations of PM10 and PM2.5 were measured as well as daily mass concentrations of heavy metals detected in PM10. Our monitoring stations (the low-pressure cascade impactor, the scanning mobility particle sizer, and the aerosol absorption photometer) were located on the third floor of a public building at Jožef Stefan Institute (JSI) (approx. 15 m above street level) adjacent to the FMF building.
Collection of samples and their analysis
Mass distribution of the particles was measured by a low-pressure cascade impactor with an inlet flow of 10 L/min. (DLPI, Dekati Ltd., Kangasala, Finland), which can classify airborne particles into 13 channels in the range from 30 nm to 10 µm, as seen in Table 1. Each stage has a defined cut diameter D50, determined as the particle diameter with a 50% collection efficiency (Marjamäki et al. 2000). The particles were collected on aluminium foils covered with the standard Apiezon L grease (Apiezon, Manchester, UK), which is used for better adhesion of particles. Mass of the foils was measured by a Mettler Toledo MT/UMT (Mettler Toledo, Columbus, OH, USA) microbalance with an accuracy of 0.1 µg. The number concentration of nanoparticles (NPs) was determined by a Scanning Mobility Particle Sizer (SMPS, model 3080 L85; TSI Co., Shoreview, MN, USA), equipped with desiccator, soft X-ray neutralizer, long differential mobility analyser (DMA, model 3081; TSI Co., Shoreview, MN, USA), and a water condensation particle counter (WCPC; model 3785; TSI Co., Shoreview, MN, USA). The electrical mobility diameter of counted particles was from 14.6 to 685.4 nm. The normalized concentration, dN/dlogDp, measured with the SMPS, is the total concentration (dN) within the measured range divided by the difference between the logarithms (dlogDp) of the lower and upper diameter of the counted particles. This makes the normalized concentration independent on the channel width of the instrument (Hinds 1982). The normalized concentration in this form is used so that results from instruments with different channel widths can be compared. The measurements were performed as successive size scans, with a 3-min duration of a single scan. An aerosol absorption photometer (Aethalometer, model AE33, Aerosol d.o.o., Ljubljana, Slovenia) (Drinovec et al. 2015) was used to measure the total concentration of black carbon. All of the equipment mentioned above was located at the JSI measuring site.
The sampling time of the SMPS and Aethalometer was from Dec 28, 2016, to Jan 3, 2017, while the DLPI was operational for 1 h, from 23:45 on Dec 31, 2016, to 00:45 on Jan 1, 2017. The DLPI was operational only during the firework activity with the goal to sample particles released mostly by the fireworks and not those that originated from other sources of air pollution.
The collected particles were analysed by inductively coupled plasma mass spectrometry (ICP-MS). For the aerosol removal from Al foils and dissolving, the aerosol particle samples were placed in PP tubes, added 5 mL of 5 M HNO3 (Suprapur, Merck) solution and exposed to ultrasonic irradiation (28/34 kHz, 80/180 W) for previously optimized time—15 min to avoid Al foil destruction. The accuracy of the applied sample preparation procedure was evaluated by analysing the LGC standard reference materials (A2 Elements on Filter Media/Work-room and B4 Elements on Filter Media/Work-room Air; National Institute of Occupational Health, Norway). The materials were prepared by spiking and are certified for the total content of 24 elements. As a blank control, Al foil with an equal surface as for the sample support was also sonicated following the same procedure. The solution was then filtered on a cellulose nitrate filter (Millipore, 0.45 µm pore size, previously washed with dissolving solution), and then all samples were diluted with ultrapure deionized water (Millipore, 0.055 µS⋅cm−1) to a total volume of 10 mL and stored at 8 °C in a refrigerator until analysis with ICP-MS was performed. For each element, the difference between mass on a stage and on the blank control was calculated. Considering air flow and duration of the measurement, mass concentrations were calculated. Agilent 8900 ICP-QQQ inductively coupled plasma mass spectrometer with a micromist nebulizer was used for the determination of the following elements—Mg, Al, V, Cr, Mn, Fe, Co, Ni, Cu, Zn, As, Rb, Sr, Sn, Ba, and Pb. The instrumental parameters were as follows: RF power, 1550 W; sampling depth, 8 mm; auxiliary gas flow, 0.90 mL/min; plasma gas flow, 15 L/min; He cell gas flow, 5 mL/min. All analytical standard stock solutions were trace grade from Merck for ICP (100 mg/L). For the calibration graph, six different standard solutions in concentrations ranging from 0.1 to 50.0 µg/L were prepared from stock standard solutions. For the calculation of concentrations of elements in the samples, the calibration graph method with blank correction was applied. An Internal Standard Mix solution from Agilent Technologies (10 mg/mL) was used as the internal standard. A stability check of the ICP-MS system was performed by using two standard solutions after every ten samples. A MassHunter workstation program with its Instrument control and Offline data analysis subprograms was used.
Statistical methods
The entire statistical analysis was performed using the computer software Program R (Team 2019). In particular, to calculate Pearson’s correlation coefficients (r) between each pair of heavy metals as well as between each heavy metal and PM10, and corresponding p-values, we use the Hmisc package (Harrell Jr 2021). To visualize the results, we use the corrplot package (Wei and Simko 2021). Hmisc package was also used to calculate Pearson’s correlation coefficients reported in Table 5.
The trends of NPs and PM10 data were investigated using modified mk package (Patakamuri and O’Brien 2021). As NPs and PM10 data is autocorrelated, we employ the modified (Block Bootstrapped) Mann–Kendall (MK) trend test and Sen’s slope estimate to find the nature of NPs and PM10 trends and significance levels. Modified version prevents the detection of a false trend (further details in (Önöz and Bayazit 2012; Patakamuri and O’Brien 2021)). As Mann–Kendall tests and Sen’s slope estimation methods are now well-known and widely used statistical tools, we will not go into details here. In short, the null hypothesis of the Mann–Kendall test is that there is no trend, whereas the alternative is that the trend is monotone. So, if p < 0.05, the null hypothesis is rejected, or in other words, we have evidence for a monotonic trend. On the other hand, Sen’s slope estimator tells something about the dynamics and magnitude of a trend (Sen 1968). Namely, a positive value indicates a positive (increasing) trend, whereas a negative value indicates a negative (decreasing) trend.
Results
Meteorological measurements
Figure 2a is drawn using publicly available data on wind speed and its direction recorded at the FMF (black arrows) and ARSO 2 (red arrows) stations. The data are presented from 21:00 on Dec 31, 2016, to 04:00 Jan 1, 2017. The speed did not exceed 0.6 m/s at any of the measurement stations, and after midnight, the wind blew in the northeast (from southwest to northeast) direction until 02:00 at the FMF station and in the southwest direction at the ARSO 2 station. After 02:00, the wind direction at the FMF station changed to southeast.
a Wind speed and direction at FMF station (black arrows) and ARSO 2 (red arrows) station from 21:00 on Dec 31, 2016, to 04:00 Jan 1, 2017. The arrows point in the direction in which the wind was blowing. b Temperature and relative humidity variations at the ARSO 2 station from Dec 28, 2016, to Jan 3, 2017
The average hourly temperature (T) and relative humidity (Rh) are shown in Fig. 2b from Dec 28, 2016, to Jan 3, 2017. As both meteorological stations (ARSO 2, FMF) recorded similar time modulations of T and Rh values, only the data from ARSO 2 station are shown. The periodical modulation of the average temperature is out of phase with the Rh. When the temperature increases, Rh decreases because the partial pressure of water increases with temperature. As evidenced in Table 5, their correlation is indeed strong, negative, and statistically significant (r = − 0.85, p < 0.001). On Jan 1, at 00:00, the temperature was − 5.6 °C and Rh was 85% at the ARSO 2 station. The lowest recorded temperature of − 8.4 °C during our campaign was on Jan 1, 2017, at 08:00.
Air pollution by PM 10 and PM 2.5 —data from public database (ARSO)
Based on public data from ARSO (ARSO), mass concentrations of PM10 measured in 1-h intervals from Dec 28, 2016, to Jan 1, 2017, are shown in Fig. 3a. The vertical dashed line marks midnight (Jan 1, 2017, at 00:00), when the intensity of the fireworks was highest. The peak in the PM10 mass concentration was on Jan 1 at 02:00 with the concentration reaching 234 µg/m3 at ARSO 1 station and 299 µg/m3 at ARSO 2 station. The average daily mass concentrations of PM10 for ARSO 1, ARSO 2, and ARSO 3 were 153, 137, and 126 µg/m3, respectively. These values were the highest average daily mass concentrations in the years 2016 and 2017. On Jan 1, 2017, the average daily mass concentration of PM2.5 measured at the ARSO 3 station was 114 µg/m3 (ARSO). This value turned out to be the highest among all the values recorded in 2016 and 2017.
The daily average mass concentrations of PM2.5 and PM10 at ARSO 3 station from Dec 1, 2016, to Jan 31, 2017, are shown in Fig. 3b. The dashed vertical line marks Jan 1, 2017, while the horizontal dash-dot line marks the maximum average daily mass concentration value of air pollution by PM10 (50 µg/m3) that should not be exceeded more than 35 times in a year as it is determined by the Slovenian government (Republic of Slovenia). The annual average mass concentration for PM2.5 set by the National directive and by the EU Air Quality Directive (2008/50/EC) (European Parliament) is 25 μg/m3 and was not exceeded neither in 2016 (22.50 µg/m3) nor in 2017 (20.24 µg/m3). It is worth noting that the WHO guideline limits the daily mass concentration of PM2.5 to 25 μg/m3 (WHO) and this value was exceeded multiple times, especially in the heating season (from November to February).
Air pollution by nanoparticles
Mass concentrations and the cumulative mass concentration of the particles collected by the DLPI are presented in Fig. 4. The largest mass (920 µg) was found on stage 6 with D50 of 400 nm. Particles captured on stages 5 (260 nm) to 8 (1 µm) add to more than 75% of all the mass. The total mass of all collected particles was 3.909 mg, which corresponds to a mass concentration of 6515 µg/m3.
Collected particles on the stages 3 and 5 with D50 of 108 nm and 260 nm, respectively, were analysed with ICP-MS. These two stages were selected because SMPS measurements have shown the largest number concentration of particles with the size from 70 to 300 nm (Fig. 5a). Mass concentrations of detected elements are presented in Table 2 in decreasing order. Because aluminium foil was used as a substrate in the DLPI, the data for aluminium are not shown. Among all the detected elements, iron (Fe), manganese (Mn), zinc (Zn), and magnesium (Mg) were detected in relatively large (μg/m3 and hundreds of ng/m3) quantities. Elements such as copper (Cu), chromium (Cr), barium (Ba), lead (Pb), nickel (Ni), vanadium (V), and strontium (Sr) were detected in smaller quantities (10–100 ng/m3), while rubidium (Rb), tin (Sn), cobalt (Co), and arsenic (As) were detected in traces (below 10 ng/m3). The amount of cobalt and arsenic is just above the detection threshold and can be neglected.
Elevated mass concentrations of different heavy metals were also measured at the ARSO 3 monitoring station (ARSO). They were determined from PM10 samples with the ICP-MS method after chemical decomposition according to the SIST EN 14,902:2005 standard. Data on daily concentrations of heavy metals (Table 3) shows that on Jan 1, 2017, mass concentrations of Al, V, Fe, Cu, Zn, Ga, Sr, Cd, Pb, Ba, and Rb strongly exceeded the average daily concentration from Jan 3 to Jan 31. In fact, the mass concentrations of Al, Ba, Sr, and Cu were more than 10 times, and Sr more than 140 times, higher than their average daily mass concentrations from Jan 3 to Jan 31, highlighting the negative side of New Year’s celebrations.
Figure 5 presents the normalized concentration of NPs given as the diameter in log scale vs time (a) and the total concentration of all NPs in the size range from 14.6 to 685.4 nm (b) for the period from Dec 28, 2016, to Jan 3, 2017. During the New Year night, the maximum value of the total concentration was reached 45 min after midnight. When the total concentration of NPs increased, the majority of the detected NPs were in the size range between 50 and 300 nm (Fig. 5a).
The Aethalometer data (Fig. 5b) shows a diurnal modulation of black carbon concentrations with higher values in the evenings. This is due to the activity of sources, such as holiday traffic and an increased use of wood and fossil fuels for heating, and due to the reduced mixing of the atmosphere and a very low mixing layer height. From Dec 28, 2016, to Jan 3, 2017, the black carbon concentrations resemble the SMPS total concentration data shown in Fig. 5b. The Pearson correlation coefficient between black carbon and SMPS measurements (taken every 3 min) is 0.88 (p < 0.001).
Statistical analysis
Correlations of heavy metals and PM 10
Intercorrelations of daily heavy metal concentrations for January 2017 are given in Table 4 and visually represented in Fig. 6. In Table 4, star symbols above the diagonal show the significance of the corresponding Pearson correlation coefficients reported under the diagonal. The heavy metals can be roughly divided into two groups (clusters) with high correlation. The first group contains Al, V, Cu, Zn, Ga, Sr, Cd, Pb, Ba, and Rb while the second one contains Cr, Mn, Fe, Ni, Co, and Mo. The correlations between the heavy metals and PM10 are shown in Table 4, last row and column. All except As are significantly positively correlated with PM10. Moreover, most of the heavy metals are strongly positively correlated with PM10, and only three (Cr, Ni, and Sb) are moderately positively correlated with PM10.
In Table 5, correlations of T, Rh, NPs, BC, and PM10 are shown. Rh, NP, BC, and PM10 are significantly positively correlated, while temperature is significantly negatively correlated with all parameters.
Additional trend tests for NPs, PM 10 (ARSO 1 and ARSO 2) data
The results show that in the period in question (from Dec 28, 2016, 00:00 to Jan 2, 2017, 23:00), all three variables (NPs, PM10 – ARSO 1, and PM10 – ARSO 2) exhibit a significant trend: NPs (BB Mann–Kendall test: τ = 0.25, p < 0.001), PM10 – ARSO 2 (BB Mann–Kendall test: τ = 0.47, p < 0.001), PM10 – ARSO 1 (Mann–Kendall test: τ = 0.47, p < 0.001). Furthermore, all these trends were positive, as suggested by a positive Sen’s slope (NP: 42.64, PM10 – ARSO 2: 0.71, PM10 – ARSO 1: 0.80), indicating that the closer the New Year was, the more polluted the air was. In our case, Sen’s slope of NP is 42.64 which means that the value of NP (i.e. number concentration of measured particles) increases, on average, each hour by 42.64 units (Sen’s slope values of PM10 are interpreted in the similar manner).
Discussion
On Jan 1, 2017, the average daily mass concentrations of PM10 at all ARSO sites reached the highest values among all the values recorded in 2016 and 2017. The measured values exceeded the maximum average daily mass concentration value of air pollution by PM10 (50 µg/m3) (ARSO 1 – 153 µg/m3; ARSO 2 – 137 µg/m3; ARSO 3 – 126 µg/m3). Average hourly mass concentrations (Fig. 3a) show that the mass concentration peaked 2 h after midnight. A time delay of 2 h is due to the low mixing speed of the air close to the ground with the air higher in the atmosphere. As seen from Fig. 2a, the wind speed did not exceed 0.6 m/s at any of the measurement stations after midnight. Also, the wind blew mostly in the northeast direction at the FMF station, while at ARSO 2 station the wind blew in southwest direction. Although this would suggest that the pollutants from the main firework event were blown away from the ARSO 2 station, it cannot be excluded that the winds higher in the atmosphere moved the pollutants closer to PM10 sampling sites. One of the most probable sources for the steep increase in mass concentrations of PM10 is multiple firework displays (the official one as well as the local fireworks) scattered across the city of Ljubljana. The increase in the average daily mass concentration of PM10 was followed by an increase in the average daily mass concentration of PM2.5 (Fig. 3b). The average daily mass concentration on Jan 1, 2017, for PM2.5 and PM10 at ARSO 3 station was 114 μg/m3 and 126 μg/m3, respectively. This suggests that most of the detected aerosols were in fact PM2.5, suggesting that mostly sub 2.5 μm particles formed during the firework displays.
As seen in Fig. 5b, elevated NPs and BC concentrations were detected every evening at the JSI site. On the days before and after the New Year fireworks, the increase in NPs and BC concentrations began at approx. 17:00 and lasted until midnight (00:00). The onset is probably due to increased traffic and wood and fossil fuel burning used for heating. Another peak in the NPs and BC concentrations can be seen during the morning rush hours, between 06:00 and 11:00. On Dec 31, 2016, the NPs and BC concentrations started increasing at approx. the same time compared to other days (on Dec 31, 2016, at 17:00) but lasted 3 h longer, until 03:00 Jan 1, 2017. The NP and BC concentrations peaked on Jan 1, 2017, at 00:45 and 00:20, respectively. Although the SMPS and the Aethalometer were stationed side by side, there is a 20-min difference in the corresponding pollution peak. This might be due to the different dispersion velocities of different pollutants associated with their size. The extended period of increased NPs and BC concentrations and the observed peak after midnight is associated with the use of fireworks. The increase in NP concentrations after midnight was most prominent for particles between 80 and 150 nm in size. Although on Jan 1, 2017, the lowest midnight and morning temperature was measured during our measurement campaign (Fig. 2b), the substantial prolongment of increased NPs and BC concentrations could not be associated solely to lower temperatures. Compared to the days before and after New Year, the midnight temperature on Jan 1, 2017, was only 1 °C lower. Although NPs and BC concentrations were elevated for the first few hours of Jan 1, 2017, the increase in concentrations was not as evident as with PM10. This is explained by agglomeration of pristine NPs, generation of oxidation products, and vapour condensation (Jimenez et al. 2009), all contributing to the increased size of particles.
As seen from Table 5, T is significantly negatively correlated with Rh, NPs, PM10, and BC measurements indicating that particle number and mass concentrations increase whenever there is a temperature drop. This is due to an increased use of wood and fossil fuels for heating. There is a significant positive correlation between NPs, PM10, and BC measurements, indicating that all particle sizes increase simultaneously as well as BC mass concentrations. The modified (Block Bootstrapped) Mann–Kendall (MK) trend tests and Sen’s slope estimates reveal a significant positive trend in PM10 and NP data, confirming that the mass and number concentrations of PM10 and NPs were rising as we approached New Year.
Mass concentrations of different particle sizes were also determined by the DLPI. The DLPI mass concentration of 6515 μg/m3 is more than 21 times higher than the mass concentration of PM10 at any ARSO station. The high mass concentration obtained with the DLPI is probably due to a more effective impactor than the PM10 sampler used at ARSO stations. The chemical analysis of the two stages of the DLPI shows a different chemical composition for each stage. Stage 3 (D50 = 108 nm) has higher mass concentrations of zinc (Zn), chromium (Cr), and nickel (Ni) compared to stage 5 (D50 = 260 nm). On the other hand, stage 5 has substantial higher mass concentrations of magnesium (Mg), barium (Ba), and lead (Pb). These measurements indicate that larger particles could originate from fireworks while smaller particles originate from other sources (traffic, wood and fossil fuel burning, industry) as magnesium, barium, and lead are known to be used in fireworks (Cao et al. 2018; Zhang et al. 2019; Pirker et al. 2020). Elevated mass concentrations of various heavy metals were also measured at ARSO 3 station, and are presented in Table 3. On Jan 1, 2017, the daily concentrations of various heavy metals in the air were substantially higher than their average concentrations measured in subsequent days from Jan 3 to Jan 31, 2017: Al 13.64 times, V 4.14 times, Mn 1.64, Fe 2.11 times, Cu 20.24 times, Zn 2.59 times, Ga 2.89 times, Sr 146.81 times, Cd 2.57 times, Pb 4.58 times, Ba 32.9 times, and Rb 3.55 times. Elevated concentrations were measured also on Jan 2, 2017. From the intercorrelations of daily heavy metal concentrations (Table 4, Fig. 6), two groups with high correlation can be formed. In one group, there are heavy metals associated with fireworks such as Al, V, Cu, Zn, Ga, Sr, Cd, Pb, Ba, and Rb (Cao et al. 2018; Zhang et al. 2019; Pirker et al. 2020). These heavy metals and their compounds are often used in fireworks as oxidants (V, Ba, Sr), fuels (Al), colouring agents (Sr, Cu, Ba, Rb), for smoke effects (Zn), for a steady and reproducible burning rate (Pb), and as impurities (Cd, V, Ga) (Dutcher et al. 1999; Moreno et al. 2010; Kong et al. 2015; Cao et al. 2018). Although most of the elements could be from other sources of air pollution, Sr and Ba are considered the best indicators of air pollution by fireworks (Cao et al. 2018). As both of these elements are significantly positively correlated with other elements from the first group, this strongly indicates that the fireworks were the source and also polluted the local atmosphere. In the second group, there are elements such as Cr, Mn, Fe, Ni, Co, and Mo which are associated with air pollution from traffic, industry, and heating. Elements such as Fe and Cr are sometimes also added to fireworks, but could originate from other sources.
An increase in air pollution after the firework event is in accordance with other reported cases (Drewnick et al. 2006; Moreno et al. 2007; Wang et al. 2007; Camilleri and Vella 2010; Joly et al. 2010; Li et al. 2017; Ambade 2018; Retama et al. 2019; Hoyos et al. 2020; Rindelaub et al. 2021). Increased concentrations of heavy metals such as Ba, Pb, Al, Sr, and Cu were observed after firework events (Moreno et al. 2007; Wang et al. 2007; Camilleri and Vella 2010; Ambade 2018), similar to measurements reported in this manuscript. Correlations between heavy metals associated with fireworks and PM10 similar to our measurements were also reported (Camilleri and Vella 2010). In multiple studies elevated PM2.5 and PM10 concentrations, up to ten times higher than background levels, were reported after a firework event (Wang et al. 2007; Ambade 2018; Retama et al. 2019). An increase in NPs and BC concentrations after a firework event was also reported (Li et al. 2017; Yuan et al. 2020).
Substantially higher concentrations of heavy metals in PM10 in the first 2 days of 2017 demonstrate the harmful consequences of fireworks to air quality. It is known that heavy metals contained in the particulate matter are associated with acute changes in cardiovascular and respiratory physiology (Rundell and Caviston 2008; Cutrufello et al. 2011). On Jan 1, 2017, Sr, Ba, and Cu mass concentrations increased the most compared to the Jan 3–Jan 31 average (Table 3). The Sr concentration in air was exceptionally higher, more than 146 times. Although strontium is not toxic (Emsley 2011), problems with bone growth may occur in children as it replaces calcium (ATSDR). Legally enforceable occupational exposure limit of soluble barium compounds in air averaged over an 8-h work day is 0.5 mg/m3 (ATSDR), which is 1000 times higher than the detected Ba concentration on Jan 1, 2017 (0.5 µg/m3). The concentration of barium in ambient air in normal conditions is estimated to be less than 0.05 μg/m3 (ATSDR), which was more than ten times exceeded after the firework event in Ljubljana. The legal airborne permissible exposure limit of Cu in the air is 1 mg/m3 (ATSDR), which is 4000 times more than the recorded concentration on Jan 1, 2017. Based on these comparisons on current limit values, one can conclude that the firework did not pollute Ljubljana Basin substantially, with exception of strontium.
Conclusions
Measurements of air pollution caused by fireworks at New Year 2016/2017 have been performed and the obtained results were combined with available public data. An increase in the number concentration of NP and mass concentration of BC and PM10 occurred 45 min, 20 min, and 2 h after midnight, respectively. The increase in mass and number concentrations soon after midnight is convincing evidence that fireworks polluted the air. In the 2016–2017 time frame, the daily mass concentrations of PM10 from all three ARSO stations were indeed the highest on Jan 1, 2017. The average daily concentrations of PM10 and PM2.5 at ARSO 3 station for Jan 1, 2017, were 126 µg/m3 and 114 µg/m3, respectively. Both values strongly exceeded the maximum daily mass concentration value for PM10 as it is determined by the Slovenian government (Republic of Slovenia) and recommended by WHO (50 µg/m3) and the WHO guideline limit of 25 μg/m3 for daily mass concentration of PM2.5. Using statistical methods, we found a significant positive correlation between PM10, NP, and BC, as well as a significant positive trend in data series suggesting a gradual increase in concentrations as New Year approached. A slight difference in mass concentrations of PM2.5 and PM10 indicates that sub 2.5 μm particles are released during firework events. The increase in NP concentrations after midnight was most prominent for particles between 80 and 150 nm in size. Besides carbon, the chemical analysis of the collected particles revealed the presence of elements typical for pyrotechnic devices, like Fe, Al, V, Cu, Zn, Ga, Sr, Cd, Pb, Ba, and Rb. The strong positive correlation between tracer elements of fireworks such as Ba and Sr with other elements from the group indicates that the increase in the mass concentration of heavy metals was a consequence of fireworks and not other anthropogenic sources. The monitoring data clearly shows that the New Year 2016/2017 firework contributed to substantial urban air pollution in Ljubljana Basin in atmospheric conditions without strong winds.
Data availability
Not applicable.
Code availability
Not applicable.
References
Ambade B (2018) The air pollution during Diwali festival by the burning of fireworks in Jamshedpur city, India. Urban Clim 26:149–160
ARSO Kakovost zraka. https://www.arso.gov.si/zrak/kakovost zraka/. Accessed 12 Mar 2021a
ARSO Map of Ljubljana. http://gis.arso.gov.si/atlasokolja/profile.aspx?id=Atlas_Okolja_AXL@Arso. Accessed 26 Feb 2021b
ARSO PM10 and PM2.5 data. https://www.ljubljana.si/sl/moja-ljubljana/varstvo-okolja/stanje-okolja/kakovost-zraka/. Accessed 26 Feb 2021c
ARSO Average PM2.5 concentrations. https://www.arso.gov.si/zrak/kakovost zraka/podatki/PM2.5_D_dec17_slo.pdf. Accessed 26 Feb 2021d
ARSO Daily concentrations of hevy metals in PM10 particles in 2017. https://www.arso.gov.si/zrak/kakovost zraka/podatki/KovineD_PM10_LJ_okt17.pdf. Accessed 26 Feb 2021e
ATSDR Public Health Statement for Strontium. https://wwwn.cdc.gov/TSP/PHS/PHS.aspx?phsid=654&toxid=120. Accessed 11 Mar 2021a
ATSDR Toxicological Profile for Barium. https://wwwn.cdc.gov/TSP/ToxProfiles/ToxProfiles.aspx?id=327&tid=57. Accessed 11 Mar 2021b
ATSDR Toxicological Profile for Copper. https://wwwn.cdc.gov/TSP/ToxProfiles/ToxProfiles.aspx?id=206&tid=37. Accessed 11 Mar 2021c
Becker JM, Iskandrian S, Conkling J (2000) Fatal and near-fatal asthma in children exposed to fireworks. Ann Allergy, Asthma Immunol 85:512–513
Berger LR, Kalishman S, Rivara FP (1985) Injuries from fireworks. Pediatrics 75:877–882
Brook RD, Brook JR, Urch B et al (2002) Inhalation of fine particulate air pollution and ozone causes acute arterial vasoconstriction in healthy adults. Circulation 105:1534–1536
Camilleri R, Vella AJ (2010) Effect of fireworks on ambient air quality in Malta. Atmos Environ 44:4521–4527
Cao X, Zhang X, Tong DQ et al (2018) Review on physicochemical properties of pollutants released from fireworks: environmental and health effects and prevention. Environ Rev 26:133–155
Curtis L, Rea W, Smith-Willis P et al (2006) Adverse health effects of outdoor air pollutants. Environ Int 32:815–830
Cutrufello PT, Rundell KW, Smoliga JM, Stylianides GA (2011) Inhaled whole exhaust and its effect on exercise performance and vascular function. Inhal Toxicol 23:658–667
Drewnick F, Hings SS, Curtius J et al (2006) Measurement of fine particulate and gas-phase species during the New Year’s fireworks 2005 in Mainz, Germany. Atmos Environ 40:4316–4327
Drinovec L, Močnik G, Zotter P et al (2015) The “dual-spot” Aethalometer: an improved measurement of aerosol black carbon with real-time loading compensation. Atmos Meas Tech 8:1965–1979
Dutcher DD, Perry KD, Cahill TA, Copeland SA (1999) Effects of indoor pyrotechnic displays on the air quality in the Houston Astrodome. J Air Waste Manage Assoc 49:156–160
Emsley J (2011) Nature’s building blocks: an AZ guide to the elements. Oxford University Press
European Parliament Directive 2008/50/EC of the European Parliament and of the Council of 21 May 2008 on ambient air quality and cleaner air for Europe. https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32008L0050. Accessed 26 Feb 2021
Fu H, Yang Z, Liu Y, Shao P (2021) Ecological and human health risk assessment of heavy metals in dust affected by fireworks during the Spring Festival in Beijing. Air Qual Atmos Heal 14:139–148. https://doi.org/10.1007/s11869-020-00920-9
Gauderman WJ, Urman R, Avol E et al (2015) Association of improved air quality with lung development in children. N Engl J Med 372:905–913
Greven FE, Vonk JM, Fischer P et al (2019) Air pollution during New Year’s fireworks and daily mortality in the Netherlands. Sci Rep 9:1–8
Hamad S, Green D, Heo J (2016) Evaluation of health risk associated with fireworks activity at Central London. Air Qual Atmos Heal 9:735–741. https://doi.org/10.1007/s11869-015-0384-x
Harrell Jr FE (2021) Hmisc: Harrell Miscellaneous. R package version 4.6–0. https://cran.r-project.org/package=Hmisc
Hickey C, Gordon C, Galdanes K et al (2020) Toxicity of particles emitted by fireworks. Part Fibre Toxicol 17:1–11
Hinds WC (1982) Aerosol technology: properties, behaviour, and measurement of airborne particles. John Wiley & Sons
Hirai K, Yamazaki Y, Okada K et al (2000) Acute eosinophilic pneumonia associated with smoke from fireworks. Intern Med 39:401–403
Hoyos CD, Herrera-Mejía L, Roldán-Henao N, Isaza A (2020) Effects of fireworks on particulate matter concentration in a narrow valley: the case of the Medellín metropolitan area. Environ Monit Assess 192:1–31
Jimenez JL, Canagaratna MR, Donahue NM, et al (2009) Evolution of organic aerosols in the atmosphere. Science (80- ) 326:1525–1529
Joly A, Smargiassi A, Kosatsky T et al (2010) Characterisation of particulate exposure during fireworks displays. Atmos Environ 44:4325–4329
Kelly FJ (2003) Oxidative stress: its role in air pollution and adverse health effects. Occup Environ Med 60:612–616
Kong SF, Li L, Li XX et al (2015) The impacts of firework burning at the Chinese Spring Festival on air quality: insights of tracers, source evolution and aging processes. Atmos Chem Phys 15:2167–2184
Li J, Xu T, Lu X et al (2017) Online single particle measurement of fireworks pollution during Chinese New Year in Nanning. J Environ Sci 53:184–195
Li W, Shi Z, Yan C et al (2013) Individual metal-bearing particles in a regional haze caused by firecracker and firework emissions. Sci Total Environ 443:464–469
Marjamäki M, Keskinen J, Chen D-R, Pui DYH (2000) Performance evaluation of the electrical low-pressure impactor (ELPI). J Aerosol Sci 31:249–261
Moreno T, Querol X, Alastuey A et al (2010) Effect of fireworks events on urban background trace metal aerosol concentrations: is the cocktail worth the show? J Hazard Mater 183:945–949
Moreno T, Querol X, Alastuey A et al (2007) Recreational atmospheric pollution episodes: inhalable metalliferous particles from firework displays. Atmos Environ 41:913–922
Ning Z, Chan KL, Wong KC et al (2013) Black carbon mass size distributions of diesel exhaust and urban aerosols measured using differential mobility analyzer in tandem with Aethalometer. Atmos Environ 80:31–40. https://doi.org/10.1016/j.atmosenv.2013.07.037
Önöz B, Bayazit M (2012) Block bootstrap for Mann-Kendall trend test of serially dependent data. Hydrol Process 26:3552–3560
Organization WH (2016) Ambient air pollution: a global assessment of exposure and burden of disease
Patakamuri SK, O’Brien N (2021) modifiedmk: modified versions of Mann Kendall and Spearman’s rho trend tests. R package version 1.6. https://cran.r-project.org/package=modifiedmk
Patrón D, Lyamani H, Titos G et al (2017) Monumental heritage exposure to urban black carbon pollution. Atmos Environ 170:22–32
Pirker L, Gradišek A, Višić B, Remškar M (2020) Nanoparticle exposure due to pyrotechnics during a football match. Atmos Environ 233:117567. https://doi.org/10.1016/j.atmosenv.2020.117567
Pöschl U (2005) Atmospheric aerosols: composition, transformation, climate and health effects. Angew Chemie Int Ed 44:7520–7540
Republic of Slovenia Uredba o kakovosti zunanjega zraka. https://www.uradni-list.si/glasilo-uradni-list-rs/vsebina/2011-01-0368/uredba-o-kakovosti-zunanjega-zraka?h=Uredba o kakovosti zunanjega zraka. Accessed 26 Feb 2021
Retama A, Neria-Hernández A, Jaimes-Palomera M, et al (2019) Fireworks: a major source of inorganic and organic aerosols during Christmas and New Year in Mexico city. Atmos Environ X 2:100013
Rindelaub JD, Davy PK, Talbot N et al (2021) The contribution of commercial fireworks to both local and personal air quality in Auckland, New Zealand. Environ Sci Pollut Res 28:21650–21660
Rundell KW, Caviston R (2008) Ultrafine and fine particulate matter inhalation decreases exercise performance in healthy subjects. J Strength Cond Res 22:2–5
Sen PK (1968) Estimates of the regression coefficient based on Kendall’s tau. J Am Stat Assoc 63:1379–1389
Statistical Office of the Republic of Slovenia Population by selected age groups and sex, municipalities, Slovenia, half-yearly. https://pxweb.stat.si/SiStatData/pxweb/en/Data/Data/05E1022S.px/. Accessed 26 Feb 2021
Team RC (2019) R: a language and environment for statistical computing [Computer software]. R Vienna, Austria: Foundation for Statistical Computing
Van Yperen DT, Van Lieshout EMM, Dijkshoorn JN et al (2021) Injuries, treatment, and impairment caused by different types of fireworks; results of a 10 year multicenter retrospective cohort study. Scand J Trauma Resusc Emerg Med 29:1–9
Wang Y, Zhuang G, Xu C, An Z (2007) The air pollution caused by the burning of fireworks during the lantern festival in Beijing. Atmos Environ 41:417–431
Wei T, Simko V (2021) R package “corrplot”: visualization of a correlation matrix (Version 0.90). https://github.com/taiyun/corrplot
WHO WHO Air quality guidelinesfor particulate matter, ozone, nitrogendioxide and sulfur dioxide. https://apps.who.int/iris/bitstream/handle/10665/69477/WHO_SDE_PHE_OEH_06.02_eng.pdf;sequence=1. Accessed 26 Feb 2021
Wisse RPL, Bijlsma WR, Stilma JS (2010) Ocular firework trauma: a systematic review on incidence, severity, outcome and prevention. Br J Ophthalmol 94:1586–1591
Yuan L, Zhang X, Feng M, et al (2020) Size-resolved hygroscopic behaviour and mixing state of submicron aerosols in a megacity of the Sichuan Basin during pollution and fireworks episodes. Atmos Environ 226:117393
Zhang J, Huang X, Chen Y et al (2019) Characterization of lead-containing atmospheric particles in a typical basin city of China: seasonal variations, potential source areas, and responses to fireworks. Sci Total Environ 661:354–363
Zhang Y, Albinet A, Petit JE et al (2020) Substantial brown carbon emissions from wintertime residential wood burning over France. Sci Total Environ 743:140752. https://doi.org/10.1016/j.scitotenv.2020.140752
Acknowledgements
COST Action CA16109 Chemical On-Line cOmpoSition and Source Apportionment of fine aerosoL, COLOSSAL. We would like to thank Māris Bērtiņš for performing the ICP-MS measurements. Velkavrh gratefully acknowledges the support from the Slovenian Research Agency Young Researchers Grant.
Funding
This research was funded by the Slovenian Research Agency, grant numbers P1-0099, P1-0285, J1-9110, J1-9186, and J1-2451.
Author information
Authors and Affiliations
Contributions
Conceptualization, M.R.; methodology, L.P. and M.R.; validation, A.O., L.D., and G.M.; formal analysis, L.P., Ž. V., A.O., and L.D.; investigation, L.P., A.O., and L.D.; resources, M.R. and G.M.; data curation, L.P., Ž.V., A.O., L.D., and G.M.; writing—original draft preparation, M.R. and L.P.; writing—review and editing, Ž.V., A.O., L.D., G.M., and M.R.; visualization, L.P. and Ž.V.; supervision, M.R.; project administration, M.R.; funding acquisition, M.R. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pirker, L., Velkavrh, Ž., Osīte, A. et al. Fireworks—a source of nanoparticles, PM2.5, PM10, and carbonaceous aerosols. Air Qual Atmos Health 15, 1275–1286 (2022). https://doi.org/10.1007/s11869-021-01142-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11869-021-01142-3