Opinion statement
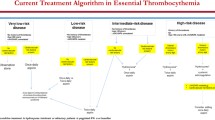
Current treatment of essential thrombocythemia (ET) should primarily prevent thrombo-hemorrhagic events, without increasing the rate of fibrotic progression or leukemic evolution, and secondarily control microvascular symptoms. Unlike other classic BCR::ABL1-negative myeloproliferative neoplasms, ET is frequently diagnosed in adolescents and young adults (AYA), defined as individuals aged 15 to 39 years, in up to 20% of patients. However, since the current risk stratification of this disease is based on models, including that of ELN, IPSET-Thrombosis and its revised version, mainly applied to an older patients’ population, international guidelines are needed that specifically consider how to evaluate the prognosis of AYAs with ET. Furthermore, although ET is the most frequent MPN among AYA subjects, there is a lack of specific recommendations on how to treat it in this subgroup of patients, as management decisions are typically extrapolated from those for the elderly. Accordingly, since AYAs with ET represent a unique disease subset defined by attenuated genetic risk, more indolent phenotype, and longer survival than their older counterparts, treatment selection requires special attention to specific issues such as the risk of fibrotic/leukemic transformation, carcinogenicity, and fertility. This review article will provide a comprehensive overview of the diagnosis, prognostic stratification, and possible therapeutic approaches for AYA patients with ET, including antiplatelets/anticoagulants and cytoreductive agents, with a focus on pregnancy management in real-life clinical practice.
Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Tefferi A, Vardiman JW. Classification and diagnosis of myeloproliferative neoplasms: the 2008 World Health Organization criteria and point-of-care diagnostic algorithms. Leukemia. 2008;22:14–22.
Tefferi A. Myeloproliferative neoplasms 2012: the John M. Bennett 80th birthday anniversary lecture. Leuk Res. 2012;36:1481–9.
Radaelli F, Colombi M, Calori R, et al. Analysis of risk factors predicting thrombotic and/or haemorrhagic complications in 306 patients with essential thrombocythemia. Hematol Oncol. 2007;25:115–20.
Moulard O, Mehta J, Fryzek J, Olivares R, Iqbal U, Mesa RA. Epidemiology of myelofibrosis, essential thrombocythemia, and polycythemia vera in the European Union. Eur J Haematol. 2014;92:289–97.
Berlin NI. Diagnosis and classification of the polycythemias. Semin Hematol. 1975;12:339–51.
Najean Y, Mugnier P, Dresch C, Rain JD. Polycythaemia vera in young people: an analysis of 58 cases diagnosed before 40 years. Br J Haematol. 1987;67:285–91.
Gugliotta L, Fiacchini M, et al. Epidemiological, diagnostic, therapeutic and prognostic aspects of essential thrombocythemia in a retrospective study of the GIMMC group in two thousand patients [abstract]. Blood. 1997;90(suppl 1):348a.
Polycythemia vera: the natural history of 1213 patients followed for 20 years. Gruppo Italiano Studio Policitemia. Ann Intern Med. 1995;123:656–64.
Alvarez-Larran A, Cervantes F, Bellosillo B, et al. Essential thrombocythemia in young individuals: frequency and risk factors for vascular events and evolution to myelofibrosis in 126 patients. Leukemia. 2007;21:1218–23.
Palandri F, Polverelli N, Ottaviani E, Castagnetti F, Baccarani M, Vianelli N. Long-term follow-up of essential thrombocythemia in young adults: treatment strategies, major thrombotic complications and pregnancy outcomes. A study of 76 patients. Haematologica. 2010;95:1038–40.
Rollison DE, Howlader N, Smith MT, et al. Epidemiology of myelodysplastic syndromes and chronic myeloproliferative disorders in the United States, 2001–2004, using data from the NAACCR and SEER programs. Blood. 2008;112:45–52.
Thiele J, Kvasnicka HM, Orazi A, et al. Essential thrombocythemia. In: Campo E, Swerdlow SH, Harris NL, Pileri S, Stein H, Jaffe ES, editors. WHO classification of tumours: pathology and genetics of tumours of haematopoietic and lymphoid tissues. IARC Press, Lyon, France; 2008. p. 48-50.
Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–405.
•• Khoury JD, Solary E, Abla O, et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms. Leukemia. 2022;36:1703–19. The new 2022 revision of WHO classification that provided updated diagnostic criteria for all BCR::ABL1-negative myeloproliferative neoplasms, including essential thrombocythemia.
•• Arber DA, Orazi A, Hasserjian RP, et al. International Consensus Classification of Myeloid Neoplasms and Acute Leukemias: integrating morphologic, clinical, and genomic data. Blood. 2022;140:1200-28. An additional classification which provided updated diagnostic criteria for all BCR::ABL1-negative myeloproliferative neoplasms, integrating clinical-morphological and molecular features.
Kralovics R, Passamonti F, Buser AS, et al. Gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352:1779–90.
Baxter EJ, Scott LM, Campbell PJ, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365:1054–61.
Tefferi A. JAK2 mutations and clinical practice in myeloproliferative neoplasms. Cancer J. 2007;13:366–71.
Levine RL, Pardanani A, Tefferi A, Gilliland DG. Role of JAK2 in the pathogenesis and therapy of myeloproliferative disorders. Nat Rev Cancer. 2007;7:673–83.
Vannucchi AM, Antonioli E, Guglielmelli P, Pardanani A, Tefferi A. Clinical correlates of JAK2V617F presence or allele burden in myeloproliferative neoplasms: a critical reappraisal. Leukemia. 2008;22:1299–307.
Passamonti F, Rumi E. Clinical relevance of JAK2 (V617F) mutant allele burden. Haematologica. 2009;94:7–10.
Carobbio A, Finazzi G, Antonioli E, et al. JAK2V617F allele burden and thrombosis: a direct comparison in essential thrombocythemia and polycythemia vera. Exp Hematol. 2009;37:1016–21.
Pikman Y, Lee BH, Mercher T, et al. MPLW515L is a novel somatic activating mutation in myelofibrosis with myeloid metaplasia. PLoS Med. 2006;3: e270.
Pardanani AD, Levine RL, Lasho T, et al. MPL515 mutations in myeloproliferative and other myeloid disorders: a study of 1182 patients. Blood. 2006;108:3472–6.
Klampfl T, Gisslinger H, Harutyunyan AS, et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N Eng J Med. 2013;369:2379–90.
Nangalia J, Massie CE, Baxter EJ, et al. Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. N Eng J Med. 2013;369:2391–405.
Cattaneo D, Croci GA, Bucelli C, et al. Triple-negative essential thrombocythemia: clinical-pathological and molecular features. A single-center cohort study. Front Oncol. 2021;11:637116.
Rotunno G, Mannarelli C, Guglielmelli P, et al. Impact of calreticulin mutations on clinical and hematological phenotype and outcome in essential thrombocythemia. Blood. 2014;123:1552–5.
Fabris S, Cattaneo D, Salerio S, et al. Impact on thrombotic risk of canonical and atypical CALR mutations in essential thrombocythemia. A single-center cohort study. Thromb Res. 2022;210:67-9.
Gangat N, Wassie E, Lasho T, et al. Mutations and thrombosis in essential thrombocythemia: prognostic interaction with age and thrombosis history. Eur J Haematol. 2015;94:31–6.
Rumi E, Pietra D, Ferretti V, et al. JAK2 or CALR mutation status defines subtypes of essential thrombocytemia with substantially different clinical course and outcomes. Blood. 2014;123:1544–51.
Chen CC, Gau JP, Chou HJ, et al. Frequencies, clinical characteristics, and outcome of somatic CALR mutations in JAK2-unmutated essential thrombocythemia. Ann Hematol. 2014;93:2029–36.
Tefferi A, Wassie EA, Guglielmelli P, et al. Type 1 versus type 2 calreticulin mutations in essential thrombocythemia: a collaborative study of 1027 patients. Am J Hematol. 2014;89:E121–4.
Perez Encinas MM, Sobas M, Gomez-Casares MT, et al. The risk of thrombosis in essential thrombocythemia is associated with the type of CALR mutation: a multicentre collaborative study. Eur J Haematol. 2021;106:371–9.
Pietra D, Rumi E, Ferretti VV, et al. Differential clinical effects of different mutation subtypes in CALR-mutant myeloproliferative neoplasms. Leukemia. 2016;30:431–8.
Cervantes F, Alvarez-Larrán A, Talarn C, Gómez M, Montserrat E. Myelofibrosis with myeloid metaplasia following essential thrombocythaemia: actuarial probability, presenting characteristics and evolution in a series of 195 patients. Br J Haematol. 2002;118:786–90.
Passamonti F, Rumi E, Arcaini L, et al. Prognostic factors for thrombosis, myelofibrosis, and leukemia in essential thrombocythemia: a study of 605 patients. Haematologica. 2008;93:1645–51.
Kiladjian JJ, Rain JD, Bernard JF, Briere J, Chomienne C, Fenaux P. Long-term incidence of hematological evolution in three French prospective studies of hydroxyurea and pipobroman in polycythemia vera and essential thrombocythemia. Semin Thromb Hemost. 2006;32:417–21.
Barbui T, Thiele J, Passamonti F, et al. Survival and disease progression in essential thrombocythemia are significantly influenced by accurate morphologic diagnosis: an international study. J Clin Oncol. 2011;29:3179–84.
Tefferi A, Elliott M. Thrombosis in myeloproliferative disorders: prevalence, prognostic factors, and the role of leukocytes and JAK2V617F. Semin Thromb Hemost. 2007;33:313–20.
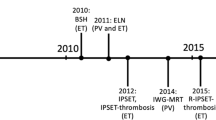
Barbui T, Tefferi A, Vannucchi AM, et al. Philadelphia chromosome-negative classical myeloproliferative neoplasms: revised management recommendations from European LeukemiaNet. Leukemia. 2018;32:1057–69.
Tefferi A, Barbui T. Polycythemia vera and essential thrombocythemia: 2021 update on diagnosis, risk-stratification and management. Am J Hematol. 2020;95:1599–613.
Ferrari A, Stark D, Peccatori FA, et al. Adolescents and young adults (AYA) with cancer: a position paper from the AYA Working Group of the European Society for Medical Oncology (ESMO) and the European Society for Paediatric Oncology (SIOPE). ESMO Open. 2021;6: 100096.
Barbui T, Barosi G, Birgegard G, et al. Philadelphia-negative classical myeloproliferative neoplasms: critical concepts and management recommendations from European LeukemiaNet. J Clin Oncol. 2011;29:761–70.
Barbui T, Finazzi G, Carobbio A, et al. Development and validation of an International Prognostic Score of thrombosis in World Health Organization-essential thrombocythemia (IPSET-thrombosis). Blood. 2012;120:5128–33.
Barbui T, Vannucchi AM, Buxhofer-Ausch V, et al. Practice-relevant revision of IPSET-thrombosis based on 1019 patients with WHO-defined essential thrombocythemia. Blood Cancer J. 2015;5: e369.
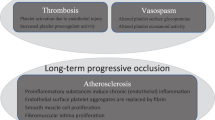
Michiels JJ. Acquired von Willebrand disease due to increasing platelet count can readily explain the paradox of thrombosis and bleeding in thrombocythemia. Clin Appl Thromb Hemost. 1999;5:147–51.
• Tefferi A, Guglielmelli P, Lasho TL, et al. Mutation-enhanced international prognostic systems for essential thrombocythaemia and polycythaemia vera. Br J Haematol. 2020;189:291–302. The most recent prognostic score available for essential thrombocythemia, which integrates clinical-laboratory and molecular data to define overall survival of patients.
Szuber N, Vallapureddy RR, Penna D, et al. Myeloproliferative neoplasms in the young: Mayo Clinic experience with 361 patients age 40 years or younger. Am J Hematol. 2018;93:1474–84.
Sobas M, Kiladjian J-J, Beauverd Y, et al. Real-world study of children and young adults with myeloproliferative neoplasms: identifying risks and unmet needs. Blood Adv. 2022;6:5171–83.
Landolfi R, Marchioli R, Kutti J, et al. Efficacy and safety of low-dose aspirin in polycythemia vera N Engl J Med. 2004;350:114-24.
Alvarez-Larran A, Pereira A, Guglielmelli P, et al. Antiplatelet therapy versus observation in low-risk essential thrombocythemia with a CALR mutation. Haematologica. 2016;101:926–31.
Chu DK, Hillis CM, Leong DP, Anand SS, Siegal DM. Benefits and risks of antithrombotic therapy in essential thrombocythemia: a systematic review. Ann Intern Med. 2017;167:170–80.
Godfrey AL, Green A, Harrison CN. Essential thrombocythemia: challenges in clinical practice and future prospects. Blood. 2022;blood.2022017625. https://doi.org/10.1182/blood.2022017625. Online ahead of print.
Dillinger JG, Sideris G, Henry P, Bal dit Sollier C, Ronez E, Drouet L. Twice daily aspirin to improve biological aspirin efficacy in patients with essential thrombocythemia. Thromb Res. 2012;129:91–4.
•• Rocca B, Tosetto A, Betti S, et al. A randomized double-blind trial of 3 aspirin regimens to optimize antiplatelet therapy in essential thrombocythemia. Blood. 2020;136:171–82. The first clinical trial that specifically evaluated the efficacy and safety profile of different doses of antiplatelet therapy in essential thrombocythemia.
Tosetto A, Rocca B, Petrucci G, et al. Association of platelet thromboxane inhibition by low-dose aspirin with platelet count and cytoreductive therapy in essential thrombocythemia. Clin Pharmacol Ther. 2022;111:939–49.
•• Alvarez-Larrán A, Sant'Antonio E, Harrison C, et al. Unmet clinical needs in the management of CALR-mutated essential thrombocythaemia: a consensus-based proposal from the European LeukemiaNet. Lancet Haematol. 2021;8:e658-65. The most recent ELN proposal for the management of patients with CALR-mutated essential thrombocythemia.
Amerikanou R, Lambert J, Alimam S. Myeloproliferative neoplasms in adolescents and young adults. Best Pract Res Clin Haematol. 2022;35: 101374.
Carobbio A, Thiele J, Passamonti F, et al. Risk factors for arterial and venous thrombosis in WHO-defined essential thrombocythemia: an international study of 891 patients. Blood. 2011;117:5857–9.
Tefferi A, Szuber N, Pardanani A, et al. Extreme thrombocytosis in low-risk essential thrombocythemia: retrospective review of vascular events and treatment strategies. Am J Hematol. 2021;96:E182–4.
Finazzi G, Carobbio A, Thiele J, et al. Incidence and risk factors for bleeding in 1104 patients with essential thrombocythemia or prefibrotic myelofibrosis diagnosed according to the 2008 WHO criteria. Leukemia. 2012;26:716–9.
Gangat N, Szuber N, Jawaid T, Hanson CA, Pardanani A, Tefferi A. Young platelet millionaires with essential thrombocythemia. Am J Hematol. 2021;96:E93–5.
Koren-Michowitz M, Lavi N, Ellis MH, Vannucchi AM, Mesa R, Harrison CN. Management of extreme thrombocytosis in myeloproliferative neoplasms: an international physician survey. Ann Hematol. 2017;96:87–92.
Godfrey AL, Campbell PJ, MacLean C, et al. Hydroxycarbamide plus aspirin versus aspirin alone in patients with essential thrombocythemia age 40 to 59 years without high-risk features. J Clin Oncol. 2018;36:3361–9.
Cortelazzo S, Finazzi G, Ruggeri M, et al. Hydroxyurea for patients with essential thrombocythemia and a high risk of thrombosis. N Engl J Med. 1995;332:1132–6.
Harrison CN, Campbell PJ, Buck G, et al. Hydroxyurea compared with anagrelide in high-risk essential thrombocythemia. N Engl J Med. 2005;353:33–45.
Hernández-Boluda JC, Alvarez-Larrán A, Gómez M, et al. Clinical evaluation of the European LeukaemiaNet criteria for clinic haematological response and resistance/intolerance to hydroxycarbamide in essential thrombocythaemia. Br J Haematol. 2011;152:81–8.
Gisslinger H, Gotic M, Holowiecki J, et al. Anagrelide compared to hydroxyurea in WHO-classified essential thrombocythemia: the ANAHYDRET Study, a randomized controlled trial. Blood. 2013;121:1720–8.
Birgegård G, Besses C, Griesshammer M, et al. Treatment of essential thrombocythemia in Europe: a prospective long-term observational study of 3649 high-risk patients in the Evaluation of Anagrelide Efficacy and Long-term Safety study. Haematologica. 2018;103:51–60.
Ahn IE, Natelson E, Rice L. Successful long-term treatment of Philadelphia chromosome-negative myeloproliferative neoplasms with combination of hydroxyurea and anagrelide. Clin Lymphoma Myeloma Leuk. 2013;13(Suppl 2):S300–4.
D’adda M, Micheletti M, Drera M, Ferrari S, Rossi G. The combined use of hydroxyurea and anagrelide allows satisfactory hematologic control in patients with chronic myeloproliferative disorders and thrombocytosis: a report on 13 patients with poor tolerance to hydroxyurea monotherapy. Leuk Lymphoma. 2008;49:2216–8.
Gugliotta L, Besses C, Griesshammer M, et al. Combination therapy of hydroxycarbamide with anagrelide in patients with essential thrombocythemia in the evaluation of Xagrid(R) efficacy and long-term safety study. Haematologica. 2014;99:679–87.
Mascarenhas J, Kosiorek HE, Prchal JT, et al. A randomized phase 3 trial of interferon-α vs hydroxyurea in polycythemia vera and essential thrombocythemia. Blood. 2022;139:2931–41.
Yacoub A, Mascarenhas J, Kosiorek H, et al. Pegylated interferon alfa-2a for polycythemia vera or essential thrombocythemia resistant or intolerant to hydroxyurea. Blood. 2019;134:1498–509.
Gerds AT, Gotlib J, Ali H, et al. Myeloproliferative neoplasms, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022;20:1033–62.
Rumi E, Cazzola M. How I treat essential thrombocythemia. Blood. 2016;128:2403-14.
Quintas-Cardama A, Kantarjian H, Manshouri T, et al. Pegylated interferon alfa-2a yields high rates of hematologic and molecular response in patients with advanced essential thrombocythemia and polycythemia vera. J Clin Oncol. 2009;27:5418–24.
Qureshi A, Kaya B, Pancham S, et al. Guidelines for the use of hydroxycarbamide in children and adults with sickle cell disease. Br J Haematol. 2018;181:460–75.
Gangat N, Wolanskyj AP, McClure RF, et al. Risk stratification for survival and leukemic transformation in essential thrombocythemia: a single institutional study of 605 patients. Leukemia. 2007;21:270–6.
Finazzi G, Ruggeri M, Rodeghiero F, Barbui T. Efficacy and safety of long-term use of hydroxyurea in young patients with essential thrombocythemia and a high risk of thrombosis. Blood. 2003;101:3749.
Choi HS, Hong J, Hwang SM, et al. Evaluation of the need for cytoreduction and its potential carcinogenicity in children and young adults with myeloproliferative neoplasms. Ann Hematol. 2021;100:2567–74.
Edahiro Y. Treatment options and pregnancy management for patients with PV and ET. Int J Hematol. 2022;115:659–71.
Iurlo A, Cattaneo D, Orofino N, Bucelli C, Fabris S, Cortelezzi A. Anagrelide and mutational status in essential thrombocythemia. BioDrugs. 2016;30:219–23.
Storen EC, Tefferi A. Long-term use of anagrelide in young patients with essential thrombocythemia. Blood. 2001;97:863–6.
Bieniaszewska M, Sobieralski P, Leszczyńska A, Dutka M. Anagrelide in essential thrombocythemia: efficacy and long-term consequences in young patient population. Leuk Res. 2022;123: 106962.
De Stefano V, Ruggeri M, Cervantes F, et al. High rate of recurrent venous thromboembolism in patients with myeloproliferative neoplasms and effect of prophylaxis with vitamin K antagonists. Leukemia. 2016;30:2032–8.
Smalberg JH, Arends LR, Valla DC, Kiladjian JJ, Janssen HL, Leebeek FW. Myeloproliferative neoplasms in Budd-Chiari syndrome and portal vein thrombosis: a meta-analysis. Blood. 2012;120:4921–8.
Sekhar M, McVinnie K, Burroughs AK. Splanchnic vein thrombosis in myeloproliferative neoplasms. Br J Haematol. 2013;162:730–47.
Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e419S – e496.
Ageno W, Dentali F, Squizzato A. How I treat splanchnic vein thrombosis. Blood. 2014;124:3685–91.
De Stefano V, Qi X, Betti S, Rossi E. Splanchnic vein thrombosis and myeloproliferative neoplasms: molecular-driven diagnosis and long-term treatment. Thromb Haemost. 2016;115:240–9.
Sant’Antonio E, Guglielmelli P, Pieri L, et al. Splanchnic vein thromboses associated with myeloproliferative neoplasms: An international, retrospective study on 518 cases. Am J Hematol. 2020;95:156–66.
How J, Story C, Ren S, et al. Practice patterns and outcomes of direct oral anticoagulant use in myeloproliferative neoplasm patients. Blood Cancer J. 2021;11:176.
Ianotto JC, Couturier MA, Galinat H, et al. Administration of direct oral anticoagulants in patients with myeloproliferative neoplasms. Int J Hematol. 2017;106:517–21.
Barbui T, De Stefano V, Carobbio A, et al. Direct oral anticoagulants for myeloproliferative neoplasms: results from an international study on 442 patients. Leukemia. 2021;35:2989–93.
Herbreteau L, Papageorgiou L, Le Clech L, et al. Benefice and pitfall of direct oral anticoagulants in very high-risk myeloproliferative neoplasms. Thromb Res. 2022;216:25–34.
Hamulyák EN, Daams JG, Leebeek FWG, et al. A systematic review of antithrombotic treatment of venous thromboembolism in patients with myeloproliferative neoplasms. Blood Adv. 2021;5:113–21.
Baysal M, Bayrak M, Eşkazan AE. Current evidence on the use of direct oral anticoagulants in patients with myeloproliferative neoplasm: a systematic review. Expert Rev Hematol. 2023;16:131–40.
Robinson SE, Harrison CN. How we manage Philadelphia-negative myeloproliferative neoplasms in pregnancy. Br J Haematol. 2020;189:625–34.
Alimam S, Bewley S, Chappell LC, et al. Pregnancy outcomes in myeloproliferative neoplasms: UK prospective cohort study. Br J Haematol. 2016;175:31–6.
Landtblom AR, Andersson TM, Johansson ALV, et al. Pregnancy and childbirth outcomes in women with myeloproliferative neoplasms—a nationwide population-based study of 342 pregnancies in Sweden. Leukemia. 2022;36:2461–7.
Maze D, Kazi S, Gupta V, et al. Association of treatments for myeloproliferative neoplasms during pregnancy with birth rates and maternal outcomes: a systematic review and meta-analysis. JAMA Netw Open. 2019;2: e1912666.
Passamonti F, Randi ML, Rumi E, et al. Increased risk of pregnancy complications in patients with essential thrombocythemia carrying the JAK2 (617V>F) mutation. Blood. 2007;110:485–9.
Rumi E, Bertozzi I, Casetti IC, et al. Impact of mutational status on pregnancy outcome in patients with essential thrombocytemia. Haematologica. 2015;100:443–5.
CLASP: a randomised trial of low-dose aspirin for the prevention and treatment of pre-eclampsia among 9364 pregnant women. CLASP (Collaborative Low-dose Aspirin Study in Pregnancy) Collaborative Group. Lancet. 1994;343:619–29.
Passamonti F, Rumi E, Randi ML, Morra E, Cazzola M. Aspirin in pregnant patients with essential thrombocythemia: a retrospective analysis of 129 pregnancies. J Thromb Haemost. 2010;8:411–3.
Gangat N, Joshi M, Shah S, et al. Pregnancy outcomes in myeloproliferative neoplasms: a Mayo Clinic report on 102 pregnancies Am J Hematol. 2020;95:E114–7.
Cincotta R, Higgins JR, Tippett C, et al. Management of essential thrombocythaemia during pregnancy. Aust N Z J Obstet Gynaecol. 2000;40:33–7.
Niittyvuopio R, Juvonen E, Kaaja R, et al. Pregnancy in essential thrombocythaemia: experience with 40 pregnancies. Eur J Haematol. 2004;73:431–6.
Griesshammer M, Struve S, Barbui T. Management of Philadelphia negative chronic myeloproliferative disorders in pregnancy. Blood Rev. 2008;22:235–45.
Melillo L, Tieghi A, Candoni A, et al. Outcome of 122 pregnancies in essential thrombocythemia patients: a report from the Italian registry. Am J Hematol. 2009;84:636–40.
Gangat N, Tefferi A. Myeloproliferative neoplasms and pregnancy: overview and practice recommendations. Am J Hematol. 2021;96:354–66.
Rottenstreich A, Kleinstern G, Amsalem H, Kalish Y. The course of acquired von Willebrand syndrome during pregnancy among patients with essential thrombocytosis. J Thromb Thrombolysis. 2018;46:304–9.
Skeith L, Carrier M, Robinson SE, Alimam S, Rodger MA. Risk of venous thromboembolism in pregnant women with essential thrombocythemia: a systematic review and meta-analysis. Blood. 2017;129:934–9.
Bistervels IM, Buchmüller A, Wiegers HMG, et al. Intermediate-dose versus low-dose low-molecular-weight heparin in pregnant and post-partum women with a history of venous thromboembolism (Highlow study): an open-label, multicentre, randomised, controlled trial. Lancet. 2022;400:1777–87.
Sant’Antonio E, Borsani O, Camerini C, et al. Philadelphia chromosome-negative myeloproliferative neoplasms in younger adults: a critical discussion of unmet medical needs, with a focus on pregnancy. Blood Rev. 2022;52: 100903.
Griesshammer M, Sadjadian P, Wille K. Contemporary management of patients with BCR-ABL1-negative myeloproliferative neoplasms during pregnancy. Expert Rev Hematol. 2018;11:697–706.
Schrickel L, Heidel FH, Sadjadian P, et al. Interferon alpha for essential thrombocythemia during 34 high-risk pregnancies: outcome and safety. J Cancer Res Clin Oncol. 2021;147:1481–91.
Puyade M, Cayssials E, Pierre F, Pourrat O. Pregnancy and myeloproliferative neoplasms: a retrospective monocentric cohort. Obstet Med. 2017;10:165–9.
Lapoirie J, Contis A, Guy A, et al. Management and outcomes of 27 pregnancies in women with myeloproliferative neoplasms. J Matern Fetal Neonatal Med. 2020;33:49–56.
Beauverd Y, Radia D, Cargo C, et al. Pegylated interferon alpha-2a for essential thrombocythemia during pregnancy: outcome and safety. A case series. Haematologica. 2016;101:e182–4.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Iurlo, A., Bucelli, C. & Cattaneo, D. Essential Thrombocythemia in Adolescents and Young Adults: Clinical Aspects, Treatment Options and Unmet Medical Needs. Curr. Treat. Options in Oncol. 24, 802–820 (2023). https://doi.org/10.1007/s11864-023-01099-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11864-023-01099-8