Abstract
Essential thrombocytosis (ET) is a rare haematological malignancy, with an incidence rate of 1.5–2.5/100,000 per year. For many patients with ET the first manifestation of their underlying disease is a thrombotic or haemorrhagic complication. A recent retrospective study revealed an incidence rate of at least 2.1% in people under 40 years presenting with an acute coronary syndrome, although the diagnosis was initially missed in all cases. Thus, cardiologists face a much higher than average incidence rate of ET in their daily practice, but seem insufficiently aware of the disease. The current review summarises symptoms, (differential) diagnosis, complications and treatment considerations of ET of relevance for a cardiologist. Typical symptoms, besides thrombosis and haemorrhage, include erythromelalgia and aquagenic pruritus, while platelets > 450 × 109/l are a diagnostic for ET once other myeloproliferative neoplasms, secondary and spurious thrombocytosis have been excluded. With regard to treatment, timing of revascularisation depends on the presence of ischaemia and concurrent platelet counts. In the presence of ischaemia, revascularisation should not be delayed and adequate platelet counts can be achieved by platelet apheresis. In the absence of ischaemia, revascularisation can be delayed until adequate platelet counts have been achieved by cytoreductive therapies. Cardiologists should be aware of/screen for possible ET.
Similar content being viewed by others
Introduction
Essential thrombocytosis/thrombocythaemia (ET) has been considered a rare underlying aetiology for acute coronary syndromes (ACS) [1, 2]. However, we recently showed a prevalence of at least 2.1% in a cohort of patients under 40 years that underwent coronary angiography (CAG) in the setting of their first ACS [3]. More importantly, as shown previously [4], this diagnosis was either missed/severely delayed (average 6 years) despite the presence of elevated thrombocytes (i.e. > 450 × 109/l) upon presentation. This observation might suggest that ET is insufficiently known among cardiologists. The present review aims at summarising the available literature on ET that is relevant, e.g. with regard to diagnosis and treatment, for the cardiologist.
Definition and epidemiology
ET is a rare chronic myeloid malignancy, having an incidence rate of 1.5–2.5/100,000 per year [5]. Together with polycythaemia vera (PV) and primary myelofibrosis (PMF), ET is one of the three myeloproliferative neoplasms (MPNs) characterised by stem-cell-derived clonal myeloproliferation with mutually exclusive Janus kinase (JAK)2V617F, calreticulin (CALR) or myeloproliferative leukaemia (MPL) mutations [6]. In ET, JAK2V617F mutations occur in about 55%, CALR mutations in 15–24% and MPL mutations in about 4% of cases. The remainder, about 20%, are so-called triple negative [6]. Median age at diagnosis of ET is in the sixth decade of life [5], with less than 20% of patients being diagnosed below age 40 years [7]. From a population point of view, ET is one of the rare (< 1%) pathologies associated with ACS [1]. From a haematological point of view, the incidence of ACS in patients with ET ranges from 2% to 31% in various studies [1, 8,9,10]. From a cardiologist’s point of view, we recently showed that the prevalence of ET can be at least 2.1% in certain groups [3]. Taking into account that ACS is considered extremely rare in ET patients under 40 years of age [1], the incidence in various older age groups remains to be elucidated.
Diagnosis, clinical features and differential diagnosis of ET
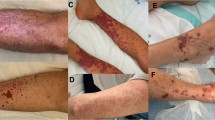
A diagnosis of ET is based on the criteria in Tab. 1 [6]. Upon diagnosis, most patients report having experienced either no (27%) or aspecific vasomotor symptoms (66%), ranging from abdominal and atypical chest pain, paraesthesia, dysaesthesia and headaches to syncope. More specific symptoms are erythromelalgia, a syndrome consisting of localised painful burning, redness, warmness and congestion in the extremities [10, 11], as well as aquagenic pruritus, which arises after contact with water [12]. Less than half (44%) report previous symptoms related to thrombosis (18%) or haemorrhage (26%). Unfortunately, some patients initially present with severe complications, such as peripheral, pulmonary, portal vein, cerebral or coronary embolisms or haemorrhagic pericardial effusion. Finally, splenomegaly (26%) and hepatomegaly (3%) were noticed in patients with abdominal pain and ET [13, 14]. Increased routine laboratory screening within the general population will increase the incidental finding of thrombocytosis. At present, thrombocytosis is encountered in 1.5–2.2% of people consulting primary care [15].
With regard to the differential diagnosis of thrombocytosis, in clinical practice 80–90% of subjects with a platelet count above 450 × 109/l do not have an essential/primary thrombocytosis, but have secondary/reactive thrombocytosis, which is an abnormally high platelet count secondary to underlying events, disease or medication [15, 16] which might be either acute/transient or chronic. In the case of secondary thrombocytosis, the platelet count is rarely > 1000 × 109/l. Second, a peripheral blood smear might differentiate primary from secondary thrombocytosis, since in contrast to secondary thrombocytosis, in which platelets appear normal, giant platelets may be observed in primary thrombocytosis [17]. In contrast to primary thrombocytosis, reactive thrombocytosis rarely results in thrombotic or haemorrhagic events [16]. Another, in fact erroneous, cause of ‘thrombocytosis’ may result from the use of automated analysers. A variety of clinical conditions may result in spuriously raised platelet counts [18, 19] when these small fragments are counted as platelets by the automated analyser. Hence, a peripheral blood smear might also differentiate primary/essential from artefactual/spurious thrombocytosis. Examples are given in Tab. 2.
Thrombotic and haemorrhagic complications associated with ET
Overall rates of complications—in various regions/settings and using different definitions of events—during long-term follow-up in patients with ET are relatively high. The incidence of thrombotic complications ranges from 9% to 84% at diagnosis and from 7% to 32% during long-term follow-up. For haemorrhage these rates range from 4% to 63% at diagnosis and from 8% to 14% during follow-up [8, 20, 21]. With regard to arterial events, cerebrovascular events (relative proportion 55–56%) were shown to be more prevalent than either coronary (22–31%) or peripheral (13–22%) embolisms [1, 8, 9]. With regard to recurrence, as many as 34% of patients with prior thrombosis experience a recurrent thrombotic event. The highest risk for recurrent events is observed within the first 2 years after the first thrombotic event and slowly declines thereafter. Antithrombotic therapy reduces the incidence of recurrent events by about 50% [9].
These numbers clearly indicate that thrombotic complications surpass haemorrhagic complications, that both types of events often coincide with the initial diagnosis of ET and that treatment of ET (after diagnosis) markedly reduces, but does not annihilate, the chance of a second event occurring. Moreover, although ET is a haematological diagnosis, these data also imply that other specialists, such as neurologists, cardiologists and vascular surgeons, should play a proactive role in the identification of ET patients and should thus actively screen for, for example, laboratory anomalies or discrepancies between the event and the risk profile of a patient.
Numerically, myocardial infarction is a rare complication of ET with an incidence rate ranging from 2% to 31% within various studies/regions/settings/periods/definitions [1, 8, 10]. Interestingly, ACS in the setting of ET has been observed in the presence and absence of underlying atherosclerosis [22, 23]. Consequently, as supported by several studies [22], even young patients with ET without cardiovascular (CV) risk factors can experience acute, even life-threatening, thrombotic events. A study that specifically focused on patients that were diagnosed with ET at a young age (i.e. a median age of 31 years) showed similar results compared to those in older adults, with arterial events (18%) being more common than venous events (6%), and cerebrovascular events (13%) more likely than coronary or peripheral embolisms (2% each) [7].
Possible mechanism for complications in ET
Both thrombotic and haemorrhagic complications have been observed in the setting of ET. Interestingly, many ET patients that present with an ACS have a normal CAG without signs of atherosclerosis [10, 22,23,24,25,26], which supports the hypothesis that vascular events can be a direct result of the haematological problem, i.e. be unrelated to pre-existing atherosclerosis [27]. In support of this, an > 80% incidence of spontaneous platelet aggregation was shown in patients with ET [28] and large thrombus burden is often described in cases of ACS in the setting of ET (e.g. [29, 30]). Platelet function tests such as prothrombin time, partial thromboplastin time and bleeding time are usually within reference ranges [30]. In those cases where no obstructive coronary artery disease is observed during CAG, it could be argued that thrombus may have resolved after initiation of antiplatelet/anticoagulation therapy combined with delayed (i.e. after several days or months) CAG (e.g. [25]). On the other hand, spasm could be provoked by provocation testing in at least two cases [25, 26], suggesting that besides hyperviscosity, endothelial dysfunction or the release of certain platelet-derived vasospasm-promoting substances, such as serotonin and thromboxane A2, might also contribute to the aetiology of ACS in the setting of ET. In support of this, Cheng and Hung [25] described a patient that experienced recurrent anginal symptoms after discontinuation of diltiazem, but no recurrence of symptoms after discontinuation of cytoreductive therapy despite platelets counts > 900 × 109/l. Both mechanisms for acute occlusion, i.e. thrombotic and vasospastic, and their possible underlying aetiologies are depicted in Fig. 1 [23].
Likewise, alterations in platelet function and composition have been implied in long-term complications of ET (see Fig. 1). For example, organised fibrin, which will replace aggregates of platelets that have become attached to the endothelial surface, may result in extensive intraluminal narrowing of coronary arteries, causing anginal symptoms [27]. Alternatively, the production of proinflammatory eicosanoids [23] and cytokines in the setting of MPNs is thought to explain the development of premature atherosclerosis (and malignancies, see below) secondary to a state of chronic low-grade (endothelial) inflammation in these patients [31]. Finally, other vascular changes, including smooth muscle cell proliferation and fibromuscular intima proliferation, have been described in arterioles as a result of thrombocytosis [11].
Risk factors for thrombotic and haemorrhagic complications in ET
Age (> 60 years), previous events, the presence of JAK2V617F, leukocytosis and long-term thrombocytosis have been identified as major risk factors for thromboembolic complications [5, 6, 9, 14]. Additionally, the traditional CV risk factors advanced age, smoking, hypertension, hyperlipidaemia and diabetes have been associated with thrombotic complications in the setting of ET [1, 5, 6]. Importantly, the presence of extreme thrombocytosis (platelets > 1000 × 109/l) was associated with a lower risk of thrombosis, possibly through the presence of acquired von Willebrand syndrome (i.e. the structural and/or functional alterations in von Willebrand factor as a result of a concurrent disorder, such as an MPN or, for example, a CV disorder such as aortic stenosis/Heyde syndrome) and consequent increased risk of bleeding [5]. Even at lower rates, platelet number does not seem to be a good predictor of thrombotic events [17]. Also in young patients (< 40 years), cardiovascular risk factors are concurrent stimuli for arterial thrombosis and the use of tobacco was shown to reduce 10-year event-free survival from 90% to 72% [7].
With regard to the risk of recurrent CV events, patients with ET have been stratified into very low-, low-, intermediate- and high-risk individuals (see Tab. 3). Low-risk individuals have an annual risk of thrombosis that is not significantly different from that of the general population at about 0.6–1.3%/year, while the risk increases to 1.8–3.7%/year in the high-risk population [5].
Haematological complications and mortality in ET patients
ET, similar to the other MPNs, can undergo several transformations. First, transformation of ET into PV has been described in about 2% of patients [32]. Next, both ET and PV can transform/progress into (post-ET/PV) myelofibrosis [33]. In ET, evolution into myelofibrosis occurs in about 3% of patients after 5 years, in 8% after 10 years, and in 15% after 15 years [34]. For PV these rates are slightly higher [35]. Finally, all three MPNs can transform directly, and for ET/PV indirectly via post-ET/PV myelofibrosis, into acute myeloid leukaemia. Overall transformation rates of the three MPNs into leukaemia are in the order of ET (2.6%), PV (3.9%) and PMF (9.3%) in 20 years [36]. Finally, patients with MPNs have a higher risk of various second malignancies, e.g. of the skin, brain, kidney and endocrine organs (odds ratio ≥ 2.5) [32, 36, 37]. As a result, patients with MPNs have reduced survival rates. Five-year survival rates for ET, PV and PMF are 89%, 88% and 45%, respectively [36]. Taken together, all MPNs have now been associated with a slightly reduced life expectancy for which age > 60, leukocytosis, male gender, the presence of concurrent adverse mutations and a history of thrombosis conferred independent risk factors [6].
ET and pregnancy
Due to abnormal thrombocyte function, pregnancy in the context of MPNs poses unique fetal and maternal challenges. In ET, about 30% of pregnancies are lost, mostly within the first trimester [38]. From a cardiological point of view, a medical history of repeated fetal losses in, notably young, patients with (recurrent) thrombotic events should raise the suspicion of underlying haematological pathology. Hence, taking a gynaecological medical history is warranted in these subjects, since it might add to the understanding of the pathophysiology of thrombotic events in low-risk individuals.
Treatment of thrombocytosis and prevention of thrombotic events
Treatment of ET should be individualised, bearing in mind all possible complications of ET ranging from thrombotic and haemorrhagic events, the presence of risk factors, and the risk of progression to myelofibrosis or myeloid leukaemia. For clinicians in Europe, the European LeukemiaNET (www.leukemia-net.org) provides guidance based on the accumulating evidence with regard to optimal treatment.
With regard to the prevention of thrombotic complications in ET, the advocated approach [6], based on risk stratification by a history of (arterial or venous) thrombosis, age > 60 years, the presence of a JAK2 mutation and cardiovascular risk factors, is presented in Tab. 3. As an exception, in the case of a definite diagnosis of coronary vasospasm, discontinuation of cytoreductive therapy may be advised in patients below 60 years and in those over 60 years in the absence of a JAK2 mutation and cardiovascular risk factors. Otherwise (see Tab. 3), antiplatelet agents, e.g. acetylsalicylic acid (ASA), are the first-line choice in the prevention of (recurrent) events, since there is little experience with P2Y12 inhibitors [21].
Next in line are cytoreductive therapies, including hydroxyurea (HU), anagrelide and interferon‑α, all of which should be initiated with monitoring of platelet count, erythrocyte and leukocyte levels. Generally, HU is the drug of first choice. In the landmark randomised clinical trial, HU was shown to reduce the risk of thrombotic complications from 10.7% to 1.6% [39]. Due to concerns regarding an increased risk of development of leukaemia during the use of HU, other drugs such as anagrelide were developed. However, in subsequent trials HU + ASA were shown to be superior to anagrelide + ASA with regard to vascular events and transformation rates [40]. For HU, there are no clear contraindications. Interferon‑α is contraindicated in patients with known cardiovascular/thyroid disease. Anagrelide, a phosphodiesterase inhibitor with positive inotropic/chronotropic effects, should be used with caution in patients with known cardiovascular disease, and its use should be accompanied by accurate monitoring of cardiac function and QT interval before and during treatment [20]. Finally, a selective JAK1/JAK2 inhibitor, ruxolitinib, has been developed and has proven its efficacy in patients with both PCV and myelofibrosis, but its use in ET seems limited [6].
Prevention of recurrent thrombotic events in ET
In a large retrospective study of patients with primary thrombocytosis who received either antiplatelet agents, anticoagulation, cytoreductive treatment or underwent phlebotomy after a first thromboembolic complication, only cytoreductive therapy resulted in a significant reduction in recurrent ACS [9]. Recently, the use of ASA twice daily has been advocated in specific individuals (Tab. 3). An alternative way to prevent recurrent arterial events might be the use of more aggressive, e.g. double antiplatelet, therapy in the first 3–4 years following an arterial thrombotic event [41]. Importantly, antiplatelet therapy plus anticoagulants should be used with caution, since their co-use was shown to result in an almost three times higher incidence of major bleeding as compared to either antiplatelet drugs or anticoagulants alone [9].
Treatment of the cardiovascular complications of ET
With regard to the treatment of ACS in the setting of ET, thrombus aspiration [24, 30], intracoronary thrombolysis [24], balloon angioplasty [30], stent placement [42, 43], coronary bypass grafting (CABG) [44] and systemic fibrinolytic/thrombolytic treatment [10] have all been described. The optimal choice of intervention and the timing thereof should be on an individual basis, taking the presence of spasm, thrombus, atherosclerosis, ongoing ischaemia and concurrent platelet counts into account. In the case of thrombotic occlusions (Fig. 2), besides double antiplatelet therapy, aspiration thrombectomy, intracoronary thrombolysis, antithrombotic therapy with, for example, heparin and/or glycoprotein IIb/IIIa receptor antagonists, and even the use of distal protection devices have all been advocated [24]. Diltiazem was used successfully in a case of coronary vasospasm in the setting of ET [25]. Finally, intracoronary imaging such as intravascular ultrasound or optical coherence tomography could be helpful for evaluating endothelial structure and the presence of atherosclerosis [24].
Flow diagram for the treatment of patients with underlying essential thrombocytosis and an acute coronary syndrome or stable angina undergoing coronary angiography (CAG). TIMI thrombolysis in myocardial infarction, DAPT double antiplatelet therapy, PCI percutaneous intervention, CABG coronary artery bypass grafting, STEMI ST-elevation myocardial infarction
The main problem with regard to percutaneous coronary interventions (PCIs) in ET patients lies in the choice of antiplatelet regimen due to the high risk of in-stent thrombosis associated with ‘thrombopathy’ [43, 45]. In-stent thromboses have been described in patients with ET after treatment with ASA monotherapy [46], ASA in combination with P2Y12 inhibition [47] and in a patient who underwent primary PCI while having a platelet count of 2100 × 109/l after initiation of ASA, P2Y12 inhibition and cytoreductive therapy [48]. Consequently, although a relation between the absolute platelet count and the risk of thrombosis has been a matter of dispute, delayed stenting (up to several weeks) to initiate (additional) antiplatelet therapy and/or achieve lower platelet counts (i.e. below 400–600 × 109/l) has been advocated [21, 39, 49]. In support of this strategy, several cases with favourable outcomes have been described when stenting of a significant stenosis, with preserved flow (i.e. thrombolysis in myocardial infarction (TIMI) grade 2 or 3), was delayed several weeks to initiate cytoreductive therapy [49], while a case series of 15 did not show an increased risk of complications in PCI patients with an average platelet count of 581 × 109/l [42].
Hence, it has been argued that revascularisation in patients with a platelet count > 400–600 × 109/l should be discussed in a multidisciplinary team, taking both the risk of thrombosis and progressive ischaemia into account (Fig. 2). In the case of ongoing ischaemia, an early invasive intervention should be performed regardless of the patient’s platelet count (see Fig. 2). In such a situation, periprocedural platelet apheresis can provide a rapid and relatively safe reduction of platelets [21]. Apheresis, however, has a short duration of action and acute stent thrombosis has been described in the setting of a PCI that was performed directly after platelet apheresis [48], supporting the notion that cytoreductive therapy needs to be started as soon as possible [21] or apheresis repeated until adequate platelet counts have been achieved.
With regard to surgery, including CABG, reduction of platelet counts to below normal levels (i.e. < 400 × 109/l) with cytoreductive or even platelet apheresis therapy (see Fig. 2) has been advocated in the perioperative setting [21]. Additionally, daily platelet counts are warranted and resumption of cytoreductive therapy is recommended as soon as the patient is able to take oral medication [21]. Conversely, it has been advised that ASA be discontinued a week before surgery if there is a high risk of bleeding or when perioperative anticoagulation is required, but can be restarted 24 h after surgery if no excessive bleeding has occurred or is anticipated [20]. Off-pump procedures have been advocated in cases of haematological disease to avoid adverse effects of cardiopulmonary bypass and surgical complications of extreme bleeding or thrombosis. Finally, bioprostheses should be preferred to avoid lifetime warfarin therapy [50].
Conclusion
Although ET is a rare haematological malignancy, its prevalence is much higher in patients presenting with either thrombotic or haemorrhaghic events, such as patients seen by a cardiologist, neurologist or vascular surgeon. Any specialist should be aware of such underlying pathology and thus actively screen for elevated thrombocytes (i.e. a platelet count > 450 × 109/l) in patients presenting with either thrombotic or haemorrhaghic events, notably in those without concomitant CV risk factors. If the platelet count continues to be elevated, a haematologist should be consulted and underlying disease should be excluded. Finally, therapeutic options with regard to treatment of ischaemia should be discussed in a multidisciplinary team, including a haematologist, prior to any intervention to prevent complications related to abnormal platelet functions.
References
Rossi C, Randi ML, Zerbinati P, Rinaldi V, Girolami A. Acute coronary disease in essential thrombocythemia and polycythemia vera. J Intern Med. 1998;244:49–53.
Cheitlin MD, McAllister HA, de Castro CM. Myocardial infarction without atherosclerosis. JAMA. 1975;231:951–9.
Kok L, Taverne LF, Verbeek EC, et al. Essential thrombocytosis in patients < 40 years old with acute coronary syndromes: a not so uncommon underlying diagnosis often overlooked. Cureus. 2022;14:e32638.
Cengiz B, Aytekin V, Bildirici U, et al. A rare cause of acute coronary syndromes in young adults—myeloproliferative neoplasms: a case series. Rev Port Cardiol (Engl Ed). 2019;38:613–7.
Barbui T, Finazzi G, Carobbio A, et al. Development and validation of an international prognostic score of thrombosis in world health organization-essential thrombocythemia (IPSET-thrombosis). Blood. 2012;120:5128–33. quiz 5252.
Tefferi A, Barbui T. Polycythemia vera and essential thrombocythemia: 2021 update on diagnosis, risk-stratification and management. Am J Hematol. 2020;95:1599–613.
Alvarez-Larrán A, Cervantes F, Bellosillo B, et al. Essential thrombocythemia in young individuals: frequency and risk factors for vascular events and evolution to myelofibrosis in 126 patients. Leukemia. 2007;21:1218–23.
Carobbio A, Thiele J, Passamonti F, et al. Risk factors for arterial and venous thrombosis in WHO-defined essential thrombocythemia: an international study of 891 patients. Blood. 2011;117:5857–9.
De Stefano V, Za T, Rossi E, et al. Recurrent thrombosis in patients with polycythemia vera and essential thrombocythemia: incidence, risk factors, and effect of treatments. Haematologica. 2008;93:372–80.
Scheffer MG, Michiels JJ, Simoons ML, Roelandt JR. Thrombocythemia and coronary artery disease. Am Heart J. 1991;122:573–6.
Michiels JJ, Abels J, Steketee J, van Vliet HH, Vuzevski VD. Erythromelalgia caused by platelet-mediated arteriolar inflammation and thrombosis in thrombocythemia. Ann Intern Med. 1985;102:466–71.
Le Gall-Ianotto C, Le Calloch R, Couturier M‑A, et al. Aquagenic pruritus in essential thrombocythemia is associated with a higher risk of thrombosis. J Thromb Haemost. 2019;17:1950–5.
Davis RB. Acute thrombotic complications of myeloproliferative disorders in young adults. Am J Clin Pathol. 1985;84:180–5.
Tefferi A, Fonseca R, Pereira DL, Hoagland HC. A long-term retrospective study of young women with essential thrombocythemia. Mayo Clin Proc. 2001;76:22–8.
Mathur A, Samaranayake S, Storrar NP, Vickers MA. Investigating thrombocytosis. BMJ. 2019;366:l4183.
Rokkam VR, Kotagiri R. Secondary thrombocytosis. 2022. http://www.ncbi.nlm.nih.gov/books/NBK560810/. Accessed 3 Apr 2022.
Petrides PE, Siegel F. Thrombotic complications in essential thrombocythemia (ET): clinical facts and biochemical riddles. Blood Cells Mol Dis. 2006;36:379–84.
Zandecki M, Genevieve F, Gerard J, Godon A. Spurious counts and spurious results on haematology analysers: a review. Part I: platelets. Int J Lab Hematol. 2007;29:4–20.
Madaan GB, Jairajpuri ZS, Hajini FF, Jetley S. Postoperative thrombocytosis: an unusual case report. Int J Appl Basic Med Res. 2015;5:225–7.
Barbui T, Barosi G, Grossi A, et al. Practice guidelines for the therapy of essential thrombocythemia. A statement from the Italian society of hematology, the Italian society of experimental hematology and the Italian group for bone marrow transplantation. Haematologica. 2004;89:215–32.
Elliott MA, Tefferi A. Thrombosis and haemorrhage in polycythaemia vera and essential thrombocythaemia. Br J Haematol. 2005;128:275–90.
Derks A, Bloemer MH. Essential thrombocythaemia as cause of myocardial infarction. Neth Heart J. 2001;9:383–5.
Douste-Blazy P, Taudou MJ, Delay M, et al. Essential thrombocythaemia and recurrent myocardial infarction. Lancet. 1984;2:992.
Khaheshi I, Memaryan M, Taherkhani M, Serati A, Movahed MR. Acute ST-segment elevation myocardial infarction as the first manifestation of essential thrombocytosis successfully treated with thrombectomy alone. Cardiovasc Interv Ther. 2016;31:275–8.
Cheng C‑W, Hung M‑J. Coronary spasm-related acute myocardial infarction in a patient with essential thrombocythemia. World J Cardiol. 2011;3:278–80.
Koh KK, Cho SK, Kim SS, Oh BH, Lee YW. Coronary vasospasm, multiple coronary thrombosis, unstable angina and essential thrombocytosis. Int J Cardiol. 1993;41:168–70.
Virmani R, Popovsky MA, Roberts WC. Thrombocytosis, coronary thrombosis and acute myocardial infarction. Am J Med. 1979;67:498–506.
Hehlmann R, Jahn M, Baumann B, Köpcke W. Essential thrombocythemia. Clinical characteristics and course of 61 cases. Cancer. 1988;61:2487–96.
Soucy-Giguère M‑C, Turgeon PY, Sénéchal M. What cardiologists should know about essential thrombocythemia and acute myocardial infarction: report of two cases and advanced heart failure therapies considerations. Int Med Case Rep J. 2019;12:253–9.
Gangadharan V, Ticku J, Kasi V. Acute myocardial infarction in a 21-year-old male due to essential thombocytosis. Cath Lab Digest. 2014;22:1–7.
Hasselbalch HC, Bjørn ME. MPNs as inflammatory diseases: the evidence, consequences, and perspectives. Mediators Inflamm. 2015;2015:102476.
Hong J, Lee JH, Byun JM, et al. Risk of disease transformation and second primary solid tumors in patients with myeloproliferative neoplasms. Blood Adv. 2019;3:3700–8.
Kundranda MN, Tibes R, Mesa RA. Transformation of a chronic myeloproliferative neoplasm to acute myelogenous leukemia: does anything work? Curr Hematol Malig Rep. 2012;7:78–86.
Cervantes F, Alvarez-Larrán A, Talarn C, Gómez M, Montserrat E. Myelofibrosis with myeloid metaplasia following essential thrombocythaemia: actuarial probability, presenting characteristics and evolution in a series of 195 patients. Br J Haematol. 2002;118:786–90.
Tefferi A, Guglielmelli P, Larson DR, et al. Long-term survival and blast transformation in molecularly annotated essential thrombocythemia, polycythemia vera, and myelofibrosis. Blood. 2014;124:2507–13. quiz 2615.
Smith CJ, Thomas JW, Ruan G, et al. A population-based study of outcomes in polycythemia vera, essential thrombocythemia, and primary myelofibrosis in the United States from 2001 to 2015: comparison with data from a Mayo Clinic single institutional series. Am J Hematol. 2021;96:E464–8.
Landtblom AR, Bower H, Andersson TM‑L, et al. Second malignancies in patients with myeloproliferative neoplasms: a population-based cohort study of 9379 patients. Leukemia. 2018;32:2203–10.
Gangat N, Tefferi A. Myeloproliferative neoplasms and pregnancy: overview and practice recommendations. Am J Hematol. 2021;96:354–66.
Cortelazzo S, Finazzi G, Ruggeri M, et al. Hydroxyurea for patients with essential thrombocythemia and a high risk of thrombosis. N Engl J Med. 1995;332:1132–7.
Harrison CN, Campbell PJ, Buck G, et al. Hydroxyurea compared with anagrelide in high-risk essential thrombocythemia. N Engl J Med. 2005;353:33–45.
Landolfi R, Di Gennaro L. Prevention of thrombosis in polycythemia vera and essential thrombocythemia. Haematologica. 2008;93:331–5.
Campo G, Valgimigli M, Carletti R, Fileti L, Ferrari R. Long-term outcome after percutaneous coronary intervention in patients with essential thrombocythemia. J Thromb Haemost. 2009;7:1235–8.
Zheng Y, Xu T, Chen L, Lin S, Chen S. Percutaneous coronary intervention in patients with essential thrombocythemia: case reports and literature review. Platelets. 2020;31:815–9.
Schölzel BE, Endeman H, Dewilde W, Yilmaz A, de Weerdt O, Ten Berg JM. Cardiac surgery in a patient with essential thrombocythemia: a case report. Neth Heart J. 2010;18:378–80.
Bošnjak I, Selthofer-Relatić K, Periša V, Steiner R. Therapeutic dilemmas in the treatment of acute coronary syndrome as manifestation of essential thrombocythaemia. J Cardiol Cases. 2013;8:168–71.
Mehran R, Aymong ED, Ashby DT, et al. Safety of an aspirin-alone regimen after intracoronary stenting with a heparin-coated stent: final results of the HOPE (HEPACOAT and an antithrombotic regimen of aspirin alone) study. Circulation. 2003;108:1078–83.
Işılak Z, Tezcan M, Atalay M, Kardeşoğlu E. PP-285 Acute myocardial infarction and subsequently in-stent thrombosis associated with occult essential thrombocytosis. Am J Cardiol. 2014;113:S120–1.
Turgut T, Harjai KJ, Edupuganti R, et al. Acute coronary occlusion and in-stent thrombosis in a patient with essential thrombocythemia. Cathet Cardiovasc Diagn. 1998;45:428–33.
Kumagai N, Mitsutake R, Miura S, et al. Acute coronary syndrome associated with essential thrombocythemia. J Cardiol. 2009;54:485–9.
Aydın U, Turk T, Ata Y, Yavuz S. Cardiac surgery in patients with essential thrombocytosis: a report of three cases. Eur Res J. 2016;2:147–50.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
R.S. Kuipers, L. Kok, R. Virmani and A. Tefferi declare that they have no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kuipers, R.S., Kok, L., Virmani, R. et al. Essential thrombocytosis: diagnosis, differential diagnosis, complications and treatment considerations of relevance for a cardiologist. Neth Heart J 31, 371–378 (2023). https://doi.org/10.1007/s12471-023-01757-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12471-023-01757-4