Abstract
There is large epidemiological evidence on the short-term health effects of O3 and NO2. These gaseous pollutants induce oxidative stress through their oxidative potential. Therefore, the evaluation of their combined oxidative capacity (Ox) has been proposed rather than studying the effect of either gas individually. To study the short-term effects of daily concentrations of O3, NO2, and Ox on mortality in Rome, in 2002–2015, daily deaths from the city mortality registry were analyzed along with O3 and NO2 levels observed in Rome and with estimated Ox and Owt (Ox, weighted by the redox potential of O3 and NO2). A Poisson regression model was used considering trends, and meteorological and population changes. The effects on mortality were estimated at lag 0–1 and 0–5 for 10 μg/m3. O3 and NO2 were associated with mortality, with the highest effects at lag 0–5, 0.81% (0.45–1.17) and 2.72% (2.07–3.37), respectively. Ox had an intermediate effect between the two gases. After adjusting for PM10, Owt had a stronger effect (1.72%; 1.14–2.30) than either gas, 0.86% (0.50–1.22) for O3 and 1.61% (1.15–2.06) for NO2. Both Ox and Owt were associated with cerebrovascular, respiratory and, to a lesser extent, cardiac mortality more than either gas. These results suggest that the use of Ox (or Owt) can provide a better assessment of the combined role of O3 and NO2 on mortality and can avoid the uncertainty of the threshold level for ozone. The brain and lungs seem to be the main targets of O3 and NO2.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The potential to cause a redox reaction refers to the capacity of environmental factors to oxidize target molecules generating reactive oxygen species (ROS) (Ayres et al. 2008; Künzli et al. 2006; Møller et al. 2014). An increase of ROS could be considered as an early effect of air pollution (Thurston et al. 2017), but also as a first defense against oxidative potential, like inflammation. Recent research shows that a small increase of ROS activates biological processes to preserve physiological functions (Schieber and Chandel 2014). Therefore, oxidative stress could be considered as beneficial at low doses and harmful at moderate to high doses (Schieber and Chandel 2014).
Gaseous pollutants, such as gaseous ions, organic components, and secondary radicals, induce oxidative stress through their oxidative potential, but few studies (Delfino et al. 2013; Pavlovic et al. 2015; Steenhof et al. 2014; Weichenthal et al. 2016; Williams et al. 2014) have dealt with this topic due to objective difficulties in estimating oxidative potential from atmospheric gases. On the other hand, agreement has been reached on the ability of particulate matter (PM) to elicit oxidative potential by means of both chemical and biochemical tests (Ayres et al. 2008). The difficulty regarding gases consists essentially in the measurement given that redox active semi-volatile compounds such as ozone (O3) and nitrogen dioxides (NO2) cannot be fully captured by filters used to sample PM (Pavlovic et al. 2015).
As a result, different approaches—such as modeling oxidative potential (Yang et al. 2015) or estimating the intensity of oxidative stress in subjects exposed to air pollutants (Kelly 2003; Rodríguez-Cotto et al. 2015; Strak et al. 2012; Yang et al. 2016)—have been adopted to assess exposure to the oxidative potential of gases. It has been observed also that estimating the oxidative potential of gaseous pollutants separately, such as O3 and NO2, which interchange rapidly in the atmosphere in daylight, might weaken the estimate of their effects because the real exposure is the result of their combined oxidant capacity (Williams et al. 2014). This last point implicitly suggests moving beyond the limits of separate estimates, by assessing the effects of combined oxidant capacity of O3 and NO2, that is hypothesized as the true exposure to both gases, combining their mechanisms of action. Another approach estimates the joint effect from a multi-pollutant model by adding their coefficients, multiplied by their respective level increases (Winquist et al. 2014).
There is large epidemiological evidence on the health effects of O3 and NO2. Long-term exposure to high levels of O3 has been related to cardiovascular (CV) and respiratory mortality (Schwartz 2016). In addition, two recent meta-analyses highlighted that both long-term (Faustini et al. 2014) and short-term exposures (Mills et al. 2016) to NO2 are associated with mortality independent of PM. Some uncertainty persists about the ozone concentration–response relationship (Bae et al. 2015), supporting the hypothesis that combined oxidant capacity of O3 and NO2 could be a better measure of exposure and could be used in the exposure assessment of these gases.
We studied the short-term effects of daily concentrations of O3, NO2, and their combined oxidant capacity (Ox) on mortality in Rome, in 2002–2015. We also assessed potential confounding induced by co-exposures to PM10 and PM2.5.
Methods
Deaths that occurred in the city of Rome (Italy) from 2002 to 2015 from natural (International Classification of Diseases, 9th revision—ICD-9: 1–799), cardiac (ICD-9 390–429), cerebrovascular (ICD-9 430–438), and respiratory causes (ICD-9 460–519) were collected from the mortality registry of Rome, which captures nearly 100% of the deaths that occur in the city among residents (personal communication of the registry operators). We selected deaths among subjects aged 35+ years only.
Hourly NO2 and O3 concentrations were available from three fixed monitoring stations of the Regional Environmental Protection Agency network, chosen to represent background levels of these pollutants throughout the whole city, for the entire period (1-1-2002 to 31-12-2015). Daily mean concentrations of PM10 (2002–2015) and PM2.5 (only available for 2006–2015) were also collected. We selected the same stations for O3 and NO2 for the whole period. Daily monitored data needed to be 75% complete for inclusion. Missing values from a monitor were imputed using the average measurements from the other monitors on the same day, weighted by the ratio of the yearly average at that monitor to the yearly average of the others (Stafoggia et al. 2010).
Combined oxidative potential (Ox) was obtained by adding hourly NO2 and O3 levels from each station. Weighted oxidative capacity (Owt) was obtained by adding gaseous concentrations, weighted by the oxidative potential of each gas according to the following formula:
Daily means of each pollutant were calculated by averaging hourly monitor-specific measurements of NO2, O3, Ox, and Owt. Finally, daily means for the whole city were estimated by averaging monitor-specific daily mean concentrations. Daily maximum 1-h levels of O3, NO2, and Ox were also calculated.
Daily temperature, humidity, and barometric pressure readings were provided by the Italian Air Force Meteorological Service. Apparent temperature was calculated from air temperature and dew point temperature from relative humidity, as indicated by Steadman (1979).
We used a Poisson regression model allowing for over-dispersion (McCullagh and Nelder 1989) to estimate the short-term effect of daily 10 μg/m3 (20 ppm) rise of O3, NO2 levels and their combined oxidant capacity on daily natural and cause-specific mortality among 35-year-old residents in Rome at a cumulative lag interval of 0–1 day (consistent with an immediate effect), 2–5 days (delayed effect), and 0–5 days (prolonged effect). We modeled time trends with penalized regression splines (Wood 2000) with a number of six effective degrees of freedom per year to control for both long-term trends and seasonality.
Both apparent temperature and barometric pressure were adjusted for by including non-linear terms. High temperatures were defined as days with lag 0–1 temperature above the median and were modeled with a penalized spline with degrees of freedom chosen by a generalized cross-validation method (Wood 2000). Low temperatures were defined as lag 1–6 days with temperature below the median and were modeled with another penalized spline. Barometric pressure was adjusted for with a penalized spline (lag 0) of the original variable (Stafoggia et al. 2010).
Influenza epidemics and population decreases during vacation periods were adjusted for using indicator variables: dichotomous variables were used for influenza days (as identified by the weekly national influenza surveillance) and for single-day holidays, while a three-level variable was used for summer vacation periods assuming value “2” in the 2-week period around the 15th of August, the value “1” from mid-July to the end of August, and the value “zero” elsewhere.
All results are expressed as percentage changes in mortality (and 95% CIs) per 10 μg/m3 increment of the pollutants.
In addition, a two-pollutant model of O3 and NO2 was fitted to estimate the independent effects of O3 and NO2. Then, the joint effect of O3 and NO2 was computed from the two-pollutant model as the sum of their coefficients multiplied by 10 (Winquist et al. 2014). Results were compared with those from the combined Ox approach.
We performed a few sensitivity analyses: (1) we expressed the main estimates of O3, NO2, and combined Ox effects by IQR increases as an alternative metric in order to allow better comparison of effects across pollutants. Interquartile ranges (IQRs) were calculated as the difference between the third and first quartile of daily concentrations or combined Ox; (2) we adjusted the effects of O3, NO2, and combined Ox for daily PM10 or PM2.5 (daily increases of 10 μg/m3 at lag 0–5); (3) we excluded the year 2003, when summer temperatures in Rome were extremely high and persisted throughout the season.
All analyses were performed using STATA software (V.13.0, 2013, Texas, USA) and R software (V.3.1.3; R Core Team, 2015. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/).
Results
Table 1 shows the distributions of the daily death counts, daily levels of ozone, nitrogen dioxide, their combined oxidant capacity (both 1-h maximum and daily 24-h mean), PM10 concentrations, and daily means of meteorological parameters. The bottom of the table displays Pearson correlation coefficients between pairs of all environmental variables.
In total, 300,644 natural deaths were included in the analysis, 30% from cardiac causes, 9% from cerebrovascular, and 6% from respiratory causes. The two gases showed similar daily means and 1-h maximum concentrations. O3 displayed higher variability than NO2, while Ox variability was halfway between the two gases. O3 and NO2 levels were negatively correlated. O3 was positively correlated with Ox, while NO2 was only marginally correlated with it. PM10 was positively correlated with NO2 and negatively with O3. The daily distribution of O3, NO2, and Ox levels (Supplementary Fig. 1) shows Ox levels higher than both gases, with NO2 making the larger proportion of Ox at lower Ox and O3 making inversely the larger proportion of Ox at higher Ox, suggesting that Ox levels might be most influenced by O3 in hot seasons. In the summer (Supplementary Table 1), the levels of O3, NO2, and Ox (1-h) increase, while the variability of all pollutants decreases and O3 (1-h) becomes positively related to PM10. Figure 1 shows the relationship between daily mean O3 and NO2 concentrations, year-round and by season. PM2.5 shows the same relationship with O3, NO2, and Ox as PM10 (Supplementary Table 2) though assessed over a shorter period (2006–2015).
Table 2 reports the results of the short-term effects of NO2, O3, and Ox on mortality. One-hour maximum ozone was associated with higher mortality, with the greatest effect seen (0.81%) at lag 0–5; NO2 (both 1-h maximum and 24-h mean) showed a stronger association with mortality than O3 at any lag, the greatest effect (2.72%) being at lag 0–5. The combined Ox levels (both 1-h and 24-h) showed greater effects than ozone and lower than NO2, at any lag interval. In the summer, effects on mortality were higher for both gases but remained much higher for NO2; Ox also presented values halfway between the individual gases, but still closer to ozone effects. No difference was found between the effects of Ox and Owt on natural mortality, except for Owt (1 h) effects in the hot season, which were higher than those of both gases.
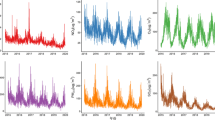
Concentration–response curves for 1-h O3, and 24-h mean NO2, Ox, and Owt (Fig. 2) show a non-linear shape between O3 and mortality at low levels (plot a), as well as between NO2 and mortality (plot b) at high levels. The concentration–response curve for Ox is suggestive of a linear relationship, while that for Owt is more similar to O3.
Association between daily O3, NO2, oxidative potential (Ox), and oxidative potential after redox weighting (Owt) with natural mortality: dose–response relationships. Pollutants at lag 0–5. a Daily 1-h maximum O3, lag 0–5; (b) daily mean NO2, lag 0–5; (c) daily mean Ox, lag 0–5; (d) daily mean Owt, lag 0–5
Results of the two-pollutant models for O3 and NO2 are reported in Fig. 3. Both O3 and NO2 were significantly associated with natural mortality, with ozone effects greater than single-pollutant models, whereas NO2 effects were attenuated. Adding PM10 (Supplementary Fig. 2) or PM2.5 (Supplementary Table 3) marginally decreased the effects of both gases and their Ox, suggesting gases and particulate effects are independent of each other. We did not find evidence of effect modification of PM10 on the Ox-natural mortality association (Fig. 4).
Associations between O3, NO2, Ox, and Owt (daily mean) with natural mortality (lag 0–5): % increase of risk (% IR), and 95% CI, per 10 mg/m3 increases in the pollutants. “Single pollutant” refers to models where O3 and NO2 are added one at a time; “two-pollutant” refers to a model where O3 and NO2 are added together; “oxidative potential” is a single-pollutant model where the sum of O3 and NO2 (un-weighted, Ox; or redox-weighted, Owt) is added as exposure term; “joint effect from two-pollutant model” uses the joint effect of O3 and NO2 computed as the sum of their coefficients multiplied by 10, from two-pollutant model
Joint association between daily mean Ox and PM10 (lag 0–5) with cause-specific mortality. a Natural mortality; (b) cardiac mortality; (c) cerebrovascular mortality; (d) respiratory mortality. All the figures were performed using R, version 3.4.2 (R Core Team 2011. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. Available: http://www.R-.project.org (accessed 18 January 2017)).
Mortality from cardiac, cerebrovascular, and respiratory diseases (Table 3) shows characteristics very similar to those of natural mortality: it increases with rising O3 (1 h only), NO2, and Ox (1-h and 24-h); the highest estimates are at lag 0–5 for all causes, and Ox shows estimates halfway between O3 and NO2. However, mortality from respiratory and cerebrovascular causes is higher than mortality from cardiac mortality and mortality from all natural causes. These effects are more evident when Owt is used. In addition, after adjusting for PM10 (Supplementary Fig. 2, Fig. 4), cerebrovascular mortality seems almost entirely due to Ox while respiratory mortality recognizes an independent contribution from Ox and PM10.
Figure 3 shows that O3 and NO2 have an independent effect on mortality when added together in a model, and the effect is very similar for both gases, but differs greatly from the single pollutant model, due to the complex relationship between these gases during the day. It is interesting in this respect to highlight that the lag 0–5 and the daily mean levels of the pollutants prove to give a smoothed but realistic measure of gases’ single effect in this study. The effect of combined oxidative potential is almost twice what was expected, when calculated both as Ox or as Owt. Finally, the joint effect obtained using the combined Ox and that of summing the estimates from the two-pollutant model show very similar results.
Sensitivity analyses show that using IQR as the metric of exposure (Supplementary Fig. 3) gives higher estimates but does not change the characteristics of O3, NO2, or Ox impact, while excluding the year 2003 gives a bit lower estimate but does not significantly modify the result.
Discussion
Our results show that ozone (1-h) and nitrogen dioxides (both 1-h and 24-h) are associated with mortality, reaching the highest effects at lag 0–5 and in the hot season. Combined Ox has an effect comprised between that of the two gases, while weighted oxidative capacity has a stronger effect on mortality than either of the two gases when 1-h levels are considered, which remains significant after adjusting for PM10 or PM2.5. Our results suggest that both Ox and Owt are associated with high cerebrovascular and respiratory mortality and, to a lesser extent, cardiac mortality, more than either gas, at both 1-h and 24-h exposures.
The effects of both O3 and NO2 on mortality today are widely recognized (Schwartz 2016). Yet their oxidative potential has been studied less, although it deserves more attention given their ability to trigger oxidative stress, which is an early effect of air pollution (Thurston et al. 2017) and, in turn, a mediator of epigenetic damage (Møller et al. 2014). Moreover, using cumulative oxidative potential of more than one pollutant streamlines the process since it is one exposure factor, yet estimates the toxicity of all the pollutants (Ito et al. 2007).
Our results show that the correlation between O3 and NO2 is basically negative, while that of O3 with PM10 is negative and between NO2 and PM10 is positive. These relationships should be interpreted by referring to the three fundamental reactions between O3 and NOx (Williams et al. 2014). These reactions interchange O3 and NO2, but lead to no net production of O3 in the absence of “smog” episodes in hot weather when the correlations between the two gases and with PM change, as we also observed in this study. Zeka and Schwartz (2004) underlined the consequence that these relationships could have on the interpretation of two-pollutant effects: when pollutants are correlated and affected by measurement errors (as in the case of air pollutants), the measurement error of the second pollutant affects that of the first and the direction of the bias depends on the sign of the pollutants’ correlations. Finally, Ito et al. (2007) offer the more general comment that if multi-pollutant effects could be predicted from the model specifications of their interactions, it is likely that multi-pollutant effects could reflect the toxicity of the mixture for which the pollutants are a surrogate, rather than the relative effects of the pollutant.
In our results, the effect on mortality of weighted oxidative potential is greater than it was from either pollutant by itself, as has been reported in previous papers (Weichenthal et al. 2016; Williams et al. 2014); however, the exposure metric differs among studies: Ox effects were larger than those of single pollutants as observed for 24-h levels in previous studies, while we found larger effects of Ox for 1-h levels. Williams et al. (2014) found higher effects for O3 than for NO2 (for both 24-h and 1-h), while we found larger effects for NO2 than for O3, in spite of the higher redox potential of O3. Possible explanations are that NO2 acts via other mechanisms on health effects (Williams et al. 2014) or even that the 1-h maximum levels of the two gases do not occur at the same time of day. On the other hand, the results we obtained for Owt are consistent with those observed in a London study of Ox (Williams et al. 2014).
We chose to study 1-h levels when exposures get the highest values and 24-h levels when the complex reactions between O3 and NO2 occur, allowing a greater assessment of population exposure. Other European studies such as APHEA-1 and 2 (Gryparis et al. 2004; Samoli et al. 2006) mostly used 8-h mobile means for both O3 and NO2; however, 8-h mobile means are only slightly lower than 1 h (Supplementary Fig. 1) in our data, and even so would not have avoided assessing O3 and NO2 at different times of the day.
Our two-pollutant analysis shows that both gases individually have an effect on mortality, with O3 having a larger impact, possibly due to its stronger oxidative potential. Previous studies do not present homogeneous results. Mortality due to NO2 did not change substantially when analyzed in the same model with O3 8-h in European studies (Gryparis et al. 2004; Samoli et al. 2006), neither was any change observed in a London study (Williams et al. 2014). These observations suggest that the effects of O3 and NO2 were independent, but mortality associated with both gases was higher when 24-h levels were used, suggesting prudence in interpreting the relative effects of O3 and NO2. In the New York study (Ito et al. 2007), adding O3 to a multi-pollutant model did not increase the low multi-collinearity of NO2 among all pollutants. In addition, using a single factor as Owt seems to give a more “true” picture of the effects of the NO2–O3 combination because it takes into account the toxicity of both gases (Williams et al. 2014).
We would add that using only one factor as Owt could provide a better estimate because it does not require assumptions of independence or linearity as a multi-pollutant analysis does. In summary, if estimating the joint effect of more pollutants in the same environment is important to assess the true impact on human health (Winquist et al. 2014), the combined oxidative potential of more pollutants presents the advantages of being a single exposure factor and at the same time of summarizing exposure to more pollutants. The high similarity with the joint effect from the two-pollutant model suggests further analyses of these approaches would be beneficial.
The concentration–response functions for O3 and NO2 suggest in our results a non-linear relationship for single pollutants, whereas Ox does not deviate significantly from linearity, thus supporting the above hypothesis that it could provide a more accurate estimate of the NO2–O3 combination. A non-linear relationship of O3 and even of Ox effects has been observed in the London study (Williams et al. 2014) and other authors (Atkinson et al. 2012; Bae et al. 2015) found clear evidence of a threshold in the relationship between ozone and mortality at very similar levels of 40 ppb and 65 μg/m3, respectively. The use of the combined oxidative potential of both gases seems to solve the problem of the threshold of ozone effects since low or zero ozone concentrations in urban areas arise because the ozone is converted to NO2. Even so, the concentration–response function between ozone and mortality still requires further assessment also to take into account the collinearity between gases.
Finally, the results of cause-specific analyses offer interesting observations. Specific-cause mortality is not yet been exhaustively explored with combined gaseous oxidative potential. One possible explanation for the stronger effects on cerebrovascular and respiratory mortality when compared with cardiovascular mortality is that the brain and the lung are specific targets for O3 and NO2 oxidative potential or are more sensitive to the oxidative potential of these gases. In addition, the almost exclusive involvement of Ox in increasing cerebrovascular mortality (compared with PM), as well as the independent contribution of both Ox and PM to increase respiratory mortality, suggests the important and diverse role of oxidative potential of gases on human health. Also, a clear seasonality of the diseases could explain some of the results. While Ox has higher effects in the hot season, all specific causes of mortality are more frequent in the cold season: more people die in the winter from cardiac disease (von Klot et al. 2012). Seasonal differences in stroke incidence are conflicting and might depend on stroke subtype rather than on season itself (Takizawa et al. 2013). Respiratory mortality is clearly influenced by the cold season: a linear relation between influenza incidence and excess mortality has been demonstrated also considering changes in mortality baselines (Goldstein et al. 2012).
There are possible limits of our analysis: first, the effect modifiers could have not been adequately addressed given that adequate evidence supports ozone-related effects for conditions we did not analyze here, such as certain genotypes, pre-existing asthma or certain nutritional deficiencies, and age (Vinikoor-Imler et al. 2014). Second, other gaseous compounds not analyzed here such as polycyclic aromatic hydrocarbons and quinones also contribute to oxidative potential.
We can conclude that combined Ox is able to capture a combined effect of O3 and NO2 on mortality that could be more representative of the true situation than that given by either pollutant individually or the two-pollutant analysis. In addition, the use of Ox can avoid the uncertainty of the threshold level for ozone. The important role that oxidative potential shows in increasing cerebrovascular and respiratory mortality suggests that these systems could be a specific target for O3 and NO2 oxidative potential.
References
Atkinson RW, Yu D, Armstrong BG, Pattenden S, Wilkinson P, Doherty RM, Heal MR, Anderson HR (2012) Concentration–response function for ozone and daily mortality: results from five urban and five rural U.K. populations. Environ Health Perspect 120:1411–1417
Ayres JG, Borm P, Cassee FR, Castranova V, Donaldson K, Ghio A, Harrison MR, Hider R, Kelly F, Kooter IM (2008) Evaluating the toxicity of airborne particulate matter and nanoparticles by measuring oxidative stress potential—a workshop report and consensus statement. Inhal Toxicol 20:75–99
Bae S, Lim YH, Kashima S, Yorifuji T, Honda Y, Kim H, Hong YC (2015) Non-linear concentration–response relationships between ambient ozone and daily mortality. PLoS One 10(6):e0129423
Delfino RJ, Staimer N, Tjoa T, Gillen DL, Schauer JJ, Shafer MM (2013) Airway inflammation and oxidative potential of air pollutant particles in a pediatric asthma panel. J Expo Sci Environ Epidemiol 23:466–473
Faustini A, Rapp R, Forastiere F (2014) Nitrogen dioxide and mortality: review and meta-analysis of long-term studies. Eur Respir J 44:744–753
Goldstein E, Viboud C, Charu V, Lipsitch M (2012) Improving the estimation of influenza-related mortality over a seasonal baseline. Epidemiology 23:829–838
Gryparis A, Forsberg B, Katsouyanni K, Analitis A, Touloumi G, Schwartz J, Samoli E, Medina S, Anderson HR, Niciu EM, Wichmann HE, Kriz B, Kosnik M, Skorkovsky J, Vonk JM, Dörtbudak Z (2004) Acute effects of ozone on mortality from the “air pollution and health: a European approach” project. Am J Respir Crit Care Med 170:1080–1087
Ito K, Thurston GD, Silverman RA (2007) Characterization of PM2.5, gaseous pollutants, and meteorological interactions in the context of time-series health effects models. J Expo Sci Environ Epidemiol 17(Suppl 2):S45–S60
Kelly FJ (2003) Oxidative stress: its role in air pollution and adverse health effects. Occup Environ Med 60:612–616
Künzli N, Mudway IS, Götschi T, Shi T, Kelly FJ, Cook S, Burney P, Forsberg B, Gauderman JW, Hazenkamp ME, Heinrich J, Jarvis D, Norbäck D, Payo-Losa F, Poli A, Sunyer J, Borm PJA (2006) Comparison of oxidative properties, light absorbance, total and elemental mass concentration of ambient PM2.5 collected at 20 European sites. Environ Health Perspect 114:684–690
McCullagh P, Nelder JA (1989) Generalized linear models, 2nd edn. Chapman & Hall/CRC, London
Mills IC, Atkinson RW, Anderson HR, Maynard RL, Strachan DP (2016) Distinguishing the associations between daily mortality and hospital admissions and nitrogen dioxide from those of particulate matter: a systematic review and meta-analysis. BMJ Open 6(7):e010751
Møller P, Danielsen PH, Karottki DG, Jantzen K, Roursgaard M, Klingberg H, Jensen DM, Christophersen DV, Hemmingsen JG, Cao Y, Loft S (2014) Oxidative stress and inflammation generated DNA damage by exposure to air pollution particles. Mutat Res Rev Mutat Res 762:133–166
Pavlovic J, Holder AL, Yelverton TL (2015) Effects of aftermarket control technologies on gas and particle phase oxidative potential from diesel engine emissions. Environ Sci Technol 49:10544–10552
Rodríguez-Cotto RI, Ortiz-Martínez MG, Jiménez-Vélez BD (2015) Organic extracts from African dust storms stimulate oxidative stress and induce inflammatory responses in human lung cells through Nrf2 but not NF-κB. Environ Toxicol Pharmacol 39:845–856
Samoli E, Aga E, Touloumi G, Nisiotis K, Forsberg B, Lefranc A, Pekkanen J, Wojtyniak B, Schindler C, Niciu E, Brunstein R, Dodic Fikfak M, Schwartz J, Katsouyanni K (2006) Short-term effects of nitrogen dioxide on mortality: an analysis within the APHEA project. Eur Respir J 27:1129–1138
Schieber M, Chandel NS (2014) ROS function in redox signaling and oxidative stress. Curr Biol 24:R453–R462
Schwartz J (2016) The year of ozone. Am J Respir Crit Care Med 193:1077–1079
Stafoggia M, Forastiere F, Faustini A, Biggeri A, Bisanti L, Cadum E, Cernigliaro A, Mallone S, Pandolfi P, Serinelli M, Tessari R, Vigotti MA, Perucci CA, EpiAir Group (2010) Susceptibility factors to ozone-related mortality—a population-based case-crossover analysis. Am J Respir Crit Care Med 182:376–384
Steadman RG (1979) The assessment of sultriness. Part I: a temperature–humidity index based on human physiology and clothing science. J Appl Meteorol 18:861–873
Steenhof M, Janssen NA, Strak M, Hoek G, Gosens I, Mudway IS, Kelly FJ, Harrison MR, Pieters RHH, Cassee F, Brunekreef B (2014) Air pollution exposure affects circulating white blood cell counts in healthy subjects: the role of particle composition, oxidative potential and gaseous pollutants—the RAPTES project. Inhal Toxicol 26:141–165
Strak M, Janssen NA, Godri KJ, Goseus I, Mudway IS, Cassee FR, Lebret E, Kelly FJ, Harrison MR, Brunekreef B, Steenhof M, Hoek G (2012) Respiratory health effects of airborne particulate matter: the role of particle size, composition, and oxidative potential—the RAPTES project. Environ Health Perspect 120:1183–1189
Takizawa S, Shibata T, Takagi S, Kobayashi S, Japan Standard Stroke Registry Study Group (2013) Seasonal variation of stroke incidence in Japan for 35631 stroke patients in the Japanese standard stroke registry, 1998–2007. J Stroke Cerebrovasc Dis 22:36–41
Thurston GD, Kipen H, Annesi-Maesano I, Balmes J, Brook RD, Cromar K, Matteis SD, Forastiere F, Forsberg B, Frampton MW, Grigg J, Heederik D, Kelly FJ, Kuenzli N, Laumbach R, Peters A, Rajagopalan ST, Rich D, Ritz B, Samet JM, Sandstrom T, Sigsgaard T, Sunyer J, Brunekreef B (2017) A joint ERS/ATS policy statement: what constitutes an adverse health effect of air pollution? An analytical framework. Eur Respir J 11(49):1
Vinikoor-Imler LC, Owens EO, Nichols JL, Ross M, Brown JS, Sacks JD (2014) Evaluating potential response-modifying factors for associations between ozone and health outcomes: a weight-of-evidence approach. Environ Health Perspect 122:1166–1176
von Klot S, Zanobetti A, Schwartz J (2012) Influenza epidemics, seasonality, and the effects of cold weather on cardiac mortality. Environ Health 11:74
Weichenthal S, Lavigne E, Evans G, Pollitt K, Burnett RT (2016) Ambient PM2.5 and risk of emergency room visits for myocardial infarction: impact of regional PM2.5 oxidative potential: a case-crossover study. Environ Health 15:46
Williams ML, Atkinson RW, Anderson HR, Kelly FJ (2014) Associations between daily mortality in London and combined oxidant capacity, ozone and nitrogen dioxide. Air Qual Atmos Health 7:407–414
Winquist A, Kirrane E, Klein M, Strickland M, Darrow LA, Sarnat SE, Gass K, Mulholland J, Russell A, Tolbert P (2014) Joint effects of ambient air pollutants on pediatric asthma emergency department visits in Atlanta, 1998–2004. Epidemiology 25:666–673
Wood SN (2000) Modelling and smoothing parameter estimation with multiple quadratic penalties. J R Stat Soc Ser B Stat Methodol 62:413–428
Yang A, Wang M, Eeftens M, Beelen R, Dons E, Leseman DLAC, Brunekreef B, Cassee FR, Janssen NAH, Hoek G (2015) Spatial variation and land use regression modeling of the oxidative potential of fine particles. Environ Health Perspect 123:1187–1192
Yang A, Janssen NA, Brunekreef B, Cassee FR, Hoek G, Gehring U (2016) Children’s respiratory health and oxidative potential of PM2.5: the PIAMA birth cohort study. Occup Environ Med 73:154–160
Zeka A, Schwartz J (2004) Estimating the independent effects of multiple pollutants in the presence of measurement error: an application of a measurement-error-resistant technique. Environ Health Perspect 112:1686–1690
Acknowledgements
We thank Margaret Becker for revising the English and Matteo Scortichini for his help with Fig. 4.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Faustini, A., Stafoggia, M., Williams, M. et al. The effect of short-term exposure to O3, NO2, and their combined oxidative potential on mortality in Rome. Air Qual Atmos Health 12, 561–571 (2019). https://doi.org/10.1007/s11869-019-00673-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11869-019-00673-0