Opinion Statement
Navigating the complex landscape of breast cancer treatment involves distinct strategies for luminal and triple-negative subtypes. While neoadjuvant chemotherapy historically dominates the approach for aggressive triple-negative tumors, recent evidence highlights the transformative impact of immunotherapy, alongside chemotherapy, in reshaping treatment paradigms. In luminal cancers, endocrine therapy, notably aromatase inhibitors, demonstrates promising outcomes in postmenopausal patients with low-grade luminal A tumors. However, integrating targeted therapies like CDK4/6 inhibitors in neoadjuvant setting remains inconclusive. Identifying predictive factors for treatment response, especially in luminal tumors, poses a challenge, emphasizing the necessity for ongoing research. A multidisciplinary approach, tailored to individual patient profiles, is crucial for maximizing efficacy while minimizing toxicity. As we strive to optimize breast cancer management, a comprehensive understanding of the distinct characteristics and treatment implications of luminal and triple-negative subtypes, including the transformative role of immunotherapy, is essential for informed decision-making and personalized care.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the past two decades, neoadjuvant chemotherapy has become a cornerstone in the management of early-stage breast cancer. Its primary objectives include enabling surgical intervention in cases of inoperable disease, then enhancing the feasibility of breast-conserving surgery even in operable tumors, at the cost of a heightened risk of local recurrence [1, 2]. Notably, there is no demonstrable survival advantage to administering the same chemotherapy before surgery compared to postoperative administration [2]. Furthermore, recent years have witnessed the neoadjuvant approach serving as a platform for evaluating novel therapeutics and predictive/prognostic biomarkers. This strategy has also facilitated the development and implementation of personalized treatment strategies, allowing for both therapeutic escalation and de-escalation based on individual response patterns. This review sequentially addresses the role and perspectives of neoadjuvant therapy in patients with early TNBC, emphasizing its significant impact. Additionally, it examines the nuanced considerations surrounding HER2-negative luminal tumors, where therapeutic decision-making is more intricate and may involve endocrine therapy in conjunction with or independently of neoadjuvant chemotherapy.
Role of Neoadjuvant Therapy
Breast Conservation
Neoadjuvant chemotherapy was initially employed in locally advanced inoperable BC. Subsequent investigations have underscored its value in augmenting rates of breast conservation, and yielding superior cosmetic outcomes [3]. In the NSABP B18 trial [1], 4 cycles of neoadjuvant AC as opposed to the same in adjuvant setting resulted in an increase in breast conservation rates from 59.8% to 67.8%. These findings are supported by a meta-analysis conducted by the EBCTCG, which synthesized individual data from 4756 patients from 10 randomized trials [2]. The meta-analysis revealed a breast conservation rate of 64.8% in the neoadjuvant arm compared to 49% in the adjuvant arm. However, this enhancement in breast conservation comes at the expense of a heightened risk of local recurrence, which escalates from 15.9% to 21.4%.
Survival: Data From Major NAC Trials
In the U.S. NSABP B18 study, [4] 1523 patients underwent randomization between primary surgery followed by 4 cycles of AC and the same chemotherapy administered before surgery. HER2 status was not tested at the time, and 57% of the tumors were HR-positive (10% with unknown HR status). The pCR rate was 13% (yT0/is N0). However, no significant difference in survival was observed at the 16-year mark. Among patients achieving a pCR, RFS and OS at 9 years stood at 85% and 75%, respectively. In the NSABP B27 trial [4], encompassing 2411 patients with operable BC, randomization occurred between three arms: 4 cycles of AC followed by surgery (804 patients), 4 cycles of AC followed by 4 cycles of docetaxel and then surgery (805 patients), and 4 cycles of AC followed by surgery followed by 4 cycles of docetaxel. The pCR rate was 14% in the AC-alone arm (1492 patients), with 10% showing no tumor and 4% demonstrating the persistence of carcinoma in situ. In contrast, the docetaxel group exhibited a higher pCR rate of 26%, comprising 19% with no tumor and 7% with carcinoma in situ (p < 0.001). Updated published data indicate that the addition of docetaxel did not significantly enhance DFS or OS. The key takeaway from these studies is that pCR emerges as a prognostic factor associated with increased DFS and OS at 5 and 9 years. The EBCTCG meta-analysis, incorporating individual data from 4756 patients across 10 trials, also indicates no survival advantage for administering the same chemotherapy before surgery rather than after [2]. The distant recurrence rate increased to 38.2% compared to 38%, and overall mortality reached 40.9% compared to 41.2% with neoadjuvant and adjuvant treatments, respectively.
Optimization of Neoadjuvant Treatment: Triple-Negative Breast Cancer
Neoadjuvant chemotherapy stands as the cornerstone in the medical management of TNBC exceeding 2 cm or displaying positive lymph nodes. However, primary surgery holds a preference in specific scenarios. Definitive pathological staging proves beneficial for cT1N0 tumors, where potential alternatives include either no chemotherapy [5] or less intensive chemotherapy options [6, 7]. This consideration is particularly applicable in patients involving comorbidities, fertility preservation concerns, or other medical constraints causing a delay in the initiation of NAC.
TNBC exhibits heightened sensitivity to cytotoxic chemotherapy, boasting the highest pCR rates among all BC subtypes. Notably, this subtype establishes the most pronounced prognostic impact between achieving a pCR (and the RCB score) and enhanced survival [8, 9]. The RCB score's assessment of residual disease is highly prognostic, delineating 5-year RFS rates of 91%, 80%, 66%, and 28% for RCB 0, 1, 2, and 3, respectively. [10, 11] Post-neoadjuvant escalation treatments have demonstrated improved survival outcomes in cases with residual disease [12, 13]. We will delve into how immunotherapy in the neoadjuvant setting has fundamentally altered practices while concurrently posing an array of questions.
Von Minckwitz et al. scrutinized several prospective studies within the GBG, encompassing 7 trials with 3332 patients. Their analysis delved into the impact of diverse neoadjuvant therapeutic modalities across different subtypes. In TN tumors, the probability of achieving pCR appeared to correlate with cumulative doses of anthracyclines (≥ 300 mg/m2 doxorubicin or equivalent) and taxanes (≥ 400 mg/m2 docetaxel or equivalent), rather than the number of cycles administered [14]. However, despite the excellent prognosis associated with patients achieving pCR, the outlook for those with residual disease remains bleak, underscoring the necessity for ongoing investigations in this BC subtype.
Increasing Dose Density in Triple-Negative Breast Cancer
TNBC exhibit a notably high responsiveness to chemotherapy, with a more pronounced reduction in induced mortality risk observed in ER-negative tumors compared to their ER + counterparts [15]. The theoretical foundation for dose intensification lies in overcoming the development of resistant tumor clones [16] and adheres to Gompertzian kinetics, predicting tumor growth to be inversely proportional to size [17]. Intensified chemotherapy emerges as a viable strategy to elevate the pCR rate [18]. This can entail a reduction in the interval between cycles or the administration of sequential full-dose chemotherapy as opposed to concurrent reduced-dose regimens. Several research teams have proposed the potential benefits of augmenting the intensity of alkylating agents in TNBC [19,20,21]. The efficacy of intensified chemotherapy in the adjuvant setting for BC patients with lymph node involvement was investigated in a phase III trial, revealing survival benefits for this high-risk population [22]. Numerous meta-analyses of literature data underscore a survival advantage for TN tumors [23] and an enhanced pCR rate when this intensified strategy is employed preoperatively [24].
The most robust evidence stems from the analysis conducted by the EBCTCG, which compiled individual data from 37,298 patients across 26 randomized trials involving neoadjuvant or adjuvant anthracycline and taxane-based chemotherapy [25]. Notably, these trials display heterogeneity, especially concerning the definition of dose intensity. Among 10,004 patients, shortening the interval from 3 to 2 weeks with the same chemotherapy resulted in a relative reduction in the risk of recurrence by 17% and specific mortality by 14%. For 11,028 patients, offering sequential chemotherapy every 3 weeks (permitting dose escalation) versus concomitant chemotherapy yielded a relative reduction in the risk of recurrence by 13% and specific mortality by 11%. Across the entire cohort of 37,298 patients, dose-dense chemotherapy translated into a 10-year absolute reduction in the risk of recurrence by 3.4% (a relative risk reduction of 14%) and an absolute reduction in the risk of all-cause death by 2.7% (a relative risk reduction of 13%). No discernible differences were observed based on hormone receptor expression. The relative benefits of the dose-dense regimen on recurrence were 14% and 15%, and on specific mortality were 13% and 14% in ER + and ER-negative populations, respectively. It is noteworthy that the HER2 status was rarely known, and if known, trastuzumab was not yet available. Consequently, a certain proportion of ER-negative patients did not fall into the TN category.
Platinum-Based Chemotherapy
The prevalence of TNBC in women with a BRCA1 mutation, the multitude of molecular alterations in TN tumors, and the histopathological similarities shared between TNBC and BRCA1-mutated BC (such as deficiencies in DNA repair systems and disruptions of homologous recombination) have sparked interest in platinum salts for this subtype [26]. Initial small retrospective series indicated the potential effectiveness of platinum salts [27, 28]. Encouraging results emerged from various phase II trials conducted by Spanish [29], German [30], and North American [31] groups. However, these trials highlighted increased toxicities, particularly hematological issues, and more frequent treatment interruptions in patients receiving platinum salts.
The phase III BrighTNess trial, which randomized 634 patients with TNBC into 3 arms (paclitaxel-carboplatin-veliparib, paclitaxel-carboplatin, and paclitaxel alone, followed by doxorubicin-cyclophosphamide for all), demonstrated that carboplatin, with or without veliparib, significantly increased the pCR rate (53% and 58%, compared to 31%) [32]. Updated data with a 4.5-year follow-up revealed that carboplatin had a substantial impact on RFS: 78% versus 68% (HR 0.57, 95% CI = 0.36–0.91; p = 0.02) [33]. Overall, in most studies, the addition of platinum salts to anthracycline and taxane-based neoadjuvant chemotherapy significantly enhanced pCR rates in TNBC [34, 35].
A noteworthy phase III study presented at San Antonio Breast Cancer Symposium in 2022 demonstrated that the benefit of carboplatin on pCR is primarily observed in patients under 50 years old [36]. This advantage extended to RFS and OS, again specifically for patients under 50 years old. A comprehensive meta-analysis incorporating 9 randomized trials with 2,109 patients indicated that the addition of carboplatin increased pCR from 37 to 52%. [37] Updated survival data from 6 of these randomized trials revealed that adding carboplatin to standard chemotherapy significantly increased RFS (HR 0.70, 95% CI = 0.56–0.89) and showed a non-significant 18% reduction in all-cause mortality (HR 0.92, 95% CI = 0.64–1.04) [38]. The use of platinum salts has gradually become a standard neoadjuvant treatment for TNBC, with the potential for more pronounced benefits in younger patients. The Keynote-522 trial, presented in the section on immunotherapy, has significantly contributed to integrating the use of platinum salts in the neoadjuvant setting into common practice.
PARP inhibitors
TNBC more commonly display homologous recombination deficiencies compared to other BC. A deeper comprehension of the biology of TNBC has unveiled new therapeutic targets, such as Poly-ADP Ribose Polymerase (PARP) inhibitors [39, 40]. In patients with BC, particularly those with BRCA1/2 mutations (primarily TNBC) [41], PARP inhibition compromises DNA repair, leading to cell death [21, 42]. Many TNBC belong to the "molecular basal" subtype, sharing characteristics with BRCA1-associated cancers, notably DNA repair deficiency. Various studies, outlined in Supplementary Table S1, have explored the role of PARP inhibitors in the neoadjuvant setting, yielding diverse outcomes.
The phase III BrighTNess trial, as mentioned earlier, underscores that the impact of the carboplatin-veliparib combination is solely driven by carboplatin, with no discernible contribution from veliparib on pCR or survival [32]. Additionally, talazoparib monotherapy underwent a non-comparative phase II trial: NeoTALA investigated the efficacy of 6 months of talazoparib monotherapy in 61 patients with a germline BRCA mutation and operable TNBC larger than 1 cm. [43] The pCR rate proved significant, reaching 45.8% in the evaluable population, comparable to conventional anthracycline and taxane-based chemotherapy [44]. It is noteworthy that 10 patients progressed during neoadjuvant treatment and subsequently switched to chemotherapy. Olaparib was pitted against carboplatin in the phase II GeparOLA trial, comparing the two in combination with paclitaxel, followed by EC [45]. Among 107 patients, 77 of them with TN tumors, harboring germline BRCA mutations or tumors exhibiting homologous recombination deficiency or somatic BRCA mutations, the pCR rate was 55.1% with olaparib compared to 48.6% with carboplatin. After 49.8 months of follow-up, the 4-year DFS rate was 76% with olaparib versus 88.5% with carboplatin. It is crucial to note the small sample size and the study's non-design for survival analyses. Niraparib is also under investigation in the neoadjuvant setting with promising results [46]. Despite these positive developments, the current evidence level is insufficient to recommend the use of PARP inhibitors in the neoadjuvant setting outside clinical trials.
Immunotherapy
Recent technological advances, particularly in "omics" sciences, have significantly enhanced our understanding of the tumor microenvironment's heterogeneity in TNBC [47, 48]. This heightened comprehension of the interplay between cancer cells and the immune system has paved the way for innovative therapeutic approaches [49, 50]. The landscape of oncology, on a broader scale, has witnessed an expansion, prominently in recent years with immune checkpoint inhibitors (ICI). A subset of TNBC exhibits PDL-1 expression on both the tumor and tumor-infiltrating lymphocytes (TILs), [51] indicating a higher mutational burden compared to other BC subtypes [52]. Pembrolizumab and atezolizumab have undergone investigation in advanced-stage TNBC. In monotherapy for advanced-stage cases, the response rate varies from 5 to 20%, contingent on PDL-1 expression [53, 54]. Several comparative trials have explored the potential of ICI in the neoadjuvant setting for TNBC [55,56,57,58].
Notably, in the case of durvalumab studied in GeparNuevo, it is intriguing to observe that, after 43.7 months of follow-up, there is a significant improvement in 3-year survival parameters (iDFS 85.6% versus 77.2%, HR 0.48, 95% CI = 0.24–0.97; OS 95.2% versus 83.5%, 95% CI = 0.08–0.72). This observation holds significance, even though the pCR rate did not see a significant increase (absolute difference of 9%, p = 0.287), and durvalumab was not continued post-surgery [59]. Two phase III trials have delved into this area. The IMpassion031 phase III trial randomized 333 patients between atezolizumab or placebo, added to a sequential chemotherapy of 12 weekly nab-paclitaxel followed by 4 cycles of dose-dense AC60 [56]. Atezolizumab was continued for up to one year. The addition of this anti-PD-L1 significantly elevated the pCR rate by 17% (41% versus 58%, p = 0.004). Unlike the metastatic context, the PD-L1 status did not appear predictive of a response. The most recent updated survival data presented at ESMO Breast 2023, after 40 months of follow-up, show no significant advantage in favor of the atezolizumab arm for DFS (HR 0.76, 95% CI = 0.44–1.21) and OS (HR 0.56, 95% CI = 0.30–1.04).
The Keynote-522 phase III trial encompassed 1174 patients with stage II or III BC, randomized in a 2:1 ratio to receive, in the neoadjuvant setting, immunotherapy with pembrolizumab or placebo with a sequential combination of carboplatin-paclitaxel followed by AC or EC every 3 weeks. [57] Patients continued pembrolizumab or placebo post-surgery for 9 cycles. The addition of pembrolizumab to chemotherapy significantly increased the pCR rate by 13.6% (64.8% versus 51.2%, p = 0.00055) and the 3-year DFS by 7.7% (84.5% versus 76.8%, HR 0.63, 95% CI = 0.48–0.82; p < 0.001) [60]. The sponsor announced in May 2024 that a significant improvement in overall survival was demonstrated at a pre-specified interim analysis.
Perspectives
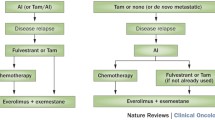
These immunotherapy approaches have been integrated into clinical practice, particularly following the outcomes of Keynote-522, yet numerous inquiries linger. Given the potential for enduring toxicities associated with immunotherapy, it is imperative to discern which patients derive the greatest benefits and who might circumvent potential consequences. Notably, the absolute benefit in terms of pCR is more pronounced in cases of lymph node involvement (Keynote-522: 20.6% versus 6.2%, and IMpassion031: 26% versus 9% for N + and N0 patients, respectively) [56, 57]. Strategies for de-escalation could be contemplated for small N0 tumors or adaptive approaches involving immunotherapy supplementation in the absence of response during neoadjuvant chemotherapy monitoring. However, predictive markers of response, such as PD-L1 status, the presence of tumor-infiltrating lymphocytes, or genomic signatures, have thus far fallen short in accurately discriminating patients necessitating immunotherapy [56, 59, 61].
Another pivotal question pertains to post-neoadjuvant treatment. In the Keynote-522 and IMpassion031 studies, immunotherapy was continued irrespective of achieving pCR. In Keynote-522, 3-year DFS was comparable in cases of pCR, whether patients received pembrolizumab or placebo (94.4% versus 92.5%), whereas 67.4% versus 56.8% in case of residual disease (pembrolizumab versus placebo, respectively) [60]. In exploratory analyses, most of the benefits are driven by the RCB-2 subgroup (HR for EFS 0.52) [62]. The utility of maintaining adjuvant immunotherapy in the event of pCR is therefore a subject of debate, considering potential toxicities that have been described even after several months of treatment. Similarly, long-term data from the GeparNuevo study, where patients did not receive durvalumab after surgery, suggest that adjuvant immunotherapy may not be imperative in case of pCR [59]. The randomized non-inferiority phase III trial OptimICE-pCR (NCT05812807) is evaluating the omission of postneoadjuvant pembrolizumab in patients achieving pCR (with a noninferiority margin of 3% in 3y-RFS rate).
The anti-TROP2 conjugated antibody sacituzumab-govitecan has demonstrated its efficacy in the metastatic setting of TNBC [63]. Ongoing studies aim to assess the potential of this drug in the neoadjuvant setting (NeoSTAR, NCT04230109) and post-neoadjuvant settings in adaptive strategies based on the presence of residual disease (SASCIA, NCT04595565, and ASPRIA, NCT04434040). With residual invasive disease after NAC, other escalation studies are ongoing. ASCENT05/OptimICE-RD (NCT05633654) evaluates the efficacy of sacituzumab-govitecan plus pembrolizumab versus pembrolizumab ± capecitabine. Datopotamab-deruxtecan is another TROP2 IgG1 attached to a topoisomerase inhibitor, evaluated in TROPION-Breast-03 (NCT0562958) with or without durvalumab versus treatment of physician’s choice in cases of residual disease.
Finally, de-escalation strategies seeking to minimize the use of anthracyclines are under investigation, such as the phase II NeoStop, which compared carboplatin docetaxel to a standard regimen including anthracyclines, with promising results (comparable efficacy with less toxicity), or the phase II NeoPACT (NCT03639948) evaluating pembrolizumab in an anthracycline-free regimen.
Optimization of Neoadjuvant Treatment: Her2-Negative Luminal Breast Cancer
The sensitivity of luminal BC to chemotherapy is comparatively lower than that observed in cases with HER2 amplification or the basal-like subtype [64]. The indication for NAC in this population is not as well-established. Common indications encompass initially inoperable tumors (presenting with inflammation, T4 or N3 stage, or extensive N2 involvement), cases where surgery needs postponement, or situations where immediate breast-conserving surgery is unfeasible due to the tumor-to-breast size ratio. pCR rates in luminal cancers generally range from less than 10% to less than 30% [65], with a high level of ER expression considered a negative predictive factor for chemotherapy response [66]. Reassessment of pCR rates after NAC has been conducted based on subtype, distinguishing between luminal A and luminal B [8, 9, 67]. Despite lower pCR rates, luminal BC, especially in the first 5 years, exhibit a more favorable prognosis compared to other subtypes.
For luminal BC, especially lobular and low-grade luminal cancers, the individual prognostic value of pCR is considerably lower [8, 9]. In contrast to HER2-positive or TN cancers, the choice of adjuvant treatment for luminal cancers is not guided by the quality of histological response; it invariably involves endocrine therapy (ET). Evaluation tools, such as the RCB, have been developed to better consider the impact of NAC in this context. RCB incorporates classical histological response elements (infiltrating residue, lymph node involvement) with quantitative aspects like residual cellularity and the size of metastatic lymph nodes [10, 68]. RCB proves invaluable in assessing potential chemotherapy benefits even in the absence of a complete response. For luminal cancers, a more specific chemotherapy response score is the CPS EG score, developed by the MD Anderson Cancer Center team. This score amalgamates initial clinical stage ("C"), pathological stage after chemotherapy ("PS"), and biological elements at the end of treatment, including ER expression ("E") and nuclear grade ("G") [69]. Seven classes (ranging from 0 to 6 points) are defined to specify post-neoadjuvant chemotherapy prognosis.
Chemotherapy and Histological Response
Around 20% of tumors in the Cortazar meta-analysis were HR + /HER2-, encompassing 2616 patients. Similar to TN or HER2-positive tumors, a noteworthy correlation has been established between pCR and survival parameters [9]. However, this correlation is less pronounced, primarily attributed to more proliferative tumors. The pCR of grade 3 HR + tumors exhibits a stronger correlation with survival parameters compared to grade 1 or 2 tumors: an HR of 0.29 (95% CI = 0.13–0.65) versus an HR of 0.47 (95% CI = 0.21–1.07) respectively [9]. An RCB 0-I score is achieved in 21.9% of cases, contrasting with 55 to 80% in other tumor subtypes [11]. Nevertheless, RCB stands out as an independent prognostic factor for RFS: each one-point increase in the RCB score multiplies the risk of relapse by 1.55, compared to 2.16 for TN and 2.09 for HER2 amplification [11]. The pCR rate for grade 3 tumors was 16.2%, in contrast to 7.5% for grade 1 or 2 tumors. Similarly, the histological type was correlated with pCR, with rates of 15.5% and 7.8% for ductal and lobular tumors respectively. The pCR rate with NAC for luminal A tumors is estimated between 7.5% and 8.9%, compared to 15% for luminal B tumors without HER2 amplification [8, 67]. Apart from the indisputable indication for locally advanced or inflammatory tumors, the benefit of NAC remains unclear for HR + tumors without HER2 amplification. The sole identified benefit is the objective of tumor reduction to facilitate conservative treatment, posing challenges in identifying predictive factors for response.
Predictive Factors for Response
Low expression (< 50%) of the ER is linked to a higher pCR rate but correlates with a less favorable long-term prognosis [66, 70]. Likewise, the absence of progesterone receptor expression serves as an independent predictive factor for pCR in multivariate analysis (OR = 0.76, p < 0.001) and represents an unfavorable and independent prognostic factor for RFS (HR 1.58, 95% CI = 1.306–1.912; p < 0.001) and OS (HR 1.80, 95% CI = 1.406–2.308; p < 0.001) [70]. This is also applicable to Ki-67. In the GeparTrio trial, pCR rates were 3.4%, 8.2%, and 18.5% for Ki-67 thresholds < 15%, between 15 and 35%, and > 35%, respectively [71].
In the adjuvant setting, recent years have witnessed the integration of molecular signatures as a decision-making tool for chemotherapy indication, particularly in a de-escalation strategy. The adjuvant RxPONDER study failed to demonstrate the benefits of adjuvant chemotherapy for postmenopausal patients with 1 to 3 invaded lymph nodes and a Recurrence Score (RS) ≤ 25 [72]. For such patients, justifying NAC with the aim of "downstaging" becomes challenging. This is where neoadjuvant ET could prove beneficial. The role of molecular signatures in the neoadjuvant setting is not firmly established and should not be recommended in routine practice today. However, consistent data indicate better responses to NAC with a high RS [73, 74]. Retrospective data suggest that neoadjuvant ET achieves superior response rates in cases of low or intermediate RS compared to high RS [75,76,77]. This information could aid in the choice between primary ET and NAC in cases requiring downstaging or form the basis for future adaptive strategies allowing therapeutic de-escalation in selected patients. An example is the WSG-ADAPT-HR + /HER2- study, where the overlay of the response at 3 weeks of initial ET based on the variation of Ki-67 and the initial RS identified patients with lymph node involvement for whom ET alone was sufficient [78]. In the absence of robust data, the use of molecular signatures in the neoadjuvant setting is not recommended outside of clinical trials.
Chemotherapy Type
In the context of HR + /HER- tumors, NAC protocol selection adheres to principles akin to those in the adjuvant setting. Typically, the chosen treatment involves the sequential combination of anthracyclines-cyclophosphamide and taxanes.
A phase III trial investigated the benefit of a dose-dense regimen in the adjuvant setting for BC patients with lymph node involvement. It demonstrated that this high-risk population, including the ER + subpopulation, experiences improved survival with dose-dense therapy [22]. An analysis of individual data from 37,298 patients across 26 randomized trials by the EBCTCG, encompassing adjuvant and neoadjuvant chemotherapy, revealed no discrepancy in the benefits of a dose-dense regimen between ER + and ER-negative populations [25]. The relative benefit on recurrence was 14% and 15%, and on specific mortality was 13% and 14%, in ER + and ER-negative populations, respectively. Consequently, this regimen is favored in this context.
In two randomized phase III trials, [79, 80] nab-paclitaxel was compared to paclitaxel in this population, yielding discordant results on pCR and showing no impact on survival [81]. Similar to TN tumors, investigation into immunotherapy with ICI is ongoing. The I-SPY2 study demonstrated that adding pembrolizumab could double the pCR rate. Initial results from the phase III Keynote-756 (NCT03725059) indicate that adding pembrolizumab to a standard chemotherapy sequence enhances the pCR rate (24.3% vs. 15.6%) in patients with grade 3, N + , or T3/T4 ER + /HER2- tumors [82]. Nivolumab is also under scrutiny in the phase III Checkmate-7FL trial (NCT04109066) and initial results are showing improvement in pCR rate from 13.8% to 24.5%, with nivolumab effect increasing with PD-L1 expression [83]. However, the relevance of this endpoint in ER + BC does not permit a definitive assessment of the strategy's value, and survival data are eagerly awaited. We must consider potential severe and/or lasting toxicities in this curative intent therapy and the recent integration of CDK4/6 inhibitors in adjuvant setting for high-risk early ER + BC. New molecular signatures for immunotherapy response, derived from the I-SPY-2 study, are currently under investigation [84]. As previously mentioned, trastuzumab deruxtecan has demonstrated efficacy in metastatic setting for the HER2low population. The ongoing phase II trial TRIO-US B-12 TALENT (NCT04553770) is randomizing trastuzumab deruxtecan ± ET. Initial results suggest clinical activity in this population, but there is some disappointment regarding pCR [85].
Neoadjuvant Endocrine Therapy
The objective of neoadjuvant ET aligns with that of chemotherapy. While ET has demonstrated its efficacy in ER + BC, its utilization in the neoadjuvant setting is less prevalent compared to chemotherapy, likely attributed to its relatively slower onset of action. Numerous studies have affirmed the clinical effectiveness of ET in ER + breast cancers, employing agents such as tamoxifen or aromatase inhibitors (AI) [64, 86]. These therapies yield response rates akin to chemotherapy but with a more favorable toxicity profile. In aggregate, neoadjuvant ET demonstrates a comparable increase in the rate of breast conservation to chemotherapy, contingent upon the duration of treatment being sufficiently extended (at least 16 weeks). Originally proposed primarily for elderly patients ineligible for chemotherapy or primary surgery, [87] this approach has proven valuable in achieving successful outcomes.
Type of Endocrine Therapy
In the neoadjuvant context, 5 randomized phase III studies in postmenopausal patients, [88,89,90,91,92] detailed in Supplementary Table S2, have compared aromatase inhibitors (AI) with tamoxifen. A meta-analysis of data from these studies, encompassing 1345 patients, reveals a significantly increased clinical response rate under AI (OR = 1.9, p = 0.009). Similarly, ultrasonographic response rates are increased (OR = 1.54, p = 0.001) (OR = 1.62, p < 0.001) [64, 93]. While a non-significant trend toward improved histological response is noted [64].
The IMPACT study, comparing tamoxifen and anastrozole and analyzing Ki67 expression variations, found no association between Ki67 and clinical or ultrasonographic response [89, 94]. However, post-treatment Ki67 values exhibited prognostic significance [95]. Building upon these findings, the Preoperative Endocrine Prognostic Index (PEPI) was developed, integrating histological (size, lymph node status) and biological (RE and Ki67 expression levels post-treatment) tumor characteristics after neoadjuvant ET. The PEPI score, derived from the P024 study comparing letrozole and tamoxifen for 16 weeks in the neoadjuvant setting, [96] assigns points to each variable, leading to a highly prognostic classification into 3 classes. Prospective validation is underway through the phase III ALTERNATE study, with extended follow-up [97].
Two studies in postmenopausal patients compared an AI, anastrozole, and fulvestrant, [98, 99] revealing no notable clinical differences or variance in breast conservation rates. The ACOSOG Z1031 study, comparing three AIs, showed no clinical differences but confirmed a substantial impact on proliferation, with a reduction in Ki67 in both luminal A and B tumors [100]. Notably, Ki-67 emerges as a prognostic marker during neoadjuvant treatment, predicting recurrence risk if Ki-67 remains > 10% after 2–4 weeks of neoadjuvant ET. The POETIC study underscores Ki-67's prognostic potential after 2 weeks of neoadjuvant AI, particularly in the HER2-negative population. A high-risk group for recurrence can be identified: if Ki-67 remains high (> 10%) after 2 weeks of AI, the 5-year recurrence risk is 21.5%, compared to 8.5% if Ki-67 transitions from high to low (< 10%) or 4.3%, if Ki-67 is initially low (< 10%) [101].
Exploring ET in premenopausal patients, a randomized study with 197 participants compared anastrozole versus tamoxifen, both combined with goserelin [92]. After 6 months of goserelin-anastrozole treatment, clinical response rates reached 70%, ultrasonographic response rates 58%, and MRI response rates 64%, surpassing outcomes with the tamoxifen-goserelin combination. Ki67 variations were more pronounced in the anastrozole arm [77].
Comparison of Endocrine Therapy and Chemotherapy
Comparative studies between NAC and neoadjuvant ET have been conducted, with 3 randomized trials elucidated in Supplementary Table S3. These trials encompassed both premenopausal and postmenopausal patients, incorporating varying durations of ET ranging from 3 to 6 months [102,103,104].
In the feasibility study NeoCENT [104], which involved 44 randomized patients, the comparison of 6 cycles of FEC100 with letrozole (administered for 4.5 to 5.7 months) revealed a comparable rate of objective response (54% versus 59%, respectively), similar variations in Ki67, and an identical rate of breast conservation.
The comprehensive analysis of these 3 trials indicates no discernible difference between NAC and ET concerning clinical, radiological, and biological response in luminal BC. A meta-analysis, pooling data from 378 patients, [64] evaluated various odds ratios related to clinical response (OR 1.08, 95% CI = 0.50–2.35; p = 0.85), radiological response (OR 1.38, 95% CI = 0.92–2.07; p = 0.12), pCR (OR 1.99, 95% CI = 0.62–6.39; p = 0.25), and breast conservation (OR 0.65, 95% CI = 0.41–1.03; p = 0.07). This meta-analysis affirms the absence of any discernible benefit of neoadjuvant chemotherapy compared to neoadjuvant ET in patients with luminal BC.
Duration of Neoadjuvant Endocrine Therapy
The principal investigations into extending neoadjuvant ET are outlined in Table 1.
Notably, pivotal trials in neoadjuvant ET have scrutinized preoperative treatment durations ranging from 3 to 4 months. [64] Emerging evidence indicates that the extension of treatment duration may lead to a heightened reduction in tumor volume. Substantiating these observations is a phase II trial involving 70 elderly patients: the median time to objective response was 3.9 months, and more than a third of responsive patients achieved maximal tumor volume reduction following a treatment duration of at least 6 months [107].
Endocrine and Targeted Therapies
Various types of targeted therapies have undergone evaluation subsequent to findings in advanced phases, particularly in randomized phase II trials. These therapies include those targeting the mTOR pathway (everolimus), [113]growth factor pathways (gefitinib) [114], and the cell cycle (palbociclib, ribociclib, abemaciclib). Regrettably, the overall outcomes have been disappointing. In a trial comparing letrozole to a letrozole-everolimus combination, a more significant clinical response was observed in the everolimus arm, accompanied by a reduction in ER and cyclin D1 expression [113]. However, these trials do not reveal an increase in the pCR rate with targeted therapy, which typically remains below 10%, nor do they show consistent impacts on breast conservation rates, which are inconsistently evaluated and reported. The most intriguing findings from these studies pertain to biological aspects. The addition of everolimus to letrozole leads to a highly significant (90%) reduction in Ki67 expression after 16 weeks of treatment, particularly in patients with tumors harboring a mutation in exon 9 of PIK3CA, while the variation is minimal in cases of mutations in exon 20 or the wild-type form [113]. Similarly, the incorporation of palbociclib, a CDK4/6 cell cycle kinase inhibitor, results in a rapid (as early as day 15) and profound (nearly 100%) decrease in Ki67 expression [115]. The key studies assessing the contribution of CDK4/6 inhibitors to neoadjuvant ET are summarized in Table 2.
The PALLET study, a phase II trial, randomized 307 patients between letrozole-palbociclib and letrozole alone. No significant differences were observed in terms of clinical response (54.3% vs. 49.5%) or progression during neoadjuvant ET (3.2% vs. 5%) [118]. In the NeoPAL study, another phase II trial, the letrozole-palbociclib combination was compared with conventional NAC (3 FEC100 followed by docetaxel) in 106 patients with luminal B or luminal A cancers with nodal involvement [117]. The study found no significant disparities in pCR rates (3.8% vs. 5.9%), histological response assessed by RCB 0-I (7.7% vs. 15.7%), clinical response (75%), or breast conservation rates (69%). Notably, a PEPI score of 0 was achieved in 17.6% in the ET arm versus 8.0% in the chemotherapy arm [117]. After 40 months of follow-up, survival data indicated comparable RFS, despite 43% of patients in the ET group not receiving adjuvant chemotherapy [122]. Similar studies on ribociclib [116, 120, 121] and abemaciclib [119] in the neoadjuvant setting have reported consistent results. However, the overall evidence suggests that the strategy of combining targeted therapies, particularly cell cycle inhibitors, with ET has not definitively demonstrated superiority over ET alone or chemotherapy. Consequently, routine clinical use is not currently supported [123].
Recovery Strategies
Capecitabine
Following a comparable post-neoadjuvant approach, the Japanese CREATE-X trial [12] randomly assigned 910 patients with non-HER2 amplified tumors and residual disease after neoadjuvant chemotherapy, including anthracyclines and taxanes, to receive either capecitabine for 6 months or a placebo. At the 5-year mark, capecitabine demonstrated a superior RFS (74.1% versus 67.6%, HR 0.70, p = 0.01) and OS (89.2% versus 83.6%, HR 0.59, p = 0.01). Notably, the OS benefit was not statistically significant in the HR + population (68%), but it was notable in patients with TN tumors (32%). The observed OS benefit in this subgroup was 78.8% versus 70.3% at 5 years (HR 0.52, 95% CI = 0.30–0.90). For residual disease after neoadjuvant chemotherapy in TNBC, the selection of post-neoadjuvant treatment remains open, given that immunotherapy was not a standard option when demonstrating the benefits of capecitabine. Capecitabine was not permitted in cases of residual disease in the Keynote-522 trial. Although not directly addressed, pembrolizumab exhibited superior 3y-RFS compared to a placebo in cases of residual disease (67.4% versus 56.8%) [60]. This means that pembrolizumab not only increased pCR rates but also improved EFS among non-responders, and it is therefore well-positioned to compete with capecitabine for this indication [62]. Currently, there is no evidence supporting the superiority of the pembrolizumab-capecitabine combination for residual disease. Reassuringly, phase II trials in the metastatic setting affirm its safety, with no emergence of new toxicity signals [124, 125].
Olaparib
Olaparib is approved for high risk TNBC and ER + BC in the scenario of a germline BRCA mutation. The phase III OlympiA trial, focusing on a high-risk recurrence setting in the BRCA-mutated population, particularly in TN tumors (82%), assessed the efficacy of 1 year of adjuvant olaparib [13]. Notably, capecitabine was excluded from OlympiA, and eligibility for patients who had undergone NAC was contingent on the presence of residual disease. Encouragingly, the 4-year results demonstrate significant outcomes in both RFS (87.5% versus 80.4%) and OS (89.8% versus 86.4%) [126].
When contemplating the optimal choice among pembrolizumab, capecitabine, and olaparib, or considering their concomitant or sequential use, current data suggest a potential preference for olaparib. The CREATE-X trial, which lacked sufficient BRCA-mutated patients, could not adequately assess the impact of capecitabine in this population, while OlympiA shows a survival benefit. The OlympiAD results in the metastatic setting can also be extrapolated with caution, where olaparib outperformed chemotherapy, including capecitabine for 45% of patients [127]. However, the olaparib-capecitabine combination appears overly toxic, particularly in terms of myelosuppression. While there is currently no evidence supporting the superiority of combining immunotherapy and PARP inhibitors in BC, early-phase trials in the metastatic setting have not revealed new toxicity signals. [128, 129]Therefore, the combination of pembrolizumab and olaparib is frequently encountered in clinical practice in cases of residual disease and constitutional BRCA mutation.
Conclusion
Neoadjuvant chemotherapy offers the prospect of enhancing breast conservation rates, albeit with an increased risk of local recurrence. In the realm of triple-negative breast cancers, the combination of anthracyclines and taxanes achieves a pCR rate of 25 to 40%. Dose dense chemotherapy schedules elevate this rate by 5%, significantly impacting survival, while platinum salts and immunotherapy each contribute to a 10 to 20% increase. Immune checkpoint inhibitors have demonstrated their survival impact in the neoadjuvant setting, particularly for younger patients, reshaping the treatment strategy. Open questions include those related to the duration of immunotherapy, choice of chemotherapy partner, and post-neoadjuvant strategies based on achieving complete response. Immunotherapy's potential to challenge the relevance of pCR as an surrogate endpoint for assessing survival in triple-negative breast cancers is a topic of ongoing exploration, and consensus on post-neoadjuvant treatment decisions in case of residual disease is lacking.
The decision-making process for a neoadjuvant treatment strategy for luminal tumors is complex, except for initially inoperable tumors. This strategy is recommended cautiously with the goal of preserving the breast when possible. Currently, no reliable predictive factors exist for neoadjuvant chemotherapy response in luminal tumors without HER2 amplification. Neoadjuvant chemotherapy's superiority over neoadjuvant endocrine therapy remains inconclusive in this population. In the case of luminal breast cancers ineligible for immediate conservative surgery, neoadjuvant endocrine therapy may be considered, particularly in postmenopausal women with low-grade luminal A breast cancer, ideally administered for over 6 months. Aromatase inhibitors in the postmenopausal population have demonstrated superiority in clinical and radiological response compared to tamoxifen. The combination of targeted therapies, such as CDK4/6 inhibitors with endocrine therapy, has not definitively proven its superiority. Biological markers and molecular analyses continue to be fundamental tools for evaluating response and understanding tumor biology in luminal cancers.
Key References
-
Geyer CE, Sikov WM, Huober J, et al. Long-term efficacy and safety of addition of carboplatin with or without veliparib to standard neoadjuvant chemotherapy in triple-negative breast cancer: 4-year follow-up data from BrighTNess, a randomized phase III trial. Ann Oncol. 2022;33(4):384-394. doi:10.1016/j.annonc.2022.01.009
-
In the BrighTNess trial, adding carboplatin to neoadjuvant chemotherapy for triple-negative breast cancer improved pathological complete response rates and showed long-term event-free survival benefit with manageable safety, supporting its use in early-stage TNBC.
-
-
Litton JK, Beck JT, Jones JM, et al. Neoadjuvant Talazoparib in Patients With Germline BRCA1/2 Mutation-Positive, Early-Stage Triple-Negative Breast Cancer: Results of a Phase II Study. Oncologist. Published online June 15, 2023:oyad139. doi:10.1093/oncolo/oyad139
-
In early-stage TNBC patients with germline BRCA mutations, neoadjuvant talazoparib monotherapy demonstrated activity with a pathologic complete response (pCR) rate of 45.8% (evaluable population) and 49.2% (intent-to-treat population), with manageable safety.
-
-
Schmid P, Cortes J, Dent R, et al. Event-free Survival with Pembrolizumab in Early Triple-Negative Breast Cancer. N Engl J Med. 2022;386(6):556-567. doi:10.1056/NEJMoa2112651
-
Neoadjuvant pembrolizumab plus chemotherapy, followed by adjuvant pembrolizumab after surgery, demonstrated significantly longer event-free survival compared to neoadjuvant chemotherapy alone in patients with early triple-negative breast cancer, with a median follow-up of 39.1 months.
-
Data Availability
No datasets were generated or analysed during the current study.
Abbreviations
- AC:
-
Doxorubicine + Cyclophosphamide
- AI:
-
Aromatase inhibitors
- BC:
-
Breast Cancer
- cCR:
-
Clinical Complete Response
- EBCTCG:
-
Early Breast Cancer Trialists' Collaborative Group
- EC:
-
Epirubicin and Cyclophosphamide
- ER:
-
Estrogen Receptors
- ET:
-
Endocrine therapy
- FEC:
-
5-Fluorouracil, epirubicin and cyclophosphamide
- GBG:
-
German Breast Group
- HR:
-
Hormone Receptors
- ICI:
-
Immune Checkpoints Inhibitors
- NAC:
-
Neoadjuvant chemotherapy
- OS:
-
Overall survival
- PARP:
-
Poly-ADP Ribose Polymerase
- PEPI:
-
Preoperative Endocrine Prognostic Index
- pCR:
-
Pathological Complete Response
- RCB:
-
Residual Cancer Burden
- RFS:
-
Recurrence-free survival
- RR:
-
Relative risk
- RS:
-
Recurrence Score
- RT:
-
Radiotherapy
- TN:
-
Triple-negative
- TNBC:
-
Triple-negative Breast Cancer
References and Recommended Reading
Fisher B, Bryant J, Wolmark N, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol. 2023;41(10):1795–808. https://doi.org/10.1200/JCO.22.02571.
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: meta-analysis of individual patient data from ten randomised trials. Lancet Oncol. 2018;19(1):27–39. https://doi.org/10.1016/S1470-2045(17)30777-5
Boughey JC, Peintinger F, Meric-Bernstam F, et al. Impact of preoperative versus postoperative chemotherapy on the extent and number of surgical procedures in patients treated in randomized clinical trials for breast cancer. Ann Surg. 2006;244(3):464–70. https://doi.org/10.1097/01.sla.0000234897.38950.5c.
Rastogi P, Anderson SJ, Bear HD, et al. Preoperative chemotherapy: updates of national surgical adjuvant breast and bowel project protocols B-18 and B-27. J Clin Oncol. 2008;26(5):778–85. https://doi.org/10.1200/JCO.2007.15.0235.
An X, Lei X, Huang R, et al. Adjuvant chemotherapy for small, lymph node-negative, triple-negative breast cancer: a single-center study and a meta-analysis of the published literature. Cancer. 2020;126(Suppl 16):3837–46. https://doi.org/10.1002/cncr.32878.
Jones S, Holmes FA, O’Shaughnessy J, et al. docetaxel with cyclophosphamide is associated with an overall survival benefit compared with doxorubicin and cyclophosphamide: 7-year follow-up of US oncology research trial 9735. J Clin Oncol. 2009;27(8):1177–83. https://doi.org/10.1200/JCO.2008.18.4028.
Blum JL, Flynn PJ, Yothers G, et al. anthracyclines in early breast cancer: the ABC Trials-USOR 06–090, NSABP B-46-I/USOR 07132, and NSABP B-49 (NRG Oncology). J Clin Oncol. 2017;35(23):2647–55. https://doi.org/10.1200/JCO.2016.71.4147.
von Minckwitz G, Untch M, Blohmer JU, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30(15):1796–804. https://doi.org/10.1200/JCO.2011.38.8595.
Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. The Lancet. 2014;384(9938):164–72. https://doi.org/10.1016/S0140-6736(13)62422-8.
Symmans WF, Wei C, Gould R, et al. Long-term prognostic risk after neoadjuvant chemotherapy associated with residual cancer burden and breast cancer subtype. J Clin Oncol. 2017;35(10):1049–60. https://doi.org/10.1200/JCO.2015.63.1010.
Yau C, Osdoit M, van der Noordaa M, et al. Residual cancer burden after neoadjuvant chemotherapy and long-term survival outcomes in breast cancer: a multicentre pooled analysis of 5161 patients. Lancet Oncol. 2022;23(1):149–60. https://doi.org/10.1016/S1470-2045(21)00589-1.
Masuda N, Lee SJ, Ohtani S, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med. 2017;376(22):2147–59. https://doi.org/10.1056/NEJMoa1612645.
Tutt ANJ, Garber JE, Kaufman B, et al. Adjuvant olaparib for patients with BRCA1- or BRCA2-mutated breast cancer. N Engl J Med. 2021;384(25):2394–405. https://doi.org/10.1056/NEJMoa2105215.
von Minckwitz G, Untch M, Nüesch E, et al. Impact of treatment characteristics on response of different breast cancer phenotypes: pooled analysis of the German neo-adjuvant chemotherapy trials. Breast Cancer Res Treat. 2011;125(1):145–56. https://doi.org/10.1007/s10549-010-1228-x.
Berry DA, Cirrincione C, Henderson IC, et al. Estrogen-receptor status and outcomes of modern chemotherapy for patients with node-positive breast cancer. JAMA. 2006;295(14):1658–67. https://doi.org/10.1001/jama.295.14.1658.
Coldman AJ, Goldie JH. Impact of dose-intense chemotherapy on the development of permanent drug resistance. Semin Oncol. 1987;14(4 Suppl 4):29–33.
Norton L. A Gompertzian model of human breast cancer growth. Cancer Res. 1988;48(24 Pt 1):7067–71.
Liedtke C, Rody A. New treatment strategies for patients with triple-negative breast cancer. Curr Opin Obstet Gynecol. 2015;27(1):77–84. https://doi.org/10.1097/GCO.0000000000000137.
Giacchetti S, Porcher R, Lehmann-Che J, et al. Long-term survival of advanced triple-negative breast cancers with a dose-intense cyclophosphamide/anthracycline neoadjuvant regimen. Br J Cancer. 2014;110(6):1413–9. https://doi.org/10.1038/bjc.2014.81.
Nieto Y, Shpall EJ. High-dose chemotherapy for high-risk primary and metastatic breast cancer: is another look warranted? Curr Opin Oncol. 2009;21(2):150–7. https://doi.org/10.1097/CCO.0b013e328324f48b.
Vollebergh MA, Lips EH, Nederlof PM, et al. An aCGH classifier derived from BRCA1-mutated breast cancer and benefit of high-dose platinum-based chemotherapy in HER2-negative breast cancer patients. Ann Oncol. 2011;22(7):1561–70. https://doi.org/10.1093/annonc/mdq624.
Del Mastro L, Poggio F, Blondeaux E, et al. Fluorouracil and dose-dense adjuvant chemotherapy in patients with early-stage breast cancer (GIM2): end-of-study results from a randomised, phase 3 trial. Lancet Oncol. 2022;23(12):1571–82. https://doi.org/10.1016/S1470-2045(22)00632-5.
Petrelli F, Cabiddu M, Coinu A, et al. Adjuvant dose-dense chemotherapy in breast cancer: a systematic review and meta-analysis of randomized trials. Breast Cancer Res Treat. 2015;151(2):251–9. https://doi.org/10.1007/s10549-015-3405-4.
Petrelli F, Coinu A, Lonati V, et al. Neoadjuvant dose-dense chemotherapy for locally advanced breast cancer: a meta-analysis of published studies. Anticancer Drugs. 2016;27(7):702–8. https://doi.org/10.1097/CAD.0000000000000369.
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Increasing the dose intensity of chemotherapy by more frequent administration or sequential scheduling: a patient-level meta-analysis of 37 298 women with early breast cancer in 26 randomised trials. Lancet. 2019;393(10179):1440–1452. https://doi.org/10.1016/S0140-6736(18)33137-4
Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010;363(20):1938–48. https://doi.org/10.1056/NEJMra1001389.
Chang HR, Glaspy J, Allison MA, et al. Differential response of triple-negative breast cancer to a docetaxel and carboplatin-based neoadjuvant treatment. Cancer. 2010;116(18):4227–37. https://doi.org/10.1002/cncr.25309.
Torrisi R, Balduzzi A, Ghisini R, et al. Tailored preoperative treatment of locally advanced triple negative (hormone receptor negative and HER2 negative) breast cancer with epirubicin, cisplatin, and infusional fluorouracil followed by weekly paclitaxel. Cancer Chemother Pharmacol. 2008;62(4):667–72. https://doi.org/10.1007/s00280-007-0652-z.
Alba E, Chacon JI, Lluch A, et al. A randomized phase II trial of platinum salts in basal-like breast cancer patients in the neoadjuvant setting. Results from the GEICAM/2006–03, multicenter study. Breast Cancer Res Treat. 2012;136(2):487–493. https://doi.org/10.1007/s10549-012-2100-y
von Minckwitz G, Schneeweiss A, Loibl S, et al. Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): a randomised phase 2 trial. Lancet Oncol. 2014;15(7):747–56. https://doi.org/10.1016/S1470-2045(14)70160-3.
Shepherd JH, Ballman K, Polley MYC, et al. CALGB 40603 (Alliance): long-term outcomes and genomic correlates of response and survival after neoadjuvant chemotherapy with or without carboplatin and bevacizumab in triple-negative breast cancer. J Clin Oncol. 2022;40(12):1323–34. https://doi.org/10.1200/JCO.21.01506.
Loibl S, O’Shaughnessy J, Untch M, et al. Addition of the PARP inhibitor veliparib plus carboplatin or carboplatin alone to standard neoadjuvant chemotherapy in triple-negative breast cancer (BrighTNess): a randomised, phase 3 trial. Lancet Oncol. 2018;19(4):497–509. https://doi.org/10.1016/S1470-2045(18)30111-6.
Geyer CE, Sikov WM, Huober J, et al. Long-term efficacy and safety of addition of carboplatin with or without veliparib to standard neoadjuvant chemotherapy in triple-negative breast cancer: 4-year follow-up data from BrighTNess, a randomized phase III trial. Ann Oncol. 2022;33(4):384–94. https://doi.org/10.1016/j.annonc.2022.01.009.
Petrelli F, Coinu A, Borgonovo K, et al. The value of platinum agents as neoadjuvant chemotherapy in triple-negative breast cancers: a systematic review and meta-analysis. Breast Cancer Res Treat. 2014;144(2):223–32. https://doi.org/10.1007/s10549-014-2876-z.
Guan X, Ma F, Fan Y, Zhu W, Hong R, Xu B. Platinum-based chemotherapy in triple-negative breast cancer: a systematic review and meta-analysis of randomized-controlled trials. Anticancer Drugs. 2015;26(8):894–901. https://doi.org/10.1097/CAD.0000000000000260.
Gupta S, Nair NS, Hawaldar R, et al. Abstract GS5–01: Addition of platinum to sequential taxane-anthracycline neoadjuvant chemotherapy in patients with triple-negative breast cancer: a phase III randomized controlled trial. Cancer Res. 2023;83(5_Supplement):GS5-01. https://doi.org/10.1158/1538-7445.SABCS22-GS5-01.
Poggio F, Bruzzone M, Ceppi M, et al. Platinum-based neoadjuvant chemotherapy in triple-negative breast cancer: a systematic review and meta-analysis. Ann Oncol. 2018;29(7):1497–508. https://doi.org/10.1093/annonc/mdy127.
Poggio F, Tagliamento M, Ceppi M, et al. Adding a platinum agent to neoadjuvant chemotherapy for triple-negative breast cancer: the end of the debate. Ann Oncol. 2022;33(3):347–9. https://doi.org/10.1016/j.annonc.2021.11.016.
Fong PC, Boss DS, Yap TA, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361(2):123–34. https://doi.org/10.1056/NEJMoa0900212.
Tutt A, Robson M, Garber JE, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet. 2010;376(9737):235–44. https://doi.org/10.1016/S0140-6736(10)60892-6.
Manié E, Vincent-Salomon A, Lehmann-Che J, et al. High frequency of TP53 mutation in BRCA1 and sporadic basal-like carcinomas but not in BRCA1 luminal breast tumors. Cancer Res. 2009;69(2):663–71. https://doi.org/10.1158/0008-5472.CAN-08-1560.
Andre F, Pusztai L. Molecular classification of breast cancer: implications for selection of adjuvant chemotherapy. Nat Clin Pract Oncol. 2006;3(11):621–32. https://doi.org/10.1038/ncponc0636.
Litton JK, Scoggins ME, Hess KR, et al. Neoadjuvant talazoparib for patients with operable breast cancer with a germline brca pathogenic variant. J Clin Oncol. 2020;38(5):388–94. https://doi.org/10.1200/JCO.19.01304.
Litton JK, Beck JT, Jones JM, et al. neoadjuvant talazoparib in patients with germline BRCA1/2 mutation-positive, early-stage triple-negative breast cancer: results of a phase ii study. Oncologist. Published online June 15, 2023:oyad139. https://doi.org/10.1093/oncolo/oyad139
Fasching PA, Link T, Hauke J, et al. Neoadjuvant paclitaxel/olaparib in comparison to paclitaxel/carboplatinum in patients with HER2-negative breast cancer and homologous recombination deficiency (GeparOLA study). Ann Oncol. 2021;32(1):49–57. https://doi.org/10.1016/j.annonc.2020.10.471.
Spring LM, Han H, Liu MC, et al. Neoadjuvant study of niraparib in patients with HER2-negative, BRCA-mutated, resectable breast cancer. Nat Cancer. 2022;3(8):927–31. https://doi.org/10.1038/s43018-022-00400-2.
Bianchini G, De Angelis C, Licata L, Gianni L. Treatment landscape of triple-negative breast cancer - expanded options, evolving needs. Nat Rev Clin Oncol. 2022;19(2):91–113. https://doi.org/10.1038/s41571-021-00565-2.
Bianchini G, Balko JM, Mayer IA, Sanders ME, Gianni L. Triple-negative breast cancer: challenges and opportunities of a heterogeneous disease. Nat Rev Clin Oncol. 2016;13(11):674–90. https://doi.org/10.1038/nrclinonc.2016.66.
Denkert C, von Minckwitz G, Darb-Esfahani S, et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018;19(1):40–50. https://doi.org/10.1016/S1470-2045(17)30904-X.
Loi S, Drubay D, Adams S, et al. Tumor-infiltrating lymphocytes and prognosis: a pooled individual patient analysis of early-stage triple-negative breast cancers. J Clin Oncol. 2019;37(7):559–69. https://doi.org/10.1200/JCO.18.01010.
Erber R, Hartmann A. Understanding PD-L1 testing in breast cancer: a practical approach. Breast Care (Basel). 2020;15(5):481–90. https://doi.org/10.1159/000510812.
O’Meara TA, Tolaney SM. Tumor mutational burden as a predictor of immunotherapy response in breast cancer. Oncotarget. 2021;12(5):394–400. https://doi.org/10.18632/oncotarget.27877
Adams S, Loi S, Toppmeyer D, et al. Pembrolizumab monotherapy for previously untreated, PD-L1-positive, metastatic triple-negative breast cancer: cohort B of the phase II KEYNOTE-086 study. Ann Oncol. 2019;30(3):405–11. https://doi.org/10.1093/annonc/mdy518.
Nanda R, Chow LQM, Dees EC, et al. pembrolizumab in patients with advanced triple-negative breast cancer: phase Ib KEYNOTE-012 study. J Clin Oncol. 2016;34(21):2460–7. https://doi.org/10.1200/JCO.2015.64.8931.
Loibl S, Untch M, Burchardi N, et al. A randomised phase II study investigating durvalumab in addition to an anthracycline taxane-based neoadjuvant therapy in early triple-negative breast cancer: clinical results and biomarker analysis of GeparNuevo study. Ann Oncol. 2019;30(8):1279–88. https://doi.org/10.1093/annonc/mdz158.
Mittendorf EA, Zhang H, Barrios CH, et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): a randomised, double-blind, phase 3 trial. Lancet. 2020;396(10257):1090–100. https://doi.org/10.1016/S0140-6736(20)31953-X.
Schmid P, Cortes J, Pusztai L, et al. Pembrolizumab for early triple-negative breast cancer. N Engl J Med. 2020;382(9):810–21. https://doi.org/10.1056/NEJMoa1910549.
Gianni L, Huang CS, Egle D, et al. Pathologic complete response (pCR) to neoadjuvant treatment with or without atezolizumab in triple-negative, early high-risk and locally advanced breast cancer: NeoTRIP Michelangelo randomized study. Ann Oncol. 2022;33(5):534–43. https://doi.org/10.1016/j.annonc.2022.02.004.
Loibl S, Schneeweiss A, Huober J, et al. Neoadjuvant durvalumab improves survival in early triple-negative breast cancer independent of pathological complete response. Ann Oncol. 2022;33(11):1149–58. https://doi.org/10.1016/j.annonc.2022.07.1940.
Schmid P, Cortes J, Dent R, et al. Event-free survival with pembrolizumab in early triple-negative breast cancer. N Engl J Med. 2022;386(6):556–67. https://doi.org/10.1056/NEJMoa2112651.
Loibl S, Sinn B, Karn T, et al. Abstract PD2–07: mRNA signatures predict response to durvalumab therapy in triple negative breast cancer (TNBC)– Results of the translational biomarker programme of the neoadjuvant double-blind placebo controlled GeparNuevo trial. Cancer Res. 2019;79(4_Supplement):PD2–07-PD2–07. https://doi.org/10.1158/1538-7445.SABCS18-PD2-07
Pusztai L, Denkert C, O’Shaughnessy J, et al. Event-free survival by residual cancer burden with pembrolizumab in early-stage TNBC: exploratory analysis from KEYNOTE-522. Ann Oncol. 2024;35(5):429–36. https://doi.org/10.1016/j.annonc.2024.02.002.
Bardia A, Hurvitz SA, Tolaney SM, et al. Sacituzumab govitecan in metastatic triple-negative breast cancer. N Engl J Med. 2021;384(16):1529–41. https://doi.org/10.1056/NEJMoa2028485.
Spring LM, Gupta A, Reynolds KL, et al. Neoadjuvant endocrine therapy for estrogen receptor-positive breast cancer: a systematic review and meta-analysis. JAMA Oncol. 2016;2(11):1477–86. https://doi.org/10.1001/jamaoncol.2016.1897.
Houssami N, Macaskill P, von Minckwitz G, Marinovich ML, Mamounas E. Meta-analysis of the association of breast cancer subtype and pathologic complete response to neoadjuvant chemotherapy. Eur J Cancer. 2012;48(18):3342–54. https://doi.org/10.1016/j.ejca.2012.05.023.
Petit T, Wilt M, Velten M, et al. Semi-quantitative evaluation of estrogen receptor expression is a strong predictive factor of pathological complete response after anthracycline-based neo-adjuvant chemotherapy in hormonal-sensitive breast cancer. Breast Cancer Res Treat. 2010;124(2):387–91. https://doi.org/10.1007/s10549-010-1142-2.
Bonnefoi H, Litière S, Piccart M, et al. Pathological complete response after neoadjuvant chemotherapy is an independent predictive factor irrespective of simplified breast cancer intrinsic subtypes: a landmark and two-step approach analyses from the EORTC 10994/BIG 1–00 phase III trial. Ann Oncol. 2014;25(6):1128–36. https://doi.org/10.1093/annonc/mdu118.
Symmans WF, Peintinger F, Hatzis C, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol. 2007;25(28):4414–22. https://doi.org/10.1200/JCO.2007.10.6823.
Mittendorf EA, Vila J, Tucker SL, et al. The neo-bioscore update for staging breast cancer treated with neoadjuvant chemotherapy: incorporation of prognostic biologic factors into staging after treatment. JAMA Oncol. 2016;2(7):929–36. https://doi.org/10.1001/jamaoncol.2015.6478.
van Mackelenbergh MT, Denkert C, Nekljudova V, et al. Outcome after neoadjuvant chemotherapy in estrogen receptor-positive and progesterone receptor-negative breast cancer patients: a pooled analysis of individual patient data from ten prospectively randomized controlled neoadjuvant trials. Breast Cancer Res Treat. 2018;167(1):59–71. https://doi.org/10.1007/s10549-017-4480-5.
Denkert C, Loibl S, Müller BM, et al. Ki67 levels as predictive and prognostic parameters in pretherapeutic breast cancer core biopsies: a translational investigation in the neoadjuvant GeparTrio trial. Ann Oncol. 2013;24(11):2786–93. https://doi.org/10.1093/annonc/mdt350.
Kalinsky K, Barlow WE, Gralow JR, et al. 21-Gene assay to inform chemotherapy benefit in node-positive breast cancer. N Engl J Med. 2021;385(25):2336–47. https://doi.org/10.1056/NEJMoa2108873.
Gianni L, Zambetti M, Clark K, et al. Gene expression profiles in paraffin-embedded core biopsy tissue predict response to chemotherapy in women with locally advanced breast cancer. J Clin Oncol. 2005;23(29):7265–77. https://doi.org/10.1200/JCO.2005.02.0818.
Whitworth P, Beitsch P, Mislowsky A, et al. Chemosensitivity and endocrine sensitivity in clinical luminal breast cancer patients in the prospective neoadjuvant breast registry symphony trial (NBRST) predicted by molecular subtyping. Ann Surg Oncol. 2017;24(3):669–75. https://doi.org/10.1245/s10434-016-5600-x.
Ueno T, Masuda N, Yamanaka T, et al. Evaluating the 21-gene assay recurrence Score® as a predictor of clinical response to 24 weeks of neoadjuvant exemestane in estrogen receptor-positive breast cancer. Int J Clin Oncol. 2014;19(4):607–13. https://doi.org/10.1007/s10147-013-0614-x.
Akashi-Tanaka S, Shimizu C, Ando M, et al. 21-Gene expression profile assay on core needle biopsies predicts responses to neoadjuvant endocrine therapy in breast cancer patients. Breast. 2009;18(3):171–4. https://doi.org/10.1016/j.breast.2009.03.005.
Iwata H, Masuda N, Sagara Y, et al. Analysis of Ki-67 expression with neoadjuvant anastrozole or tamoxifen in patients receiving goserelin for premenopausal breast cancer. Cancer. 2013;119(4):704–13. https://doi.org/10.1002/cncr.27818.
Nitz UA, Gluz O, Kümmel S, et al. Endocrine therapy response and 21-gene expression assay for therapy guidance in HR+/HER2- early breast cancer. J Clin Oncol. 2022;40(23):2557–67. https://doi.org/10.1200/JCO.21.02759.
Untch M, Jackisch C, Schneeweiss A, et al. Nab-paclitaxel versus solvent-based paclitaxel in neoadjuvant chemotherapy for early breast cancer (GeparSepto-GBG 69): a randomised, phase 3 trial. Lancet Oncol. 2016;17(3):345–56. https://doi.org/10.1016/S1470-2045(15)00542-2.
Gianni L, Mansutti M, Anton A, et al. Comparing neoadjuvant nab-paclitaxel vs paclitaxel both followed by anthracycline regimens in women with ERBB2/HER2-negative breast cancer-the evaluating treatment with neoadjuvant abraxane (ETNA) trial: a randomized phase 3 clinical trial. JAMA Oncol. 2018;4(3):302–8. https://doi.org/10.1001/jamaoncol.2017.4612.
Untch M, Jackisch C, Schneeweiss A, et al. NAB-paclitaxel improves disease-free survival in early breast cancer: GBG 69-GeparSepto. J Clin Oncol. 2019;37(25):2226–34. https://doi.org/10.1200/JCO.18.01842.
Cardoso F, Bardia A, Andre F, et al. KEYNOTE-756: Randomized, double-blind, phase 3 study of pembrolizumab vs placebo combined with neoadjuvant chemotherapy and adjuvant endocrine therapy for high-risk, early-stage estrogen receptor–positive, human epidermal growth factor receptor 2–negative (ER+/HER2−) breast cancer. JCO. 2019;37(15_suppl):TPS601-TPS601. https://doi.org/10.1200/JCO.2019.37.15_suppl.TPS601
Loi S, Curigliano G, Salgado R, et al. Abstract GS01–01: Biomarker Results in High-risk Estrogen Receptor Positive, Human Epidermal Growth Factor Receptor 2 Negative Primary Breast Cancer Following Neoadjuvant Chemotherapy ± Nivolumab: an Exploratory Analysis of CheckMate 7FL. Cancer Research. 2024;84(9_Supplement):GS01–01-GS01–01. https://doi.org/10.1158/1538-7445.SABCS23-GS01-01
Mittempergher L, Kuilman MM, Barcaru A, et al. The ImPrint immune signature to identify patients with high-risk early breast cancer who may benefit from PD1 checkpoint inhibition in I-SPY2. JCO. 2022;40(16_suppl):514–514. https://doi.org/10.1200/JCO.2022.40.16_suppl.514
Bardia A, Hurvitz S, Press MF, et al. Abstract GS2–03: GS2–03 TRIO-US B-12 TALENT: Neoadjuvant trastuzumab deruxtecan with or without anastrozole for HER2-low, HR+ early stage breast cancer. Cancer Res. 2023;83(5_Supplement):GS2–03-GS2–03. https://doi.org/10.1158/1538-7445.SABCS22-GS2-03
Barroso-Sousa R, Silva DDAFR, Alessi JVM, Mano MS. Neoadjuvant endocrine therapy in breast cancer: current role and future perspectives. Ecancermedicalscience. 2016;10:609. https://doi.org/10.3332/ecancer.2016.609
Sella T, Weiss A, Mittendorf EA, et al. Neoadjuvant endocrine therapy in clinical practice: a review. JAMA Oncol. 2021;7(11):1700–8. https://doi.org/10.1001/jamaoncol.2021.2132.
Eiermann W, Paepke S, Appfelstaedt J, et al. Preoperative treatment of postmenopausal breast cancer patients with letrozole: A randomized double-blind multicenter study. Ann Oncol. 2001;12(11):1527–32. https://doi.org/10.1023/a:1013128213451.
Smith IE, Dowsett M, Ebbs SR, et al. Neoadjuvant treatment of postmenopausal breast cancer with anastrozole, tamoxifen, or both in combination: the Immediate preoperative anastrozole, tamoxifen, or combined with tamoxifen (IMPACT) multicenter double-blind randomized trial. J Clin Oncol. 2005;23(22):5108–16. https://doi.org/10.1200/JCO.2005.04.005.
Semiglazov V, Kletsel A, Semiglazov V, et al. Exemestane (E) vs tamoxifen (T) as neoadjuvant endocrine therapy for postmenopausal women with ER+ breast cancer (T2N1–2, T3N0–1, T4N0M0). JCO. 2005;23(16_suppl):530–530. https://doi.org/10.1200/jco.2005.23.16_suppl.530
Cataliotti L, Buzdar AU, Noguchi S, et al. Comparison of anastrozole versus tamoxifen as preoperative therapy in postmenopausal women with hormone receptor-positive breast cancer: the Pre-Operative “Arimidex” Compared to Tamoxifen (PROACT) trial. Cancer. 2006;106(10):2095–103. https://doi.org/10.1002/cncr.21872.
Masuda N, Sagara Y, Kinoshita T, et al. Neoadjuvant anastrozole versus tamoxifen in patients receiving goserelin for premenopausal breast cancer (STAGE): a double-blind, randomised phase 3 trial. Lancet Oncol. 2012;13(4):345–52. https://doi.org/10.1016/S1470-2045(11)70373-4.
Leal F, Liutti VT, Antunes dos Santos VC, et al. Neoadjuvant endocrine therapy for resectable breast cancer: a systematic review and meta-analysis. Breast. 2015;24(4):406–412. https://doi.org/10.1016/j.breast.2015.03.004
Dowsett M, Ebbs SR, Dixon JM, et al. Biomarker changes during neoadjuvant anastrozole, tamoxifen, or the combination: influence of hormonal status and HER-2 in breast cancer–a study from the IMPACT trialists. J Clin Oncol. 2005;23(11):2477–92. https://doi.org/10.1200/JCO.2005.07.559.
Dowsett M, Smith IE, Ebbs SR, et al. Prognostic value of Ki67 expression after short-term presurgical endocrine therapy for primary breast cancer. J Natl Cancer Inst. 2007;99(2):167–70. https://doi.org/10.1093/jnci/djk020.
Ellis MJ, Tao Y, Luo J, et al. Outcome prediction for estrogen receptor-positive breast cancer based on postneoadjuvant endocrine therapy tumor characteristics. J Natl Cancer Inst. 2008;100(19):1380–8. https://doi.org/10.1093/jnci/djn309.
Suman VJ, Ellis MJ, Ma CX. The ALTERNATE trial: assessing a biomarker driven strategy for the treatment of post-menopausal women with ER+/Her2- invasive breast cancer. Chin Clin Oncol. 2015;4(3):34. https://doi.org/10.3978/j.issn.2304-3865.2015.09.01.
Lerebours F, Rivera S, Mouret-Reynier MA, et al. Randomized phase 2 neoadjuvant trial evaluating anastrozole and fulvestrant efficacy for postmenopausal, estrogen receptor-positive, human epidermal growth factor receptor 2-negative breast cancer patients: Results of the UNICANCER CARMINA 02 French trial (UCBG 0609). Cancer. 2016;122(19):3032–40. https://doi.org/10.1002/cncr.30143.
Quenel-Tueux N, Debled M, Rudewicz J, et al. Clinical and genomic analysis of a randomised phase II study evaluating anastrozole and fulvestrant in postmenopausal patients treated for large operable or locally advanced hormone-receptor-positive breast cancer. Br J Cancer. 2015;113(4):585–94. https://doi.org/10.1038/bjc.2015.247.
Ellis MJ, Suman VJ, Hoog J, et al. Randomized phase II neoadjuvant comparison between letrozole, anastrozole, and exemestane for postmenopausal women with estrogen receptor-rich stage 2 to 3 breast cancer: clinical and biomarker outcomes and predictive value of the baseline PAM50-based intrinsic subtype–ACOSOG Z1031. J Clin Oncol. 2011;29(17):2342–9. https://doi.org/10.1200/JCO.2010.31.6950.
Smith I, Robertson J, Kilburn L, et al. Long-term outcome and prognostic value of Ki67 after perioperative endocrine therapy in postmenopausal women with hormone-sensitive early breast cancer (POETIC): an open-label, multicentre, parallel-group, randomised, phase 3 trial. Lancet Oncol. 2020;21(11):1443–54. https://doi.org/10.1016/S1470-2045(20)30458-7.
Semiglazov VF, Semiglazov VV, Dashyan GA, et al. Phase 2 randomized trial of primary endocrine therapy versus chemotherapy in postmenopausal patients with estrogen receptor-positive breast cancer. Cancer. 2007;110(2):244–54. https://doi.org/10.1002/cncr.22789.
Alba E, Calvo L, Albanell J, et al. Chemotherapy (CT) and hormonotherapy (HT) as neoadjuvant treatment in luminal breast cancer patients: results from the GEICAM/2006-03, a multicenter, randomized, phase-II study. Ann Oncol. 2012;23(12):3069–74. https://doi.org/10.1093/annonc/mds132.
Palmieri C, Cleator S, Kilburn LS, et al. NEOCENT: a randomised feasibility and translational study comparing neoadjuvant endocrine therapy with chemotherapy in ER-rich postmenopausal primary breast cancer. Breast Cancer Res Treat. 2014;148(3):581–90. https://doi.org/10.1007/s10549-014-3183-4.
Krainick-Strobel UE, Lichtenegger W, Wallwiener D, et al. Neoadjuvant letrozole in postmenopausal estrogen and/or progesterone receptor positive breast cancer: a phase IIb/III trial to investigate optimal duration of preoperative endocrine therapy. BMC Cancer. 2008;8:62. https://doi.org/10.1186/1471-2407-8-62.
Dixon JM, Renshaw L, Macaskill EJ, et al. Increase in response rate by prolonged treatment with neoadjuvant letrozole. Breast Cancer Res Treat. 2009;113(1):145–51. https://doi.org/10.1007/s10549-008-9915-6.
Llombart-Cussac A, Guerrero Á, Galán A, et al. Phase II trial with letrozole to maximum response as primary systemic therapy in postmenopausal patients with ER/PgR[+] operable breast cancer. Clin Transl Oncol. 2012;14(2):125–31. https://doi.org/10.1007/s12094-012-0771-9.
Allevi G, Strina C, Andreis D, et al. Increased pathological complete response rate after a long-term neoadjuvant letrozole treatment in postmenopausal oestrogen and/or progesterone receptor-positive breast cancer. Br J Cancer. 2013;108(8):1587–92. https://doi.org/10.1038/bjc.2013.151.
Hojo T, Kinoshita T, Imoto S, et al. Use of the neo-adjuvant exemestane in post-menopausal estrogen receptor-positive breast cancer: a randomized phase II trial (PTEX46) to investigate the optimal duration of preoperative endocrine therapy. Breast. 2013;22(3):263–7. https://doi.org/10.1016/j.breast.2013.03.002.
Carpenter R, Doughty JC, Cordiner C, et al. Optimum duration of neoadjuvant letrozole to permit breast conserving surgery. Breast Cancer Res Treat. 2014;144(3):569–76. https://doi.org/10.1007/s10549-014-2835-8.
Fontein DBY, Charehbili A, Nortier JWR, et al. Efficacy of six month neoadjuvant endocrine therapy in postmenopausal, hormone receptor-positive breast cancer patients–a phase II trial. Eur J Cancer. 2014;50(13):2190–200. https://doi.org/10.1016/j.ejca.2014.05.010.
Rusz O, Vörös A, Varga Z, et al. One-year neoadjuvant endocrine therapy in breast cancer. Pathol Oncol Res. 2015;21(4):977–84. https://doi.org/10.1007/s12253-015-9911-1.
Baselga J, Semiglazov V, van Dam P, et al. Phase II randomized study of neoadjuvant everolimus plus letrozole compared with placebo plus letrozole in patients with estrogen receptor-positive breast cancer. J Clin Oncol. 2009;27(16):2630–7. https://doi.org/10.1200/JCO.2008.18.8391.
Smith IE, Walsh G, Skene A, et al. A phase II placebo-controlled trial of neoadjuvant anastrozole alone or with gefitinib in early breast cancer. J Clin Oncol. 2007;25(25):3816–22. https://doi.org/10.1200/JCO.2006.09.6578.
Ma CX, Gao F, Luo J, et al. NeoPalAna: neoadjuvant palbociclib, a Cyclin-Dependent Kinase 4/6 Inhibitor, and anastrozole for clinical Stage 2 or 3 estrogen receptor-positive breast cancer. Clin Cancer Res. 2017;23(15):4055–65. https://doi.org/10.1158/1078-0432.CCR-16-3206.
Curigliano G, Gómez Pardo P, Meric-Bernstam F, et al. Ribociclib plus letrozole in early breast cancer: a presurgical, window-of-opportunity study. Breast. 2016;28:191–8. https://doi.org/10.1016/j.breast.2016.06.008.
Cottu P, D’Hondt V, Dureau S, et al. Letrozole and palbociclib versus chemotherapy as neoadjuvant therapy of high-risk luminal breast cancer. Ann Oncol. 2018;29(12):2334–40. https://doi.org/10.1093/annonc/mdy448.
Johnston S, Puhalla S, Wheatley D, et al. Randomized Phase II study evaluating palbociclib in addition to letrozole as neoadjuvant therapy in estrogen receptor-positive early breast cancer: pallet trial. J Clin Oncol. 2019;37(3):178–89. https://doi.org/10.1200/JCO.18.01624.
Hurvitz SA, Martin M, Press MF, et al. Potent cell-cycle inhibition and upregulation of immune response with abemaciclib and anastrozole in neoMONARCH, pshase II neoadjuvant study in HR+/HER2- breast cancer. Clin Cancer Res. 2020;26(3):566–80. https://doi.org/10.1158/1078-0432.CCR-19-1425.
Prat A, Saura C, Pascual T, et al. Ribociclib plus letrozole versus chemotherapy for postmenopausal women with hormone receptor-positive, HER2-negative, luminal B breast cancer (CORALLEEN): an open-label, multicentre, randomised, phase 2 trial. Lancet Oncol. 2020;21(1):33–43. https://doi.org/10.1016/S1470-2045(19)30786-7.
Khan QJ, O’Dea A, Bardia A, et al. Letrozole + ribociclib versus letrozole + placebo as neoadjuvant therapy for ER+ breast cancer (FELINE trial). JCO. 2020;38(15_suppl):505–505. https://doi.org/10.1200/JCO.2020.38.15_suppl.505
Delaloge S, Dureau S, D’Hondt V, et al. Survival outcomes after neoadjuvant letrozole and palbociclib versus third generation chemotherapy for patients with high-risk oestrogen receptor-positive HER2-negative breast cancer. Eur J Cancer. 2022;166:300–8. https://doi.org/10.1016/j.ejca.2022.01.014.
Guan Y, Shen G, Fang Q, et al. Cyclin-dependent kinase 4 and 6 inhibitors in combination with neoadjuvant endocrine therapy in estrogen receptor-positive early breast cancer: a systematic review and meta-analysis. Clin Exp Med. 2023;23(2):245–54. https://doi.org/10.1007/s10238-022-00814-3.
Shah AN, Flaum L, Helenowski I, et al. Phase II study of pembrolizumab and capecitabine for triple negative and hormone receptor-positive, HER2−negative endocrine-refractory metastatic breast cancer. J Immunother Cancer. 2020;8(1):e000173. https://doi.org/10.1136/jitc-2019-000173.
Page D, Pucilowska J, Bennetts L, et al. Abstract P2–09–03: Updated efficacy of first or second-line pembrolizumab (pembro) plus capecitabine (cape) in metastatic triple negative breast cancer (mTNBC) and correlations with baseline lymphocyte and naïve CD4+ T-cell count. Cancer Research. 2019;79(4_Supplement):P2–09–03-P2–09–03. https://doi.org/10.1158/1538-7445.SABCS18-P2-09-03
Geyer CE, Garber JE, Gelber RD, et al. Overall survival in the OlympiA phase III trial of adjuvant olaparib in patients with germline pathogenic variants in BRCA1/2 and high-risk, early breast cancer. Ann Oncol. 2022;33(12):1250–68. https://doi.org/10.1016/j.annonc.2022.09.159.
Robson M, Im SA, Senkus E, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017;377(6):523–33. https://doi.org/10.1056/NEJMoa1706450.
Domchek SM, Postel-Vinay S, Im SA, et al. Olaparib and durvalumab in patients with germline BRCA-mutated metastatic breast cancer (MEDIOLA): an open-label, multicentre, phase 1/2, basket study. Lancet Oncol. 2020;21(9):1155–64. https://doi.org/10.1016/S1470-2045(20)30324-7.
Vinayak S, Tolaney SM, Schwartzberg L, et al. Open-label clinical trial of niraparib combined with pembrolizumab for treatment of advanced or metastatic triple-negative breast cancer. JAMA Oncol. 2019;5(8):1132. https://doi.org/10.1001/jamaoncol.2019.1029.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. H.B wrote the first draft of the manuscript. T.P. supervised the course of the article. M.E. critically reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Disclosure
HB, ME and TP had no conflict of interest associated with this publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Bischoff, H., Espié, M. & Petit, T. Neoadjuvant Therapy: Current Landscape and Future Horizons for ER-Positive/HER2-Negative and Triple-Negative Early Breast Cancer. Curr. Treat. Options in Oncol. (2024). https://doi.org/10.1007/s11864-024-01251-y
Accepted:
Published:
DOI: https://doi.org/10.1007/s11864-024-01251-y