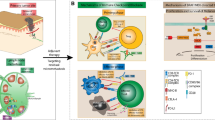
Opinion statement
Melanoma is one of the deadliest malignancies. Its incidence has been significantly increasing in most countries in recent decades. Acral melanoma (AM), a peculiar subgroup of melanoma occurring on the palms, soles, and nails, is the main subtype of melanoma in people of color and is extremely rare in Caucasians. Although great progress has been made in melanoma treatment in recent years, patients with AM have shown limited benefit from current therapies and thus consequently have worse overall survival rates. Achieving durable therapeutic responses in this high-risk melanoma subtype represents one of the greatest challenges in the field. The frequency of BRAF mutations in AM is much lower than that in cutaneous melanoma, which prevents most AM patients from receiving treatment with BRAF inhibitors. However, AM has more frequent mutations such as KIT and CDK4/6, so targeted therapy may still improve the survival of some AM patients in the future. AM may be less susceptible to immune checkpoint inhibitors because of the poor immunogenicity. Therefore, how to enhance the immune response to the tumor cells may be the key to the application of immune checkpoint inhibitors in advanced AM. Anti-angiogenic drugs, albumin paclitaxel, or interferons are thought to enhance the effectiveness of immune checkpoint inhibitors. Combination therapies based on the backbone of PD-1 are more likely to provide greater clinical benefits. Understanding the molecular landscapes and immune microenvironment of AM will help optimize our combinatory strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Melanoma is classified into four major subtypes: skin melanomas without chronic sun-induced damage; skin melanomas with chronic sun-induced damage; mucosal melanomas; acral melanomas [1]. Acral melanoma (AM) is rare in Caucasians (1–7%) [2,3,4,5] but has a higher incidence in non-White individuals, accounting for up to 50–58% of all melanomas in Asians [6] and even more (60–70%) in Blacks [7].
Although AM causes a large number of deaths in Europe and the USA and exhibits unique clinical and biological characteristics, due to its relative rarity, there are few related studies, and it is often overlooked. Hence, the understanding of AM is still limited. Herein, we reviewed the therapeutic status of AM and proposed potential therapeutic strategies based on its genomics and tumor immune microenvironment characteristics.
Current status
Chemotherapy
For patients with metastatic melanoma, dacarbazine was the most commonly used chemotherapy drug for decades but its efficacy was not ideal [8,9,10,11]. Besides failed dacarbazine-based therapy, very little is known about salvage chemotherapy in metastatic AM patients. For example, a clinical trial in China confirmed the efficacy and safety of albumin paclitaxel + carboplatin to treat metastatic melanoma. This study showed that the disease control rate (DCR) in AM patients was 81.3%, the median progression-free survival (PFS) was 6 months, and the median overall survival (OS) was 17 months [12]. Besides this study, there are few studies regarding other chemotherapy drugs to treat AM.
Immunotherapy
Immune checkpoint inhibitors
Since 2011, many immune checkpoint inhibitors (ICIs) have been approved by relevant authorities in the USA, Europe, and China for melanoma treatments. These drugs mainly include ipilimumab, pembrolizumab, nivolumab, and torpalimab [13]. Notably, AM patients respond much worse to ICIs than CM patients [14,15,16,17].
Nivolumab, pembrolizumab, and torpalimab monotherapy can lead to better clinical outcomes in advanced AM patients compared to ipilimumab [18••]. Previous studies have shown that for advanced AM patients who received first-line treatment with ipilimumab, the objective response rate (ORR) was 17.8%, the median PFS was 6.9 months, and the median OS was 38.7 months [19]. For advanced AM patients who received post-line treatment with ipilimumab, the ORR was 11.4%, the median PFS was 2.5 months, and the median OS was 7.1–16.7 months [20, 21]. In contrast, advanced AM patients who received first-line anti-PD-1 monotherapy had an ORR of 34.0–40.0%, median PFS of 3.1–9.2 months, and median OS of 18.6–60.1 months [19, 22]. Advanced AM patients who received post-line therapy with anti-PD-1 monotherapy had an ORR of 14.0–32.0%, median PFS of 3.2–4.1 months, and median OS of 16.9–25.8 months [17, 22,23,24,25,26,27].
Notably, AM patients at different primary sites might respond differently to ICIs. For example, one retrospective study in Japan with 193 advanced AM patients who received nivolumab or pembrolizumab (nail apparatus = 70; palm and sole = 123), of which 143 were first-line treatments, showed that the ORRs of the palm and sole group and the nail apparatus group were 21.1 and 8.6%, respectively, and the median OS were 22.3 and 12.8 months, respectively [28•].
Oncolytic virus
Talimogene laherparepvec (T-VEC) is an oncolytic virus that induces tumor-specific T-cell responses via reduction of virally mediated suppression of antigen presentation, stimulation of viral pathogenicity, and enhancement of tumor-selective replication [29,30,31]. Recent case studies have confirmed the efficacy of this drug in AM patients [32].
Additionally, a phase II clinical trial evaluated the safety and efficacy of OrionX010 (a herpes simplex virus type I oncolytic virus) with 26 unresectable melanoma patients in China, of which 18 (69.2%) were AM patients. In this trial, AM patients presented a median PFS of 3.0 months and a median OS of 19.2 months [33•].
Imiquimod
Imiquimod is a Toll-like receptor 7 (TLR7) agonist. It promotes the induction of CD4+ T cells and the antitumor response of CD8+ T cells by activating TLR7 located on antigen-presenting cells and shifts the immune response to a direction mediated by the T helper 1 (Th1) cells [34, 35]. A retrospective analysis, which included 20 cases of melanoma patients (AM=10) with locoregional cutaneous metastases of melanoma (LCMM), evaluated the response of LCMM to cryotherapy combined with 5% imiquimod local treatment. Regarding locoregional response, 13 patients (65%) responded to treatment, eight (40%) of these completely and five (25%) partially. In assessing overall response, three patients (15%) had complete response and one patient (5%) had stable disease [36].
Molecular targeted therapy
The efficacy of targeted therapy in AM patients has been previously demonstrated. The main signaling pathways known as abnormal during AM onset include MAPK, PI3K/AKT/PTEN, JAK/STAT3, MDM2/TP53, WNT, MCR1-MITF, TERT, and WNT/CDK4/CDKN2A [37]. To date, approximately half (42–55%) of AMs studied have BRAF, RAS, or NF1 mutations, besides triple-wild-type (TWT) mutations [38]. TWT driver mutations include genetic alterations in various genes, such as KIT, CCND1, CTNNB1, KDR(VEGFR2), MDM2, BCL2, AKT3, IDH1, GNAS, CDK4, CDKN2A, MITF, PTEN, RB1, TP53, APC, ERBB2, ERBB3, NUAK2, ABCB5, and TERT [37,38,39,40,41,42,43,44,45].
BRAF/MEK inhibitors
The frequency of BRAF mutations in AM is low (only 15–20%) [46,47,48,49], which limits the use of BRAF inhibitors in AM patients. Common BRAF inhibitors include vemurafenib, dabrafenib, and encorafenib [50•]. Recently, 20 Chinese AM patients with a BRAF mutation that received vemurafenib presented an ORR of 69.2%, a median PFS of 5.4 months, and a median OS of 11.7 months [51].
The combination of BRAF and MEK inhibitors has been widely used to treat BRAF-mutant melanoma patients, achieving satisfactory results [52]. The current MEK inhibitors used to treat melanoma mainly include trametinib, cobimetinib, and bindetinib [53]. For example, a phase II clinical trial in China observed long-term survival outcomes for unresectable or metastatic acral/cutaneous melanoma patients, including 12 AM patients who presented an ORR of 83.3% and a 3-year OS of 35.7% [54•]. Another retrospective analysis included 112 advanced melanoma patients (11 AM and 3 mucosal melanoma patients) who received a combination of BRAF and MEK inhibitors. In this study, AM and mucosal melanoma patients presented an ORR of 64.3% [55].
KIT inhibitors
KIT mutations and/or amplifications are more common in AM than those in other melanoma types (10–20%) [56, 57]. Currently, common KIT inhibitors include imatinib, sunitinib, dasatinib, and nilotinib [58], but only imatinib and nilotinib are effective in AM. A phase II clinical trial in China evaluating the effectiveness of imatinib in 43 metastatic melanoma patients (AM = 21) harboring c-Kit mutation or amplification showed a median PFS of 3.5 months, a 6-month PFS rate of 36.6%, and DCR of 53.5% [59]. Similarly, a retrospective analysis of 78 metastatic melanoma patients (AM = 42) with c-Kit mutation or amplification treated with imatinib presented median OS and PFS of all patients of 13.1 and 4.2 months, respectively. The ORR and DCR were 21.8% and 60.3%, respectively [60]. Notably, a phase II clinical trial showed that imatinib was ineffective in patients with only KIT amplification (the best overall response rate was 0%) [61]. Moreover, previous phase II clinical studies have shown an ORR of 25–32% and a DCR of 74–80% in advanced AM patients treated with nilotinib [62, 63]. Additionally, previous studies showed that sunitinib and dasatinib were less effective to treat advanced AM patients with KIT mutations [64, 65].
CDK4/6 inhibitors
The CDK4/CCND1 mutation and amplification are often present in AM, suggesting that CDK4/6 inhibitors can be used for treatment. In a recent phase II trial, 15 advanced AM patients with genetic aberrations in the CDK pathway were treated with palbociclib. Three (20.0%) patients achieved tumor shrinkage at 8 weeks, including one with confirmed partial response. The median PFS was 2.2 months, and the median OS was 9.5 months [66•]. Additionally, trials with the CDK inhibitor dinaciclib for the treatment of advanced melanoma, including AM, have been completed but the results were not published (NCT00937937).
Targeted therapy for NRAS-mutant AM
Currently, there are no drugs that directly target NRAS mutations. NRAS regulates the PI3K/Akt cascade and BRAF activation, resulting in subsequent activation of the MAPK pathway. Therefore, NRAS mutation is a genetic mutation contributing to acquired BRAF inhibitor resistance [67••]. The current treatments of melanoma patients with NRAS mutations are primarily focused on the use of MEK inhibitors to target key signal transduction pathways of the MAPK pathway [68]. Binimetinib was the first MEK inhibitor to show activity in the treatment of NRAS-mutant melanomas, but the effects were not ideal [60, 69, 70]. Clinical trials with the novel MEK inhibitor HL-085 for the treatment of advanced melanoma are being recruited (NCT05217303, NCT05263453, NCT03973151). Additionally, the potential therapeutic value of MEK inhibitors combined with inhibition of downstream effectors (MAPK, PI3K, or CDK4/6) or upstream effectors (RTK, STK19) for melanomas with NRAS mutations has been demonstrated, but the choice remains controversial [71]. There is currently a lack of reports regarding treatments of AM with NRAS mutations.
Combination therapies
Combination of immunotherapies
Interferon-α (IFN-α) stimulates the secretion of IP-10 (CXCL10), recruits effector T cells to the tumor microenvironment, and upregulates the expression of MHC-1 molecules on the surface of tumor cells, thereby enhancing the anti-tumor effects of CD8+T cells in the tumor microenvironment [72]. Additionally, IFN-α upregulates the expression of PD-L1 [73]. A retrospective analysis in China suggested that prior therapy with PEG-IFN-α improved the median recurrence-free survival of adjuvant pembrolizumab in resectable advanced melanoma [74•]. These results indicated the feasibility of IFN-α combined with PD-1 inhibitors to treat advanced AM.
Furthermore, CTLA-4 and PD-1 can inhibit anti-tumor immunity through different mechanisms [75]. At the 2021 ESMO Annual Meeting, a retrospective study evaluated the efficacy of combination therapy with PD-1 and CTLA4 inhibitors versus single ICIs in 256 advanced AM patients. Among them, 151, 51, and 54 received anti-PD1, anti-CTLA4, and anti-PD1 and anti-CTLA4 combination therapy, respectively. The median follow-up was 8.1 years, the ORRs were 26, 12, and 44%, respectively, and the median PFS were 7.0, 4.9, and 7.3 months, respectively [76]. The combination of ICIs was superior to ICI alone for ORR, but not for PFS or OS. A phase Ib clinical trial in China (NCT04197882) confirmed the feasibility of the oncolytic virus OrionX010 combined with toripalimab to treat 24 patients with resectable stage IIIB-IVM1a AM. In this trial, 81% of patients showed pathologic responses, and 33% presented radiographic responses. The median follow-up time was 8.9 months and no patient presented recurrence. The recurrence-free survival assessment is ongoing.
Combination of chemotherapy and immunotherapy
Temozolomide can enhance the antitumor activity of pembrolizumab by depleting or inhibiting regulatory T cells (Tregs) in the tumor microenvironment [77, 78]. A multicenter retrospective analysis in China with 69 metastatic melanoma patients (28 cases of AM) presented an ORR and median PFS for pembrolizumab plus temozolomide significantly superior to pembrolizumab or temozolomide alone [79•]. These results partly suggested that anti-PD-1 combined with temozolomide can be used as a first-line treatment option for unresectable advanced melanoma, including AM. Another retrospective analysis with 20 advanced AM patients who received treatment with a PD-1 inhibitor plus albumin paclitaxel presented an ORR of 20% and a DCR of 75% [80•].
Combination of targeted therapy and immunotherapy
Inhibition of BRAF and MEK can exert immunomodulatory effects and enhance anti-tumor immunity [81,82,83,84]. Increased expression of PD-1 and its ligand, PD-L1, has been reported in advanced melanoma patients treated with BRAF inhibitors [85]. Additionally, MEK inhibitors protect tumor-infiltrating CD8+ T cells from death caused by T-cell receptors(TCR) stimulation [86]. Besides the IMspire150 trial, the keynote-022 and COMBI-I trials did not demonstrate that compared with targeted drugs, PD-(L)1 monoclonal antibody combined with BRAF and MEK inhibitors increased the PFS in BRAF mutation-positive advanced melanoma patients [87,88,89]. However, no reports on AM are currently available.
Combination of immunotherapy with antiangiogenic therapy
Anti-angiogenic drugs can improve patient response to ICIs by promoting antitumor immunity [90••]. A Chinese clinical trial evaluating the safety and efficacy of camrelizumab combined with apatinib in advanced AM patients is in progress (NCT03955354). Preliminary results showed that among the 27 AM patients, the ORR and DCR were 22.2% and 77.8%, respectively. The median PFS was 8.0 months and the 1-year durable response rate was 83.3%.
Combination of chemotherapy with antiangiogenic therapy
The combination of anti-angiogenesis therapy and chemotherapy might produce a synergistic antitumor effect. A phase III clinical trial in China with 110 metastatic melanoma patients (54 AM patients) showed that, compared with the dacarbazine group, the Endostar plus dacarbazine group had significant improvements in median PFS (4.5 months vs 1.5 months) and median OS (12.0 months vs 8.0 months) [91]. A phase II clinical trial in China with 29 patients (8 AM cases) evaluated the efficacy and safety of apatinib combined with temozolomide in advanced melanoma patients whose immunotherapy failed. The subgroup analysis showed a median PFS of 4.0 months and a median OS of 10.1 months in AM patients [92•]. Phase I trial of this regimen combined with anti-PD-1 treatment for AM is in progress (NCT04397770).
Targeted combination therapy
The efficacy of the combination of BRAF and MEK inhibitors in advanced AM patients has been described above. Notably, for melanomas with NRAS mutations, inhibition of MEK alone is not sufficient to completely inhibit the activation of downstream signaling mediated by NRAS through CDK4 [93]. Similarly, in vitro studies confirmed that overexpression of cyclins D1 and CDK4 mediates resistance to BRAF inhibitors [94]. A previous study confirmed that CDK4/6 inhibitors can overcome acquired resistance to BRAF/MEK inhibitors [95]. These results provided a theoretical basis for BRAF/MEK inhibitors combined with CDK4/6 inhibitors to treat advanced melanoma. However, another report showed that palbociclib combined with BRAF and MEK inhibitors might not work after BRAF inhibitor resistance is acquired [96].
Future directions
Over the past decade, with the introduction of immune checkpoint inhibitors and targeted drugs, the prognosis of advanced/metastatic melanoma patients has dramatically improved. For example, their 5-year overall survival rate substantially rose from less than 10% to up to 40–50% [97]. However, due to the unique genomic and tumor immune microenvironment characteristics of AM, the efficacy of ICIs and targeted drugs in advanced AM patients is not ideal, and the treatment is still facing difficulties. Hence, exploring new treatment strategies is urgent.
Immunotherapy
Acral melanoma has a unique tumor microenvironment, including relatively low expression of PD-L1 [98,98,100], a decreased number of tumor-infiltrating lymphocytes (TILs) [101,102,103,104], and a high neutrophil-lymphocyte ratio (NLR) [105]. This can also lead to poor efficacy of current ICI treatments in advanced AM patients. How to enhance a patient’s anti-tumor immune response by targeting new immune checkpoints as well as combination therapy might be the key to future immunotherapies.
Novel immune checkpoints
Due to the lack of studies with sufficient samples, the expression levels of immune checkpoints in the AM immune microenvironment remain unclear. Only one small-sample study showed that immune cells related to AM expressed multiple immune checkpoints, including PD-1, LAG-3, CTLA-4, VISTA, TIGIT, TIM-3, and ADORA2. Compared to CM, the expression of VISTA and ADORA2 was increased in AM, and the expression of TIGIT was similar. Although the expression of TIM-3 was significantly lower in AM than that in CM, it was still expressed in 29.2% of myeloid cells. For AM, VISTA, ADORA2, TIGIT, and TIM-3 might become novel immune checkpoints with research value in the future [106••].
Adenosine A2A receptor (ADORA2) can inhibit the accumulation of CD8+ T and NK cells in the tumor microenvironment [107,108,109], suggesting that ADORA2 might become a new immune checkpoint for AM treatment. Multiple antagonists of ADORA2 have been developed, with early indications that these compounds can effectively work combined with anti-PD-1 with responses in patients who derived no benefit from prior anti-PD-1/PD-L1 therapy [110].
VISTA (V-domain immunoglobin suppressor of T cell activation) is a type I transmembrane protein that has a high degree of structural homology with PD-L1 and is highly expressed in multiple immune cells [111, 112]. Preclinical studies have shown that inhibiting VISTA can inhibit the proliferation of CD4+ and CD8+ T cells [112]. The enhanced expression of VISTA in melanoma cells can also lead to increased PD-L1 expression in tumor-associated macrophages, increased Treg infiltration, and reduced MHC expression on dendritic cells [113]. Additionally, the expression of VISTA is related to acquired resistance to anti-PD-1 treatment [114, 115]. The results above indicated that the combination therapy of anti-VISTA and anti-PD-1 might be a potential and valuable treatment. For example, CA-170, a small-molecule dual antagonist of VISTA/PD-L1, is currently in phase I clinical trial (NCT02812875).
The T cell immunoreceptor with immunoglobulin and ITIM domain (TIGIT) is an inhibitory immune checkpoint upregulated in tumor antigen-specific CD8+ T cells and TILs of melanoma patients and is co-expressed with PD-1. TIGIT binds to two ligands, CD155 and CD112, that are expressed by melanoma cells and antigen-presenting cells to downregulate T and NK cell functions [116, 117]. Dual PD-1/TIGIT blockade also enhances the proliferation and function of tumor antigen-specific CD8+ T cells and TILs isolated from melanoma patients compared to single PD-1 blockade [118]. There is also evidence that increased expression of CD155 is associated with resistance to anti-PD-1 therapy [119]. Therefore, combining PD-1 and TIGIT inhibitors might be a potential strategy to treat AM patients. Currently, two related phase I/II studies are in progress (NCT04305041, NCT04305054).
The abnormal expression of T cell immunoglobulin and mucin domain 3 (TIM-3) in tumor tissues is often closely related to T cell depletion [120,121,122,123,124]. Previous studies have shown that, compared with T cells only expressing PD-1, T cells expressing both TIM-3 and PD-1 are more inhibited [125, 126]. Additionally, the upregulation of TIM-3 expression is related to the acquired resistance to anti-PD-1/PD-L1 treatment [127]. These results provided a theoretical basis for TIM-3 inhibitors combined with anti-PD-1/PD-L1 drugs in the treatment of advanced tumors. There are currently two ongoing clinical trials exploring the role of combined blockade of TIM-3 and PD-1 in advanced melanoma (NCT04139902, NCT04370704). However, multiple ligands of TIM-3 result in a single TIM-3 inhibitor that might not completely block the effects of TIM-3 [128]. Moreover, trametinib, a MEK inhibitor, can promote the expression of TIM-3, resulting in decreased CD8 + T cells, while anti-TIM-3 monoclonal antibody enhances anti-tumor immune function by stimulating CD8+ T cells, and reverses the depletion of T and NK cells in the trametinib-induced immune microenvironment. This suggested that trametinib combined with anti-TIM-3 drugs might also be a potential choice for melanoma treatments [129].
Additionally, the potential value of various new inhibitory immune checkpoints (BTLA [130,131,132], B7 family [133,134,135,136,137], IDO-1 [138, 139], LAG-3 [140, 141]) and costimulatory molecules (GITR [142,143,144,145], ICOS [146,147,148], OX40 [149,150,151], 4-1BB [152,153,154], CD40 [155, 156], CD27 [157,158,159]) has been confirmed for melanoma treatments. The efficacy and safety of some inhibitory checkpoints and costimulatory molecules in melanoma treatments, alone or combined with ICIs, have been preliminarily elucidated in early clinical trials [160,161,162,163,164,165,166,167,168,169,170,171,172,173] and others are in progress (NCT04773951, NCT04137900, NCT02554812). Currently, no large-sample study comparing the expression levels of these immune checkpoints in AM and CM tissues is available. Hence, the role of these immune checkpoints in AM treatments remains to be verified by further clinical trials.
Adoptive cell therapy
Adoptive cell therapy consists of the isolation of TILs from the excised tumor, expansion of these cells by interleukin-2 (IL-2) treatment, and reinfusion into lympho-depleted patients with IL-2 treatment. At present, although adoptive cell therapy (ACT) has achieved certain efficacy in advanced CM patients, there is a lack of clinical trials and retrospective analysis for AM patients to confirm its applicability in the treatment of advanced AM. Only a very small sample of clinical trials in Japan showed that this therapy might have a certain effect on advanced AM after treatment with ICIs [174].
Chimeric antigen receptor-engineered T cell therapy
The chimeric antigen receptor (CAR) structure consists of a single-chain variable fragment derived from a monoclonal antibody targeting a cancer-specific antigen, intracellular segment, signaling domain derived from TCR, and one or more co-stimulatory sequences (CD28, OX40, or 4-1BB) [175]. CAR can bind antigens to cancer cells and activate T cells [176]. CAR T-cell therapy had success in treating patients with hematologic diseases. However, little is known about its efficacy in AM patients.
GD2 gangliosides are sialic acid-containing glycosphingolipids that are overexpressed on several solid tumors, including melanoma [177]. A preclinical study with lesion samples from 288 melanoma patients evaluated the ability of anti-GD2/4-1BB CAR T cells to kill ganglioside GD2+ melanoma cells. Among the 288 samples, 49.3% (142/288) demonstrated positive staining for ganglioside GD2. Its expression was relatively more frequent in acral (50.0%) and mucosal (56.3%) melanomas than that in CSD (14.3%) and non-CSD (33.3%) melanomas. The median OS of patients exhibiting ganglioside GD2 expression was significantly shorter than those without ganglioside GD2 expression (31 months vs 47.1 months) [152]. This study provided a theoretical basis for the treatment of advanced AM with anti-GD2/4-1BB CAR T cells.
Vaccines
Vaccines for melanoma treatment might be used as antigen whole tumor cells, RNA or DNA, single or multiple peptides, or APCs displaying the target antigen [178]. At present, clinical trials of a large number of vaccines combined with ICIs in the treatment of advanced CM are in progress.
Tebentafusp
Tebentafusp is a bispecific protein consisting of a high-affinity T-cell receptor fused to an anti-CD3 effector that can redirect T cells to target glycoprotein 100-positive cells [179]. A phase III clinical trial showed that treatment with tebentafusp resulted in longer overall survival than therapy with single-agent pembrolizumab, ipilimumab, or dacarbazine among previously untreated patients with metastatic uveal melanoma [180]. Glycoprotein 100 (Gp-100) is a transmembrane glycoprotein, highly expressed in normal melanocytes and melanoma cells. HMB-45, which recognizes Glycoprotein 100 (Gp-100), has been repeatedly proven to be a sensitive and relatively specific marker of melanomas. Eighty percent of AMs stained with HMB-45 in a previous study [181]. Furthermore, tebentafusp potently activated antitumor immune responses in patients with metastatic melanoma [182]. These suggested that tebentafusp might be a potential choice for advanced AM treatment.
Targeted therapy
Antibody-drug conjugates
Antibody-drug conjugates (ADCs) combine a monoclonal antibody (mAb) with a cytotoxic agent, allowing its specific delivery to targeted tumor cells overexpressing cognate tumor-associated antigens (TAAs) [183]. Previous studies have confirmed the antitumor activity and safety of ADCs such as glembatumumab vedotin and DEDN6526A in patients with advanced non-AM [184,185,186,187]. In addition, many clinical trials of ADC in the treatment of advanced melanoma are underway [188]. We look forward to the efficacy of ADCs in patients with advanced AM.
Emerging gene mutations
Besides low tumor mutational burden (TMB) [40] and low gene expression associated with antigen presentation and T cell inflammation [189, 190], AM has other unique genomic characteristics. Compared to CM, the frequency of BRAF (18% vs 46%) and RAS (21% vs 31%) mutations is lower in AM, but the frequency of NF1 mutation is higher (23% vs 10%), and the proportion of TWT is also higher (38% vs. 11%). Mutations in c-kit, NOTCH2, TYRP1, and PTEN, as well as oncogenic amplification of genes including TERT, CDK4, MDM2, CCND1, PAK1, and GAB2, are more common in AM than those in CM [191, 192••]. Novel targeted drugs targeting common mutations/amplification of AM might be potential targeted therapies for AM in the future.
Previous studies have shown that TERT aberrations are observed in 41% of AM patients, and in vitro TERT inhibition has cytotoxic effects on AM cells [193]. Another Chinese study showed that the incidence of TERT copy number gain in AM (61.5%) was higher than that in other melanoma subtypes [194], suggesting that TERT inhibition might be a potential therapeutic strategy for AM.
A study showed that 47.5% of all specimens presented an increase in the CCND1 copy number. This increase was associated with the Breslow thickness in AM [195]. This suggested that CCND1 might also be a potential target for AM treatment. NUAK2 participates in the regulation of proliferation and migration of melanoma cells by regulating the cell cycle [196]. A study showed that NUAK2 is negatively correlated with the OS and PFS of AM patients, and this effect is higher than that of CM [44], suggesting that NUAK2 has potential value for AM treatment. Previous studies have also reported that CDK4/6 inhibition can induce and maintain the T cell inflammatory microenvironment [197], and enhance the efficacy of PD-L1 checkpoint blockade [198, 199]. Additionally, genetic abnormalities in the CDK4 pathway are associated with innate resistance to anti-PD-1 therapy [200•]. These results provided a theoretical basis for CDK4/6 inhibitors combined with anti-PD-(L)1 antibody to treat advanced AM.
Cancer cells are often defective in DNA damage response and repair (DDR) and highly depend on other DNA repair pathways to avoid lethal DNA damage. Alterations in DDR genes have been shown to promote the expression of PD-L1, elevate the count of TILs, increase the TMB, and enhance immunogenicity via an increased neoantigen load, which are also potential determinants of the response to ICI treatments [201, 202]. Ataxia-telangiectasia and Rad3-related protein kinase (ATR) are essential components of DDR [203]. Recent clinical trials have confirmed that ceralasertib, an oral ATR inhibitor, combined with durvalumab/paclitaxel is effective in advanced melanoma (including AM) [204•, 205]. Moreover, PARP inhibitors have been proved to have therapeutic potential for tumors with DDR defects (including ATM and ATR mutations) [206, 207], and might also be used to treat advanced AM in the future. MDM2 can inhibit DRR by inhibiting p53 function [208]. The amplification of MDM2 is related to the excessive progression of tumors in metastatic AM patients after treatment with ICIs, suggesting that it might also become a potential target for advanced AM treatment in the future [209].
Furthermore, EP300 encodes the histone acetyltransferase paralogue p300 that manipulates different cellular processes (e.g., proliferation, apoptosis, and DNA repair) and promotes tumor growth through its downstream oncogene target MITF [210]. Recently, a study in China showed that, compared with other subtypes, EP300 copies in limb melanoma increased more frequently, and 30% (70/233) of AM lesion samples carried the copy number gains of the EP300-MITF axis. Additionally, AM with copy number gains of the EP300 pathway tended to be more aggressive [211]. Therefore, the EP300-MITF pathway might become a potential clinical target. Another study showed that the p300 inhibitor C646 can overcome the resistance of melanoma cells to BRAF inhibitors in vitro and in vivo, which also provides a theoretical basis for targeted combination therapy [212]. Additionally, HDAC inhibitors have been previously shown to inhibit the expression of MITF [213], and combined with nivolumab in the treatment of advanced melanoma it achieved good ORR [214], and related clinical trials are under recruitment (NCT04674683).
A preclinical study in China showed that the higher infiltration of cancer-associated fibroblasts was related to innate resistance of AM to PD-1 inhibitors, and the FAK inhibitor defactinib enhanced the efficacy of anti-PD-1 antibodies. This study provided a basis for the combination of FAK inhibitors and PD-1 inhibitors in the treatment of advanced AM [215].
The feasibility of using PI3K/Akt/mTOR inhibitors [216], ERK inhibitors [217], or WNT inhibitors [218] to treat AM is currently being investigated.
Conclusions
At present, AM lacks effective intervention measures. The unique genomic and tumor immune microenvironment characteristics of AM are responsible for its poor response to current immunotherapy and other systemic treatments, and combined treatment is more likely to provide long-term clinical benefits. The clinical efficacy and safety of multiple combined therapies are currently being extensively studied. With an in-depth understanding of the genomic and tumor immune microenvironment characteristics of AM, it is possible to develop effective therapeutic drugs or methods and improve the survival of patients.
Data availability:
Not applicable.
Code availability:
Not applicable.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Lv J, Dai B, Kong Y, Shen X, Kong J. Acral melanoma in Chinese: a clinicopathological and prognostic study of 142 cases. Sci Rep. 2016;6:31432.
Cormier JN, Xing Y, Ding M, Lee JE, Mansfield PF, Gershenwald JE, Ross MI, Du XL. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166(17):1907–14.
Shaw JH, Koea JB. Acral (volar-subungual) melanoma in Auckland. New Zealand. Br J Surg. 1988;75(1):69–72.
Cascinelli N, Zurrida S, Galimberti V, Bartoli C, Bufalino R, Del Prato I, Mascheroni L, Testori A, Clemente C. Acral lentiginous melanoma. A histological type without prognostic significance. J Dermatol Surg Oncol. 1994;20(12):817–22.
Ridgeway CA, Hieken TJ, Ronan SG, Kim DK, Das Gupta TK. Acral lentiginous melanoma. Arch Surg. 1995;130(1):88–92.
Chang JW. Acral melanoma: a unique disease in Asia. JAMA Dermatol. 2013;149(11):1272–3.
Hudson DA, Krige JE. Melanoma in black South Africans. J Am Coll Surg. 1995;180(1):65–71.
Middleton MR, Grob JJ, Aaronson N, Fierlbeck G, Tilgen W, Seiter S, Gore M, Aamdal S, Cebon J, Coates A, Dreno B, Henz M, Schadendorf D, Kapp A, Weiss J, Fraass U, Statkevich P, Muller M, Thatcher N. Randomized phase III study of temozolomide versus dacarbazine in the treatment of patients with advanced metastatic malignant melanoma. J Clin Oncol. 2000;18(1):158–66.
Falkson CI, Ibrahim J, Kirkwood JM, Coates AS, Atkins MB, Blum RH. Phase III trial of dacarbazine versus dacarbazine with interferon alpha-2b versus dacarbazine with tamoxifen versus dacarbazine with interferon alpha-2b and tamoxifen in patients with metastatic malignant melanoma: an Eastern Cooperative Oncology Group study. J Clin Oncol. 1998;16(5):1743–51.
Avril MF, Aamdal S, Grob JJ, Hauschild A, Mohr P, Bonerandi JJ, Weichenthal M, Neuber K, Bieber T, Gilde K, Guillem Porta V, Fra J, Bonneterre J, Saïag P, Kamanabrou D, Pehamberger H, Sufliarsky J, Gonzalez Larriba JL, Scherrer A, Menu Y. Fotemustine compared with dacarbazine in patients with disseminated malignant melanoma: a phase III study. J Clin Oncol. 2004;22(6):1118–25.
Yi JH, Yi SY, Lee HR, Lee SI, Lim DH, Kim JH, Park KW, Lee J. Dacarbazine-based chemotherapy as first-line treatment in noncutaneous metastatic melanoma: multicenter, retrospective analysis in Asia. Melanoma Res. 2011;21(3):223–7.
Guo YQ, Ding Y, Li DD, Li JJ, Peng RQ, Wen XZ, Zhang X, Zhang XS. Efficacy and safety of nab-paclitaxel combined with carboplatin in Chinese patients with melanoma. Med Oncol. 2015;32(9):234.
Leonardi GC, Candido S, Falzone L, Spandidos DA, Libra M. Cutaneous melanoma and the immunotherapy revolution (Review). Int J Oncol. 2020;57(3):609–18.
Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, Larkin J, Lorigan P, Neyns B, Blank CU, Hamid O, Mateus C, Shapira-Frommer R, Kosh M, Zhou H, et al. KEYNOTE-006 investigators. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372(26):2521–32.
Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, Ferrucci PF, Hill A, Wagstaff J, Carlino MS, Haanen JB, Maio M, Marquez-Rodas I, McArthur GA, Ascierto PA, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34.
Hazarika M, Chuk MK, Theoret MR, Mushti S, He K, Weis SL, Putman AH, Helms WS, Cao X, Li H, Zhao H, Zhao L, Welch J, Graham L, Libeg M, Sridhara R, Keegan P, Pazdur R. U.S. FDA approval summary: nivolumab for treatment of unresectable or metastatic melanoma following progression on ipilimumab. Clin Cancer Res. 2017;23:3484–8.
Shoushtari AN, Munhoz RR, Kuk D, Ott PA, Johnson DB, Tsai KK, Rapisuwon S, Eroglu Z, Sullivan RJ, Luke JJ, Gangadhar TC, Salama AK, Clark V, Burias C, Puzanov I, Atkins MB, Algazi AP, Ribas A, Wolchok JD, Postow MA. The efficacy of anti-PD-1 agents in acral and mucosal melanoma. Cancer. 2016;122(21):3354–62.
•• Zheng Q, Li J, Zhang H, Wang Y, Zhang S. Immune checkpoint inhibitors in advanced acral melanoma: a systematic review. Front Oncol. 2020;10:602705. This reference is of outstanding importance because it systematically analyzes the therapeutic effects and safety profile of ICI treatments in advanced AM, and paves the way for further research on the treatment of advanced AM.
Saberian C, Ludford K, Roszik J, Gruschkus S, Johnson DH, Bernatchez C, et al. Analysis of tumor mutation burden (TMB), PD-L1 status and clinical outcomes with checkpoint inhibitors (CPI) in acral melanoma (AM). Pigment Cell Melanoma Res. 2020;33(1):226–7.
Johnson DB, Peng C, Abramson RG, Ye F, Zhao S, Wolchok JD, Sosman JA, Carvajal RD, Ariyan CE. Clinical activity of ipilimumab in acral melanoma: a retrospective review. Oncologist. 2015;20(6):648–52.
Yamazaki N, Kiyohara Y, Uhara H, Tsuchida T, Maruyama K, Shakunaga N, Itakura E, Komoto A. Real-world safety and efficacy data of ipilimumab in Japanese radically unresectable malignant melanoma patients: a postmarketing surveillance. J Dermatol. 2020;47(8):834–48.
van Not OJ, de Meza MM, van den Eertwegh AJM, Haanen JB, Blank CU, Aarts MJB, van den Berkmortel FWPJ, van Breeschoten J, de Groot JB, Hospers GAP, Ismail RK, Kapiteijn E, Piersma D, van Rijn RS, Stevense-den Boer MAM, van der Veldt AAM, Vreugdenhil G, Bonenkamp HJ, Boers-Sonderen MJ, et al. Response to immune checkpoint inhibitors in acral melanoma: a nationwide cohort study. Eur J Cancer. 2022;167:70–80.
Si L, Zhang X, Shu Y, Pan H, Wu D, Liu J, Lou F, Mao L, Wang X, Wen X, Gu Y, Zhu L, Lan S, Cai X, Diede SJ, Zhou Y, Ge J, Li J, Wu H, Guo J. A phase Ib study of pembrolizumab as second-line therapy for chinese patients with advanced or metastatic melanoma (KEYNOTE-151). Transl Oncol. 2019;12(6):828–35.
Nathan P, Ascierto PA, Haanen J, Espinosa E, Demidov L, Garbe C, Guida M, Lorigan P, Chiarion-Sileni V, Gogas H, Maio M, Fierro MT, Hoeller C, Terheyden P, Gutzmer R, Guren TK, Bafaloukos D, Rutkowski P, Plummer R, et al. Safety and efficacy of nivolumab in patients with rare melanoma subtypes who progressed on or after ipilimumab treatment: a single-arm, open-label, phase II study (CheckMate 172). Eur J Cancer. 2019;119:168–78.
Tang B, Chi Z, Chen Y, Liu X, Wu D, Chen J, Song X, Wang W, Dong L, Song H, Wu H, Feng H, Yao S, Qin S, Zhang X, Guo J. Safety, efficacy, and biomarker analysis of toripalimab in previously treated advanced melanoma: results of the POLARIS-01 Multicenter Phase II Trial. Clin Cancer Res. 2020;26(16):4250–9.
Zaremba A, Murali R, Jansen P, Möller I, Sucker A, Paschen A, Zimmer L, Livingstone E, Brinker TJ, Hadaschik E, Franklin C, Roesch A, Ugurel S, Schadendorf D, Griewank KG, Cosgarea I. Clinical and genetic analysis of melanomas arising in acral sites. Eur J Cancer. 2019;119:66–76.
Tang B, Yan X, Sheng X, Si L, Cui C, Kong Y, Mao L, Lian B, Bai X, Wang X, Li S, Zhou L, Yu J, Dai J, Wang K, Hu J, Dong L, Song H, Wu H, et al. Safety and clinical activity with an anti-PD-1 antibody JS001 in advanced melanoma or urologic cancer patients. J Hematol Oncol. 2019;12(1):7.
• Nakamura Y, Namikawa K, Yoshino K, Yoshikawa S, Uchi H, Goto K, Nakamura Y, Fukushima S, Kiniwa Y, Takenouchi T, Uhara H, Kawai T, Hatta N, Funakoshi T, Teramoto Y, Otsuka A, Doi H, Ogata D, Matsushita S, et al. Anti-PD1 checkpoint inhibitor therapy in acral melanoma: a multicenter study of 193 Japanese patients. Ann Oncol. 2020;31(9):1198–206. This reference is of importance because it clearly shows that the objective response to anti-PD-1 treatment in AM patients is significantly affected by primary tumor location.
Andtbacka RH, Kaufman HL, Collichio F, Amatruda T, Senzer N, Chesney J, Delman KA, Spitler LE, Puzanov I, Agarwala SS, Milhem M, Cranmer L, Curti B, Lewis K, Ross M, Guthrie T, Linette GP, Daniels GA, Harrington K, et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol. 2015;33(25):2780–8.
Hughes T, Coffin RS, Lilley CE, Ponce R, Kaufman HL. Critical analysis of an oncolytic herpesvirus encoding granulocyte-macrophage colony stimulating factor for the treatment of malignant melanoma. Oncolytic Virother. 2014;3:11–20.
Rehman H, Silk AW, Kane MP, Kaufman HL. Into the clinic: talimogene laherparepvec (T-VEC), a first-in-class intratumoral oncolytic viral therapy. J Immunother Cancer. 2016;4:53.
Franke V, Smeets PMG, van der Wal JE, van Akkooi ACJ. Complete response to talimogene laherparepvec in a primary acral lentiginous melanoma. Melanoma Res. 2020;30:548–51.
• Cui C, Wang X, Lian B, Ji Q, Zhou L, Chi Z, Si L, Sheng X, Kong Y, Yu J, Li S, Mao L, Tang B, Dai J, Yan X, Bai X, Andtbacka R, Guo J. OrienX010, an oncolytic virus, in patients with unresectable stage IIIC-IV melanoma: a phase Ib study. J Immunother Cancer. 2022;10(4):e004307. This reference is of importance because it clearly shows that OrienX010 oncolytic virotherapy has a tolerable safety profile with antitumor effects in both injected and non-injected metastases and warrants further evaluation in patients with acral melanoma.
Hemmi H, Kaisho T, Takeuchi O, Sato S, Sanjo H, Hoshino K, Horiuchi T, Tomizawa H, Takeda K, Akira S. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002;3(2):196–200.
Narayan R, Nguyen H, Bentow JJ, Moy L, Lee DK, Greger S, Haskell J, Vanchinathan V, Chang PL, Tsui S, Konishi T, Comin-Anduix B, Dauphine C, Vargas HI, Economou JS, Ribas A, Bruhn KW, Craft N. Immunomodulation by imiquimod in patients with high-risk primary melanoma. J Invest Dermatol. 2012;132(1):163–9.
Rivas-Tolosa N, Ortiz-Brugués A, Toledo-Pastrana T, et al. Local cryosurgery and imiquimod: a successful combination for the treatment of locoregional cutaneous metastasis of melanoma: a case series. J Dermatol. 2016;43(5):553–6.
Tod BM, Schneider JW, Bowcock AM, Visser WI, Kotze MJ. The tumor genetics of acral melanoma: what should a dermatologist know? JAAD Int. 2020;1(2):135–47.
Merkel EA, Gerami P. Malignant melanoma of sun-protected sites: a review of clinical, histological, and molecular features. Lab Invest. 2017;97(6):630–5.
Rabbie R, Ferguson P, Molina-Aguilar C, Adams DJ, Robles-Espinoza CD. Melanoma subtypes: genomic profiles, prognostic molecular markers and therapeutic possibilities. J Pathol. 2019;247(5):539–51.
Furney SJ, Turajlic S, Stamp G, Thomas JM, Hayes A, Strauss D, Gavrielides M, Xing W, Gore M, Larkin J, Marais R. The mutational burden of acral melanoma revealed by whole-genome sequencing and comparative analysis. Pigment Cell Melanoma Res. 2014;27(5):835–8.
Leichsenring J, Stögbauer F, Volckmar AL, Buchhalter I, Oliveira C, Kirchner M, Fröhling S, Hassel J, Enk A, Schirmacher P, Endris V, Penzel R, Stenzinger A. Genetic profiling of melanoma in routine diagnostics: assay performance and molecular characteristics in a consecutive series of 274 cases. Pathology. 2018;50(7):703–10.
Moon KR, Choi YD, Kim JM, Jin S, Shin MH, Shim HJ, Lee JB, Yun SJ. Genetic alterations in primary acral melanoma and acral melanocytic nevus in Korea: common mutated genes show distinct cytomorphological features. J Invest Dermatol. 2018;138(4):933–45.
Darmawan CC, Jo G, Montenegro SE, Kwak Y, Cheol L, Cho KH, Mun JH. Early detection of acral melanoma: a review of clinical, dermoscopic, histopathologic, and molecular characteristics. J Am Acad Dermatol. 2019;81(3):805–12.
Namiki T, Coelho SG, Hearing VJ. NUAK2: an emerging acral melanoma oncogene. Oncotarget. 2011;2(9):695–704.
Vásquez-Moctezuma I, Meraz-Ríos MA, Villanueva-López CG, Magaña M, Martínez-Macias R, Sánchez-González DJ, García-Sierra F, Herrera-González NE. ATP-binding cassette transporter ABCB5 gene is expressed with variability in malignant melanoma. Actas Dermosifiliogr. 2010;101(4):341–8.
Curtin JA, Fridlyand J, Kageshita T, Patel HN, Busam KJ, Kutzner H, Cho KH, Aiba S, Bröcker EB, LeBoit PE, Pinkel D, Bastian BC. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353(20):2135–47.
Greaves WO, Verma S, Patel KP, Davies MA, Barkoh BA, Galbincea JM, Yao H, Lazar AJ, Aldape KD, Medeiros LJ, Luthra R. Frequency and spectrum of BRAF mutations in a retrospective, single-institution study of 1112 cases of melanoma. J Mol Diagn. 2013;15(2):220–6.
Oyama S, Funasaka Y, Watanabe A, Takizawa T, Kawana S, Saeki H. BRAF, KIT and NRAS mutations and expression of c-KIT, phosphorylated extracellular signal-regulated kinase and phosphorylated AKT in Japanese melanoma patients. J Dermatol. 2015;42(5):477–84.
Sheen YS, Tan KT, Tse KP, Liao YH, Lin MH, Chen JS, Liau JY, Tseng YJ, Lee CH, Hong CH, Liao JB, Chang HT, Chu CY. Genetic alterations in primary melanoma in Taiwan. Br J Dermatol. 2020;182(5):1205–13.
• Chen YA, Teer JK, Eroglu Z, Wu JY, Koomen JM, Karreth FA, Messina JL, KSM S. Translational pathology, genomics and the development of systemic therapies for acral melanoma. Semin Cancer Biol. 2020;61:149–57. This reference is of importance because it describes the clinical characteristics of acral melanoma and outlines the genetic basis of acral melanoma development.
Bai X, Si L, Chi Z, Sheng X, Cui C, Kong Y, Dai J, Mao LL, Wang X, Li SM, Tang B, Lian B, Zhou L, Yan X, Guo J. Efficacy and tolerability of vemurafenib in BRAF-mutant acral and mucosal melanoma. J Clin Oncol. 2017;35(15_suppl):e21017–7.
Long GV, Flaherty KT, Stroyakovskiy D, Gogas H, Levchenko E, de Braud F, Larkin J, Garbe C, Jouary T, Hauschild A, Chiarion-Sileni V, Lebbe C, Mandalà M, Millward M, Arance A, Bondarenko I, JBAG H, Hansson J, Utikal J, et al. Dabrafenib plus trametinib versus dabrafenib monotherapy in patients with metastatic BRAF V600E/K-mutant melanoma: long-term survival and safety analysis of a phase 3 study. Ann Oncol. 2017;28(7):1631–9.
Grimaldi AM, Simeone E, Festino L, Vanella V, Strudel M, Ascierto PA. MEK inhibitors in the treatment of metastatic melanoma and solid tumors. Am J Clin Dermatol. 2017;18(6):745–54.
• Mao L, Ding Y, Bai X, Sheng X, Dai J, Chi Z, Cui C, Kong Y, Fan Y, Xu Y, Wang X, Tang B, Lian B, Yan X, Li S, Zhou L, Wei X, Li C, Guo J, et al. Overall survival of patients with unresectable or metastatic BRAF V600-mutant acral/cutaneous melanoma administered dabrafenib plus trametinib: long-term follow-up of a multicenter, single-arm phase IIa trial. Front Oncol. 2021;11:720044. This reference is of importance because it confirms the durable and robust antitumor activity and safety of dabrafenib combined with trametinib in Chinese patients with BRAF V600 mutation-positive melanoma, including acral melanoma patients.
Fujisawa Y, Ito T, Kato H, Irie H, Kaji T, Maekawa T, Asai J, Yamamoto Y, Fujimura T, Nakai Y, Yasuda M, Matsuyama K, Muto I, Matsushita S, Uchi H, Nakamura Y, Uehara J, Yoshino K. Outcome of combination therapy using BRAF and MEK inhibitors among Asian patients with advanced melanoma: an analysis of 112 cases. Eur J Cancer. 2021;145:210–20.
Curtin JA, Busam K, Pinkel D, Bastian BC. Somatic activation of KIT in distinct subtypes of melanoma. J Clin Oncol. 2006;24(26):4340–6.
Omholt K, Grafström E, Kanter-Lewensohn L, Hansson J, Ragnarsson-Olding BK. KIT pathway alterations in mucosal melanomas of the vulva and other sites. Clin Cancer Res. 2011;17(12):3933–42.
Meng D, Carvajal RD. KIT as an oncogenic driver in melanoma: an update on clinical development. Am J Clin Dermatol. 2019;20(3):315–23.
Guo J, Si L, Kong Y, Flaherty KT, Xu X, Zhu Y, Corless CL, Li L, Li H, Sheng X, Cui C, Chi Z, Li S, Han M, Mao L, Lin X, Du N, Zhang X, Li J, et al. Phase II, open-label, single-arm trial of imatinib mesylate in patients with metastatic melanoma harboring c-Kit mutation or amplification. J Clin Oncol. 2011;29(21):2904–9.
Wei X, Mao L, Chi Z, Sheng X, Cui C, Kong Y, Dai J, Wang X, Li S, Tang B, Lian B, Yan X, Bai X, Zhou L, Guo J, Si L. Efficacy evaluation of imatinib for the treatment of melanoma: evidence from a retrospective study. Oncol Res. 2019;27(4):495–501.
Hodi FS, Corless CL, Giobbie-Hurder A, Fletcher JA, Zhu M, Marino-Enriquez A, Friedlander P, Gonzalez R, Weber JS, Gajewski TF, O'Day SJ, Kim KB, Lawrence D, Flaherty KT, Luke JJ, Collichio FA, Ernstoff MS, Heinrich MC, Beadling C, et al. Imatinib for melanomas harboring mutationally activated or amplified KIT arising on mucosal, acral, and chronically sun-damaged skin. J Clin Oncol. 2013;31(26):3182–90.
Guo J, Carvajal RD, Dummer R, Hauschild A, Daud A, Bastian BC, Markovic SN, Queirolo P, Arance A, Berking C, Camargo V, Herchenhorn D, Petrella TM, Schadendorf D, Sharfman W, Testori A, Novick S, Hertle S, Nourry C, et al. Efficacy and safety of nilotinib in patients with KIT-mutated metastatic or inoperable melanoma: final results from the global, single-arm, phase II TEAM trial. Ann Oncol. 2017;28(6):1380–7.
Lee SJ, Kim TM, Kim YJ, Jang KT, Lee HJ, Lee SN, Ahn MS, Hwang IG, Lee S, Lee MH, Lee J. Phase II trial of nilotinib in patients with metastatic malignant melanoma harboring KIT gene aberration: a multicenter trial of Korean Cancer Study Group (UN10-06). Oncologist. 2015;20(11):1312–9.
Buchbinder EI, Sosman JA, Lawrence DP, McDermott DF, Ramaiya NH, Van den Abbeele AD, Linette GP, Giobbie-Hurder A, Hodi FS. Phase 2 study of sunitinib in patients with metastatic mucosal or acral melanoma. Cancer. 2015;121(22):4007–15.
Kalinsky K, Lee S, Rubin KM, Lawrence DP, Iafrarte AJ, Borger DR, Margolin KA, Leitao MM Jr, Tarhini AA, Koon HB, Pecora AL, Jaslowski AJ, Cohen GI, Kuzel TM, Lao CD, Kirkwood JM. A phase 2 trial of dasatinib in patients with locally advanced or stage IV mucosal, acral, or vulvovaginal melanoma: a trial of the ECOG-ACRIN Cancer Research Group (E2607). Cancer. 2017;123(14):2688–97.
• Mao L, Dai J, Cao Y, Bai X, Sheng X, Chi Z, Cui C, Kong Y, Zhang Y, Wu L, Wang X, Tang B, Lian B, Yan X, Li S, Zhou L, Wei X, Li C, Qi Z, et al. Palbociclib in advanced acral melanoma with genetic aberrations in the cyclin-dependent kinase 4 pathway. Eur J Cancer. 2021;(148):297–306. This reference is of importance because it clearly shows that palbociclib monotherapy demonstrated preliminary efficacy and an acceptable safety profile in advanced AM patients with CDK4 pathway aberrations.
•• Proietti I, Skroza N, Bernardini N, Tolino E, Balduzzi V, Marchesiello A, Michelini S, Volpe S, Mambrin A, Mangino G, Romeo G, Maddalena P, Rees C, Potenza C. Mechanisms of acquired BRAF inhibitor resistance in melanoma: a systematic review. Cancers (Basel). 2020;12(10):2801. This reference is of outstanding importance because it examines all of the potential ways that melanoma cells develop resistance to BRAF inhibitors to develop strategies for achieving the full therapeutic potential of contemporary treatments in patients with melanoma. In the future, it may be possible to personalize combination therapy towards the specific resistance pathway in individual patients.
Cheng Y, Tian H. Current development status of MEK inhibitors. Molecules. 2017;22(10):1551.
Dummer R, Schadendorf D, Ascierto PA, Arance A, Dutriaux C, Di Giacomo AM, Rutkowski P, Del Vecchio M, Gutzmer R, Mandala M, Thomas L, Demidov L, Garbe C, Hogg D, Liszkay G, Queirolo P, Wasserman E, Ford J, Weill M, et al. Binimetinib versus dacarbazine in patients with advanced NRAS-mutant melanoma (NEMO): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2017;18(4):435–45.
Ascierto PA, Schadendorf D, Berking C, Agarwala SS, van Herpen CM, Queirolo P, Blank CU, Hauschild A, Beck JT, St-Pierre A, Niazi F, Wandel S, Peters M, Zubel A, Dummer R. MEK162 for patients with advanced melanoma harbouring NRAS or Val600 BRAF mutations: a non-randomised, open-label phase 2 study. Lancet Oncol. 2013;14(3):249–56.
Delyon J, Lebbe C, Dumaz N. Targeted therapies in melanoma beyond BRAF: targeting NRAS-mutated and KIT-mutated melanoma. Curr Opin Oncol. 2020;32(2):79–84.
Guo J, Xiao Y, Iyer R, Lu X, Lake M, Ladror U, Harlan J, Samanta T, Tomlinson M, Bukofzer G, Donawho C, Shoemaker A, Huang TH. Empowering therapeutic antibodies with IFN-α for cancer immunotherapy. PLoS One. 2019;14(8):e0219829.
Musella M, Galassi C, Manduca N, Sistigu A. The yin and yang of type I IFNs in cancer promotion and immune activation. Biology (Basel). 2021;10(9):856.
• Jia DD, Niu Y, Zhu H, Wang S, Ma T, Li T. Prior therapy with Pegylated-interferon alfa-2b improves the efficacy of adjuvant pembrolizumab in resectable advanced melanoma. Front Oncol. 2021;11:675873. This reference is of importance because it indicates that prior PEG-IFN-α could enhance the efficacy of adjuvant pembrolizumab. The long-lasting effects of PEG-IFN-α provide a new rationale for designing combination or sequential immunotherapy.
Buchbinder EI, Desai A. CTLA-4 and PD-1 pathways: similarities, differences, and implications of their inhibition. Am J Clin Oncol. 2016;39(1):98–106.
Bhave P, Ahmed T, Shoushtari AN, Zaremba A, Versluis JM, Mangana J, Weichenthal M, Si L, Lesimple T, Robert C, Trojaniello C, Wicky A, Heywood R, Tran L, Batty K, Stansfeld A, Lebbe C, Schwarze JK, Mooradian M. Carlino M.1047P Efficacy of checkpoint inhibitors (CPIs) in acral melanoma (AM)[J]. Annals of Oncology. 2021;32:S876–7.
Ridolfi L, Petrini M, Granato AM, Gentilcore G, Simeone E, Ascierto PA, Pancisi E, Ancarani V, Fiammenghi L, Guidoboni M, de Rosa F, Valmorri L, Scarpi E, Nicoletti SV, Baravelli S, Riccobon A, Ridolfi R. Low-dose temozolomide before dendritic-cell vaccination reduces (specifically) CD4+CD25++Foxp3+ regulatory T-cells in advanced melanoma patients. J Transl Med. 2013;11:135.
Banissi C, Ghiringhelli F, Chen L, Carpentier AF. Treg depletion with a low-dose metronomic temozolomide regimen in a rat glioma model. Cancer Immunol Immunother. 2009;58(10):1627–34.
• Hu T, Sun W, Xu Y, Qu X, Jin Y, Luo Z, Chen Y. Combination of pembrolizumab plus temozolomide therapy in unresectable and advanced melanoma: a multicenter retrospective analysis in China. Ann Transl Med. 2021;9(21):1625. This reference is of importance because it indicates that combining anti-PD-1 with temozolomide has better efficacy than temozolomide-based chemotherapy or anti-PD-1 alone for advanced melanoma treatment without increasing toxicity.
• Li JJ, Wang JH, Dingv Y, Li DD, Wen XZ, Zhao JJ, Jiang H, Liu X, Huang FX, Zhang XS. Efficacy and safety of anti-PD-1 inhibitor combined with nab-paclitaxel in Chinese patients with refractory melanoma. J Cancer Res Clin Oncol. 2022;148(5):1159–69. This reference is of importance because it indicates that Nab-paclitaxel combined with PD-1 antibody is a well-tolerated and effective regimen for patients with refractory melanoma.
Ascierto PA, Dummer R. Immunological effects of BRAF+MEK inhibition. Oncoimmunology. 2018;7(9):e1468955.
Wilmott JS, Long GV, Howle JR, Haydu LE, Sharma RN, Thompson JF, Kefford RF, Hersey P, Scolyer RA. Selective BRAF inhibitors induce marked T-cell infiltration into human metastatic melanoma. Clin Cancer Res. 2012;18(5):1386–94.
Deken MA, Gadiot J, Jordanova ES, Lacroix R, van Gool M, Kroon P, Pineda C, Geukes Foppen MH, Scolyer R, Song JY, Verbrugge I, Hoeller C, Dummer R, Haanen JB, Long GV, Blank CU. Targeting the MAPK and PI3K pathways in combination with PD1 blockade in melanoma. Oncoimmunology. 2016;5(12):e1238557.
Homet Moreno B, Mok S, Comin-Anduix B, Hu-Lieskovan S, Ribas A. Combined treatment with dabrafenib and trametinib with immune-stimulating antibodies for BRAF mutant melanoma. Oncoimmunology. 2015;5(7):e1052212.
Frederick DT, Piris A, Cogdill AP, Cooper ZA, Lezcano C, Ferrone CR, Mitra D, Boni A, Newton LP, Liu C, Peng W, Sullivan RJ, Lawrence DP, Hodi FS, Overwijk WW, Lizée G, Murphy GF, Hwu P, Flaherty KT, et al. BRAF inhibition is associated with enhanced melanoma antigen expression and a more favorable tumor microenvironment in patients with metastatic melanoma. Clin Cancer Res. 2013;19(5):1225–31.
Ebert PJR, Cheung J, Yang Y, McNamara E, Hong R, Moskalenko M, Gould SE, Maecker H, Irving BA, Kim JM, Belvin M, Mellman I. MAP kinase inhibition promotes T cell and anti-tumor activity in combination with PD-L1 checkpoint blockade. Immunity. 2016;44(3):609–21.
Ferrucci PF, Di Giacomo AM, Del Vecchio M, Atkinson V, Schmidt H, Schachter J, Queirolo P, Long GV, Stephens R, Svane IM, Lotem M, Abu-Amna M, Gasal E, Ghori R, Diede SJ, Croydon ES, Ribas A, Ascierto PA. KEYNOTE-022 international team. KEYNOTE-022 part 3: a randomized, double-blind, phase 2 study of pembrolizumab, dabrafenib, and trametinib in BRAF-mutant melanoma. J Immunother Cancer. 2020;8(2):e001806.
Gutzmer R, Stroyakovskiy D, Gogas H, Robert C, Lewis K, Protsenko S, Pereira RP, Eigentler T, Rutkowski P, Demidov L, Manikhas GM, Yan Y, Huang KC, Uyei A, McNally V, McArthur GA, Ascierto PA. Atezolizumab, vemurafenib, and cobimetinib as first-line treatment for unresectable advanced BRAFV600 mutation-positive melanoma (IMspire150): primary analysis of the randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2020;395(10240):1835–44.
Dummer R, Long GV, Robert C, Tawbi HA, Flaherty KT, Ascierto PA, Nathan PD, Rutkowski P, Leonov O, Dutriaux C, Mandalà M, Lorigan P, Ferrucci PF, Grob JJ, Meyer N, Gogas H, Stroyakovskiy D, Arance A, Brase JC, et al. Randomized phase III trial evaluating spartalizumab plus dabrafenib and trametinib for BRAF V600-mutant unresectable or metastatic melanoma. J Clin Oncol. 2022;40(13):1428–38.
•• Hack SP, Zhu AX, Wang Y. Augmenting anticancer immunity through combined targeting of angiogenic and PD-1/PD-L1 pathways: challenges and opportunities. Front Immunol. 2020;11:598877. This reference is of outstanding importance because it clearly explains the mechanisms underpinning VEGF-mediated immunosuppression and how these can be therapeutically abrogated by combined VEGF and PD-(L)1 blockade in patients with cancer to augment antitumor immunity.
Cui C, Mao L, Chi Z, Si L, Sheng X, Kong Y, Li S, Lian B, Gu K, Tao M, Song X, Lin T, Ren X, Qin S, Guo J. A phase II, randomized, double-blind, placebo-controlled multicenter trial of Endostar in patients with metastatic melanoma. Mol Ther. 2013;21(7):1456–63.
• Zhou L, Yang Y, Si L, Chi Z, Sheng X, Lian B, Wang X, Tang B, Mao L, Yan X, Li S, Bai X, Guo J, Cui C. Phase II study of apatinib combined with temozolomide in patients with advanced melanoma after failure of immunotherapy. Melanoma Res. 2022;32(3):142–9. This reference is of importance because it indicates that apatinib plus temozolomide demonstrated promising efficacy and manageable safety profile in patients with advanced melanoma after progression on immunotherapy.
Kwong LN, Costello JC, Liu H, Jiang S, Helms TL, Langsdorf AE, Jakubosky D, Genovese G, Muller FL, Jeong JH, Bender RP, Chu GC, Flaherty KT, Wargo JA, Collins JJ, Chin L. Oncogenic NRAS signaling differentially regulates survival and proliferation in melanoma. Nat Med. 2012;18(10):1503–10.
Smalley KS, Lioni M, Dalla Palma M, Xiao M, Desai B, Egyhazi S, Hansson J, Wu H, King AJ, Van Belle P, Elder DE, Flaherty KT, Herlyn M, Nathanson KL. Increased cyclin D1 expression can mediate BRAF inhibitor resistance in BRAF V600E-mutated melanomas. Mol Cancer Ther. 2008;7(9):2876–83.
Nassar KW, Hintzsche JD, Bagby SM, Espinoza V, Langouët-Astrié C, Amato CM, Chimed TS, Fujita M, Robinson W, Tan AC, Schweppe RE. Targeting CDK4/6 represents a therapeutic vulnerability in acquired BRAF/MEK inhibitor-resistant melanoma. Mol Cancer Ther. 2021;20(10):2049–60.
Martin CA, Cullinane C, Kirby L, Abuhammad S, Lelliott EJ, Waldeck K, Young RJ, Brajanovski N, Cameron DP, Walker R, Sanij E, Poortinga G, Hannan RD, Pearson RB, Hicks RJ, McArthur GA, Sheppard KE. Palbociclib synergizes with BRAF and MEK inhibitors in treatment naïve melanoma but not after the development of BRAF inhibitor resistance. Int J Cancer. 2018;142(10):2139–52.
Schadendorf D, van Akkooi ACJ, Berking C, Griewank KG, Gutzmer R, Hauschild A, Stang A, Roesch A, Ugurel S. Melanoma. Lancet. 2018;392(10151):971–84. 30238891
Patel SP, Kurzrock R. PD-L1 expression as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther. 2015;14(4):847–56.
Munhoz RR, Postow MA. Clinical development of PD-1 in advanced melanoma. Cancer J. 2018;24(1):7–14.
Kaunitz GJ, Cottrell TR, Lilo M, Muthappan V, Esandrio J, Berry S, Xu H, Ogurtsova A, Anders RA, Fischer AH, Kraft S, Gerstenblith MR, Thompson CL, Honda K, Cuda JD, Eberhart CG, Handa JT, Lipson EJ, Taube JM. Melanoma subtypes demonstrate distinct PD-L1 expression profiles. Lab Invest. 2017;97(9):1063–71.
Castaneda CA, Castillo M, Torres-Cabala C, Bernabe LA, Casavilca S, Villegas V, Sanchez J, de la Cruz M, Dunstan J, Cotrina JM, Gomez HL, Chavez C, Landa-Baella MP, Tello K, Felix BF, Abugattas J. Relationship between tumor-associated immune infiltrate and p16 staining over clinicopathological features in acral lentiginous melanoma. Clin Transl Oncol. 2019;21(9):1127–34.
Castaneda CA, Torres-Cabala C, Castillo M, Villegas V, Casavilca S, Cano L, Sanchez J, Dunstan J, Calderon G, De La Cruz M, Cotrina JM, Gomez HL, Galvez R, Abugattas J. Tumor infiltrating lymphocytes in acral lentiginous melanoma: a study of a large cohort of cases from Latin America. Clin Transl Oncol. 2017;19(12):1478–88.
Edwards J, Wilmott JS, Madore J, Gide TN, Quek C, Tasker A, Ferguson A, Chen J, Hewavisenti R, Hersey P, Gebhardt T, Weninger W, Britton WJ, Saw RPM, Thompson JF, Menzies AM, Long GV, Scolyer RA, Palendira U. CD103+ tumor-resident CD8+ T cells are associated with improved survival in immunotherapy-naïve melanoma patients and expand significantly during anti-PD-1 treatment. Clin Cancer Res. 2018;24(13):3036–45.
Edwards J, Ferguson PM, Lo SN. Pires da Silva I, Colebatch AJ, Lee H, Saw RPM, Thompson JF, Menzies AM, Long GV, Newell F, Pearson JV, Waddell N, Hayward NK, Johansson PA, Mann GJ, Scolyer RA, Palendira U, Wilmott JS. Tumor mutation burden and structural chromosomal aberrations are not associated with T-cell density or patient survival in acral, mucosal, and cutaneous melanomas. Cancer Immunol Res. 2020;8(11):1346–53.
Lee J, Lee SJ, Kim K, Kim ST, Jang KT, Lee J. Comprehensive molecular and clinical characterization of Asian melanoma patients treated with anti-PD-1 antibody. BMC Cancer. 2019;19(1):805.
•• Li J, Smalley I, Chen Z, Wu JY, Phadke MS, Teer JK, Nguyen T, Karreth FA, Koomen JM, Sarnaik AA, Zager JS, Khushalani NI, Tarhini AA, Sondak VK, Rodriguez PC, Messina JL, Chen YA, KSM S. Single-cell characterization of the cellular landscape of acral melanoma identifies novel targets for immunotherapy. Clin Cancer Res. 2022;28(10):2131–46. This reference is of outstanding importance because it provides the first single-cell analysis of primary and metastatic acral melanomas and identifies fewer effector CD8 T cells and NK cells and an absence of γδ T cells compared to non-acral cutaneous melanoma. Moreover, this reference further identifies both VISTA, TIGIT, and immunosuppressive adenosine signaling as targetable immune checkpoints in acral melanoma.
Sitkovsky MV, Hatfield S, Abbott R, Belikoff B, Lukashev D, Ohta A. Hostile, hypoxia-A2-adenosinergic tumor biology as the next barrier to overcome for tumor immunologists. Cancer Immunol Res. 2014;2(7):598–605.
Ohta A, Gorelik E, Prasad SJ, Ronchese F, Lukashev D, Wong MK, Huang X, Caldwell S, Liu K, Smith P, Chen JF, Jackson EK, Apasov S, Abrams S, Sitkovsky M. A2A adenosine receptor protects tumors from antitumor T cells. Proc Natl Acad Sci U S A. 2006;103(35):13132–7.
Young A, Ngiow SF, Barkauskas DS, Sult E, Hay C, Blake SJ, Huang Q, Liu J, Takeda K, Teng MWL, Sachsenmeier K, Smyth MJ. Co-inhibition of CD73 and A2AR adenosine signaling improves anti-tumor immune responses. Cancer Cell. 2016;30(3):391–403.
Fong L, Hotson A, Powderly JD, Sznol M, Heist RS, Choueiri TK, George S, Hughes BGM, Hellmann MD, Shepard DR, Rini BI, Kummar S, Weise AM, Riese MJ, Markman B, Emens LA, Mahadevan D, Luke JJ, Laport G, et al. Adenosine 2A receptor blockade as an immunotherapy for treatment-refractory renal cell cancer. Cancer Discov. 2020;10(1):40–53.
Huang X, Zhang X, Li E, Zhang G, Wang X, Tang T, Bai X, Liang T. VISTA: an immune regulatory protein checking tumor and immune cells in cancer immunotherapy. J Hematol Oncol. 2020;13(1):83.
Lines JL, Pantazi E, Mak J, Sempere LF, Wang L, O'Connell S, Ceeraz S, Suriawinata AA, Yan S, Ernstoff MS, Noelle R. VISTA is an immune checkpoint molecule for human T cells. Cancer Res. 2014;74(7):1924–32.
Rosenbaum SR, Knecht M, Mollaee M, Zhong Z, Erkes DA, McCue PA, Chervoneva I, Berger AC, Lo JA, Fisher DE, Gershenwald JE, Davies MA, Purwin TJ, Aplin AE. FOXD3 regulates VISTA expression in melanoma. Cell Rep. 2020;30(2):510-524.e6.
Kakavand H, Jackett LA, Menzies AM, Gide TN, Carlino MS, Saw RPM, Thompson JF, Wilmott JS, Long GV, Scolyer RA. Negative immune checkpoint regulation by VISTA: a mechanism of acquired resistance to anti-PD-1 therapy in metastatic melanoma patients. Mod Pathol. 2017;30(12):1666–76.
Kuklinski LF, Yan S, Li Z, Fisher JL, Cheng C, Noelle RJ, Angeles CV, Turk MJ, Ernstoff MS. VISTA expression on tumor-infiltrating inflammatory cells in primary cutaneous melanoma correlates with poor disease-specific survival. Cancer Immunol Immunother. 2018;67(7):1113–21.
Stanietsky N, Simic H, Arapovic J, Toporik A, Levy O, Novik A, Levine Z, Beiman M, Dassa L, Achdout H, Stern-Ginossar N, Tsukerman P, Jonjic S, Mandelboim O. The interaction of TIGIT with PVR and PVRL2 inhibits human NK cell cytotoxicity. Proc Natl Acad Sci U S A. 2009;106(42):17858–63.
Chauvin JM, Zarour HM. TIGIT in cancer immunotherapy. J Immunother Cancer. 2020;8(2):e000957.
Chauvin JM, Pagliano O, Fourcade J, Sun Z, Wang H, Sander C, Kirkwood JM, Chen TH, Maurer M, Korman AJ, Zarour HM. TIGIT and PD-1 impair tumor antigen-specific CD8+ T cells in melanoma patients. J Clin Invest. 2015;125(5):2046–58.
Lepletier A, Madore J, O'Donnell JS, Johnston RL, Li XY, McDonald E, Ahern E, Kuchel A, Eastgate M, Pearson SA, Mallardo D, Ascierto PA, Massi D, Merelli B, Mandala M, Wilmott JS, Menzies AM, Leduc C, Stagg J, et al. Tumor CD155 expression is associated with resistance to anti-PD1 immunotherapy in metastatic melanoma. Clin Cancer Res. 2020;26(14):3671–81.
Piao YR, Piao LZ, Zhu LH, Jin ZH, Dong XZ. Prognostic value of T cell immunoglobulin mucin-3 in prostate cancer. Asian Pac J Cancer Prev. 2013;14(6):3897–901.
Yuan J, Jiang B, Zhao H, Huang Q. Prognostic implication of TIM-3 in clear cell renal cell carcinoma. Neoplasma. 2014;61(1):35–40.
Zhou E, Huang Q, Wang J, Fang C, Yang L, Zhu M, Chen J, Chen L, Dong M. Up-regulation of Tim-3 is associated with poor prognosis of patients with colon cancer. Int J Clin Exp Pathol. 2015;8(7):8018–27.
Yang M, Yu Q, Liu J, Fu W, Cao Y, Yu L, Shao S, Wang X, Niu H, Wang Y. T-cell immunoglobulin mucin-3 expression in bladder urothelial carcinoma: clinicopathologic correlations and association with survival. J Surg Oncol. 2015;112(4):430–5.
Cao Y, Zhou X, Huang X, Li Q, Gao L, Jiang L, Huang M, Zhou J. Tim-3 expression in cervical cancer promotes tumor metastasis. PLoS One. 2013;8(1):e53834.
Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 2010;207(10):2187–94.
Jin HT, Anderson AC, Tan WG, West EE, Ha SJ, Araki K, Freeman GJ, Kuchroo VK, Ahmed R. Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proc Natl Acad Sci U S A. 2010;107(33):14733–8.
Tian T, Li Z. Targeting Tim-3 in cancer with resistance to PD-1/PD-L1 blockade. Front Oncol. 2021;11:731175.
Das M, Zhu C, Kuchroo VK. Tim-3 and its role in regulating anti-tumor immunity. Immunol Rev. 2017;276(1):97–111.
Liu Y, Cai P, Wang N, Zhang Q, Chen F, Shi L, Zhang Y, Wang L, Hu L. Combined blockade of Tim-3 and MEK inhibitor enhances the efficacy against melanoma. Biochem Biophys Res Commun. 2017;484(2):378–84.
Haymaker CL, Wu RC, Ritthipichai K, Bernatchez C, Forget MA, Chen JQ, Liu H, Wang E, Marincola F, Hwu P, Radvanyi LG. BTLA marks a less-differentiated tumor-infiltrating lymphocyte subset in melanoma with enhanced survival properties. Oncoimmunology. 2015;4(8):e1014246.
Fourcade J, Sun Z, Pagliano O, Guillaume P, Luescher IF, Sander C, Kirkwood JM, Olive D, Kuchroo V, Zarour HM. CD8(+) T cells specific for tumor antigens can be rendered dysfunctional by the tumor microenvironment through upregulation of the inhibitory receptors BTLA and PD-1. Cancer Res. 2012;72(4):887–96.
Dong X, Song J, Chen B, Qi Y, Jiang W, Li H, Zheng D, Wang Y, Zhang X, Liu H. Exploration of the prognostic and immunotherapeutic value of B and T lymphocyte attenuator in skin cutaneous melanoma. Front Oncol. 2021;10:592811.
Seliger B. B7-H abnormalities in melanoma and clinical relevance. Methods Mol Biol. 2014;1102:367–80.
Janakiram M, Chinai JM, Fineberg S, Fiser A, Montagna C, Medavarapu R, Castano E, Jeon H, Ohaegbulam KC, Zhao R, Zhao A, Almo SC, Sparano JA, Zang X. Expression, clinical significance, and receptor identification of the newest B7 family member HHLA2 protein. Clin Cancer Res. 2015;21(10):2359–66.
Lee YH, Martin-Orozco N, Zheng P, Li J, Zhang P, Tan H, Park HJ, Jeong M, Chang SH, Kim BS, Xiong W, Zang W, Guo L, Liu Y, Dong ZJ, Overwijk WW, Hwu P, Yi Q, Kwak L, et al. Inhibition of the B7-H3 immune checkpoint limits tumor growth by enhancing cytotoxic lymphocyte function. Cell Res. 2017;27(8):1034–45.
Flem-Karlsen K, Tekle C, Andersson Y, Flatmark K, Fodstad Ø, Nunes-Xavier CE. Immunoregulatory protein B7-H3 promotes growth and decreases sensitivity to therapy in metastatic melanoma cells. Pigment Cell Melanoma Res. 2017;30(5):467–76.
Quandt D, Fiedler E, Boettcher D, Marsch WC, Seliger B. B7-h4 expression in human melanoma: its association with patients’ survival and antitumor immune response. Clin Cancer Res. 2011;17(10):3100–11.
Wen H, Liu Y, Wang S, Wang T, Zhang G, Chen X, Li Y, Cui H, Lai F, Sheng L. Design and synthesis of indoleamine 2,3-dioxygenase 1 inhibitors and evaluation of their use as anti-tumor agents. Molecules. 2019;24(11):2124.
Iga N, Otsuka A, Hirata M, Kataoka TR, Irie H, Nakashima C, Matsushita S, Uchi H, Yamamoto Y, Funakoshi T, Fujisawa Y, Yoshino K, Fujimura T, Hata H, Ishida Y, Kabashima K. Variable indoleamine 2,3-dioxygenase expression in acral/mucosal melanoma and its possible link to immunotherapy. Cancer Sci. 2019;110(11):3434–41.
Chocarro L, Blanco E, Zuazo M, Arasanz H, Bocanegra A, Fernández-Rubio L, Morente P, Fernández-Hinojal G, Echaide M, Garnica M, Ramos P, Vera R, Kochan G, Escors D. Understanding LAG-3 signaling. Int J Mol Sci. 2021;22(10):5282.
Woo SR, Turnis ME, Goldberg MV, Bankoti J, Selby M, Nirschl CJ, Bettini ML, Gravano DM, Vogel P, Liu CL, Tangsombatvisit S, Grosso JF, Netto G, Smeltzer MP, Chaux A, Utz PJ, Workman CJ, Pardoll DM, Korman AJ, et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012;72(4):917–27.
Leyland R, Watkins A, Mulgrew KA, Holoweckyj N, Bamber L, Tigue NJ, Offer E, Andrews J, Yan L, Mullins S, Oberst MD, Coates Ulrichsen J, Leinster DA, McGlinchey K, Young L, Morrow M, Hammond SA, Mallinder P, Herath A, et al. A novel murine GITR ligand fusion protein induces antitumor activity as a monotherapy that is further enhanced in combination with an OX40 agonist. Clin Cancer Res. 2017;23(13):3416–27.
Scirka B, Szurek E, Pietrzak M, Rempala G, Kisielow P, Ignatowicz L, Miazek A. Anti-GITR antibody treatment increases TCR repertoire diversity of regulatory but not effector T cells engaged in the immune response against B16 melanoma. Arch Immunol Ther Exp (Warsz). 2017;65(6):553–64.
Mahne AE, Mauze S, Joyce-Shaikh B, Xia J, Bowman EP, Beebe AM, Cua DJ, Jain R. Dual roles for regulatory T-cell depletion and costimulatory signaling in agonistic GITR targeting for tumor immunotherapy. Cancer Res. 2017;77(5):1108–18.
Knee DA, Hewes B, Brogdon JL. Rationale for anti-GITR cancer immunotherapy. Eur J Cancer. 2016;67:1–10.
Clemente N, Boggio E, Gigliotti LC, Raineri D, Ferrara B, Miglio G, Argenziano M, Chiocchetti A, Cappellano G, Trotta F, Caldera F, Capucchio MT, Yagi J, Rojo MJ, Renò F, Cavalli R, Dianzani C, Dianzani U. Immunotherapy of experimental melanoma with ICOS-Fc loaded in biocompatible and biodegradable nanoparticles. J Control Release. 2020;320:112–24.
Fromm G, de Silva S, Giffin L, Xu X, Rose J, Schreiber TH. Gp96-Ig/costimulator (OX40L, ICOSL, or 4-1BBL) combination vaccine improves T-cell priming and enhances immunity, memory, and tumor elimination. Cancer Immunol Res. 2016;4(9):766–78.
Aspord C, Leccia MT, Charles J, Plumas J. Plasmacytoid dendritic cells support melanoma progression by promoting Th2 and regulatory immunity through OX40L and ICOSL. Cancer Immunol Res. 2013;1(6):402–15.
Kimura T, Fukushima S, Okada E, Kuriyama H, Kanemaru H, Kadohisa-Tsuruta M, Kubo Y, Nakahara S, Tokuzumi A, Kajihara I, Makino K, Miyashita A, Aoi J, Makino T, Tsukamoto H, Nishimura Y, Inozume T, Zhang R, Uemura Y, et al. Induced pluripotent stem cell-derived myeloid cells expressing OX40 ligand amplify antigen-specific T cells in advanced melanoma. Pigment Cell Melanoma Res. 2020;33(5):744–55.
Peng W, Williams LJ, Xu C, Melendez B, McKenzie JA, Chen Y, Jackson HL, Voo KS, Mbofung RM, Leahey SE, Wang J, Lizee G, Tawbi HA, Davies MA, Hoos A, Smothers J, Srinivasan R, Paul EM, Yanamandra N, Hwu P. Anti-OX40 antibody directly enhances the function of tumor-reactive CD8+ T cells and synergizes with PI3Kβ inhibition in PTEN loss melanoma. Clin Cancer Res. 2019;25(21):6406–16.
Roszik J, Markovits E, Dobosz P, Layani A, Slabodnik-Kaner K, Baruch EN, Ben-Betzalel G, Grimm E, Berger R, Sidi Y, Schachter J, Shapira-Frommer R, Avni D, Markel G, Leibowitz-Amit R. TNFSF4 (OX40L) expression and survival in locally advanced and metastatic melanoma. Cancer Immunol Immunother. 2019;68(9):1493–500.
Yu J, Wu X, Yan J, Yu H, Xu L, Chi Z, Sheng X, Si L, Cui C, Dai J, Ma M, Xu T, Kong Y, Guo J. Anti-GD2/4-1BB chimeric antigen receptor T cell therapy for the treatment of Chinese melanoma patients. J Hematol Oncol. 2018;11(1):1.
Simeone E, Ascierto PA. Immunomodulating antibodies in the treatment of metastatic melanoma: the experience with anti-CTLA-4, anti-CD137, and anti-PD1. J Immunotoxicol. 2012;9(3):241–7.
Li SY, Liu Y. Immunotherapy of melanoma with the immune costimulatory monoclonal antibodies targeting CD137. Clin Pharmacol. 2013;5(Suppl 1):47–53.
Soong RS, Song L, Trieu J, Lee SY, He L, Tsai YC, Wu TC, Hung CF. Direct T cell activation via CD40 ligand generates high avidity CD8+ T cells capable of breaking immunological tolerance for the control of tumors. PLoS One. 2014;9(3):e93162.
Yan C, Richmond A. Hiding in the dark: pan-cancer characterization of expression and clinical relevance of CD40 to immune checkpoint blockade therapy. Mol Cancer. 2021;20(1):146.
Buchan SL, Fallatah M, Thirdborough SM, Taraban VY, Rogel A, Thomas LJ, Penfold CA, He LZ, Curran MA, Keler T, Al-Shamkhani A. PD-1 blockade and CD27 stimulation activate distinct transcriptional programs that synergize for CD8+ T-cell-driven antitumor immunity. Clin Cancer Res. 2018;24(10):2383–94.
Pich C, Sarrabayrouse G, Teiti I, Mariamé B, Rochaix P, Lamant L, Favre G, Maisongrosse V, Tilkin-Mariamé AF. Melanoma-expressed CD70 is involved in invasion and metastasis. Br J Cancer. 2016;114(1):63–70.
Pich C, Teiti I, Sarrabayrouse G, Gallardo F, Gence R, Tilkin-Mariamé AF. Melanoma expressed-CD70 is regulated by RhoA and MAPK pathways without affecting vemurafenib treatment activity. PLoS One. 2016;11(2):e0148095.
Bajor DL, Mick R, Riese MJ, Huang AC, Sullivan B, Richman LP, Torigian DA, George SM, Stelekati E, Chen F, Melenhorst JJ, Lacey SF, Xu X, Wherry EJ, Gangadhar TC, Amaravadi RK, Schuchter LM, Vonderheide RH. Long-term outcomes of a phase I study of agonist CD40 antibody and CTLA-4 blockade in patients with metastatic melanoma. Oncoimmunology. 2018;7(10):e1468956.
Diab A, Hamid O, Thompson JA, Ros W, Eskens FALM, Doi T, Hu-Lieskovan S, Klempner SJ, Ganguly B, Fleener C, Wang X, Joh T, Liao K, Salek-Ardakani S, Taylor CT, Chou J, El-Khoueiry AB. A phase I, open-label, dose-escalation study of the OX40 agonist ivuxolimab in patients with locally advanced or metastatic cancers. Clin Cancer Res. 2022;28(1):71–83.
Burris HA, Infante JR, Ansell SM, Nemunaitis JJ, Weiss GR, Villalobos VM, Sikic BI, Taylor MH, Northfelt DW, Carson WE 3rd, Hawthorne TR, Davis TA, Yellin MJ, Keler T, Bullock T. Safety and activity of varlilumab, a novel and first-in-class agonist anti-CD27 antibody, in patients with advanced solid tumors. J Clin Oncol. 2017;35(18):2028–36.
Aggarwal C, Prawira A, Antonia S, Rahma O, Tolcher A, Cohen RB, Lou Y, Hauke R, Vogelzang N, Zandberg DP, Kalebasty AR, Atkinson V, Adjei AA, Seetharam M, Birnbaum A, Weickhardt A, Ganju V, Joshua AM, Cavallo R, et al. Dual checkpoint targeting of B7-H3 and PD-1 with enoblituzumab and pembrolizumab in advanced solid tumors: interim results from a multicenter phase I/II trial. J Immunother Cancer. 2022;10(4):e004424.
Booth L, Roberts JL, Sander C, Lalani AS, Kirkwood JM, Hancock JF, Poklepovic A, Dent P. Neratinib and entinostat combine to rapidly reduce the expression of K-RAS, N-RAS, Gαq and Gα11 and kill uveal melanoma cells. Cancer Biol Ther. 2019;20(5):700–10.
Geva R, Voskoboynik M, Dobrenkov K, Mayawala K, Gwo J, Wnek R, Chartash E, Long GV. First-in-human phase 1 study of MK-1248, an anti-glucocorticoid-induced tumor necrosis factor receptor agonist monoclonal antibody, as monotherapy or with pembrolizumab in patients with advanced solid tumors. Cancer. 2020;126(22):4926–35.
Piha-Paul SA, Geva R, Tan TJ, Lim DW, Hierro C, Doi T, Rahma O, Lesokhin A, Luke JJ, Otero J, Nardi L, Singh A, Xyrafas A, Chen X, Mataraza J, Bedard PL. First-in-human phase I/Ib open-label dose-escalation study of GWN323 (anti-GITR) as a single agent and in combination with spartalizumab (anti-PD-1) in patients with advanced solid tumors and lymphomas. J Immunother Cancer. 2021;9(8):e002863.
Heinhuis KM, Carlino M, Joerger M, Di Nicola M, Meniawy T, Rottey S, Moreno V, Gazzah A, Delord JP, Paz-Ares L, Britschgi C, Schilder RJ, O'Byrne K, Curigliano G, Romano E, Patah P, Wang R, Liu Y, Bajaj G, Siu LL. Safety, tolerability, and potential clinical activity of a glucocorticoid-induced TNF receptor-related protein agonist alone or in combination with nivolumab for patients with advanced solid tumors: a phase 1/2a dose-escalation and cohort-expansion clinical trial. JAMA Oncol. 2020;6(1):100–7.
Papadopoulos KP, Autio K, Golan T, Dobrenkov K, Chartash E, Chen Q, Wnek R, Long GV. Phase I study of MK-4166, an anti-human glucocorticoid-induced TNF receptor antibody, alone or with pembrolizumab in advanced solid tumors. Clin Cancer Res. 2021;27(7):1904–11.
Tawbi HA, Schadendorf D, Lipson EJ, Ascierto PA, Matamala L, Castillo Gutiérrez E, Rutkowski P, Gogas HJ, Lao CD, De Menezes JJ, Dalle S, Arance A, Grob JJ, Srivastava S, Abaskharoun M, Hamilton M, Keidel S, Simonsen KL, Sobiesk AM, et al. Long GV; RELATIVITY-047 Investigators. Relatlimab and nivolumab versus nivolumab in untreated advanced melanoma. N Engl J Med. 2022;386(1):24–34.
Hamid O, Wang D, Kim TM, et al. Clinical activity of fianlimab (REGN3767), a human anti-LAG-3 monoclonal antibody, combined with cemiplimab (anti-PD-1) in patients (pts) with advanced melanoma. J Clin Oncology. 2021;39(15_suppl):9515–5.
Long GV, Dummer R, Hamid O, Gajewski TF, Caglevic C, Dalle S, Arance A, Carlino MS, Grob JJ, Kim TM, Demidov L, Robert C, Larkin J, Anderson JR, Maleski J, Jones M, Diede SJ, Mitchell TC. Epacadostat plus pembrolizumab versus placebo plus pembrolizumab in patients with unresectable or metastatic melanoma (ECHO-301/KEYNOTE-252): a phase 3, randomised, double-blind study. Lancet Oncol. 2019;20(8):1083–97.
Daud A, Saleh MN, Hu J, et al. Epacadostat plus nivolumab for advanced melanoma: updated phase 2 results of the ECHO-204 study. J Clin Oncology. 2018;36(15_suppl):9511–1.
Zakharia Y, McWilliams RR, Rixe O, Drabick J, Shaheen MF, Grossmann KF, Kolhe R, Pacholczyk R, Sadek R, Tennant LL, Smith CM, Kennedy EP, Link CJ Jr, Vahanian NN, Yu J, Shen SS, Brincks EL, Rossi GR, Munn D, Milhem M. Phase II trial of the IDO pathway inhibitor indoximod plus pembrolizumab for the treatment of patients with advanced melanoma. J Immunother Cancer. 2021;9(6):e002057.
Hirai I, Funakoshi T, Kamijuku H, Fukuda K, Mori M, Sakurai M, Koda Y, Kato J, Mori T, Watanabe N, Noji S, Yaguchi T, Iwata T, Ohta S, Fujita T, Tanosaki R, Handa M, Okamoto S, Amagai M, Kawakami Y. Adoptive cell therapy using tumor-infiltrating lymphocytes for melanoma refractory to immune-checkpoint inhibitors. Cancer Sci. 2021;112(8):3163–72.
Simon B, Uslu U. CAR-T cell therapy in melanoma: a future success story? Exp Dermatol. 2018;27(12):1315–21.
Waldman AD, Fritz JM, Lenardo MJ. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol. 2020;20(11):651–68.
Suzuki M, Cheung NK. Disialoganglioside GD2 as a therapeutic target for human diseases. Expert Opin Ther Targets. 2015;19(3):349–62.
Kwak M, Leick KM, Melssen MM, Slingluff CL Jr. Vaccine strategy in melanoma. Surg Oncol Clin N Am. 2019;28(3):337–51.
Martinez-Perez D, Viñal D, Solares I, Espinosa E, Feliu J. Gp-100 as a novel therapeutic target in uveal melanoma. Cancers (Basel). 2021;13(23):5968.
Nathan P, Hassel JC, Rutkowski P, et al. Overall survival benefit with tebentafusp in metastatic uveal melanoma. N Engl J Med. 2021;385(13):1196–206.
Kim YC, Lee MG, Choe SW, Lee MC, Chung HG, Cho SH. Acral lentiginous melanoma: an immunohistochemical study of 20 cases. Int J Dermatol. 2003;42(2):123–9.
Middleton MR, McAlpine C, Woodcock VK, et al. Tebentafusp, a TCR/anti-CD3 bispecific fusion protein targeting gp100, potently activated antitumor immune responses in patients with metastatic melanoma. Clin Cancer Res. 2020;26(22):5869–78.
Biteghe FAN, Chalomie NET, Mungra N, et al. Antibody-based immunotherapy: alternative approaches for the treatment of metastatic melanoma. Biomedicines. 2020;8(9):327.
Ott PA, Pavlick AC, Johnson DB, et al. A phase 2 study of glembatumumab vedotin, an antibody-drug conjugate targeting glycoprotein NMB, in patients with advanced melanoma. Cancer. 2019;125(7):1113–23.
Sandhu S, McNeil CM, LoRusso P, et al. Phase I study of the anti-endothelin B receptor antibody-drug conjugate DEDN6526A in patients with metastatic or unresectable cutaneous, mucosal, or uveal melanoma. Invest New Drugs. 2020;38(3):844–54.
Ott PA, Hamid O, Pavlick AC, et al. Phase I/II study of the antibody-drug conjugate glembatumumab vedotin in patients with advanced melanoma. J Clin Oncol. 2014;32(32):3659–66.
Hasanov M, Rioth MJ, Kendra K, et al. A phase II study of glembatumumab vedotin for metastatic uveal melanoma. Cancers (Basel). 2020;12(8):2270.
Anderson TS, Wooster AL, La-Beck NM, Saha D, Lowe DB. Antibody-drug conjugates: an evolving approach for melanoma treatment. Melanoma Res. 2021;31(1):1–17.
Shoushtari AN, Bao R, Luke JJ. PD-1 blockade in Chinese versus Western patients with melanoma. Clin Cancer Res. 2020;26(16):4171–3.
Liu D, Schilling B, Liu D, Sucker A, Livingstone E, Jerby-Arnon L, Zimmer L, Gutzmer R, Satzger I, Loquai C, Grabbe S, Vokes N, Margolis CA, Conway J, He MX, Elmarakeby H, Dietlein F, Miao D, Tracy A, et al. Integrative molecular and clinical modeling of clinical outcomes to PD1 blockade in patients with metastatic melanoma. Nat Med. 2019;25(12):1916–27.
Hayward NK, Wilmott JS, Waddell N, Johansson PA, Field MA, Nones K, Patch AM, Kakavand H, Alexandrov LB, Burke H, Jakrot V, Kazakoff S, Holmes O, Leonard C, Sabarinathan R, Mularoni L, Wood S, Xu Q, Waddell N, et al. Whole-genome landscapes of major melanoma subtypes. Nature. 2017;545(7653):175–80.
•• Newell F, Wilmott JS, Johansson PA, Nones K, Addala V, Mukhopadhyay P, Broit N, Amato CM, Van Gulick R, Kazakoff SH, Patch AM, Koufariotis LT, Lakis V, Leonard C, Wood S, Holmes O, Xu Q, Lewis K, Medina T, et al. Whole-genome sequencing of acral melanoma reveals genomic complexity and diversity. Nat Commun. 2020;11(1):5259. This reference is of outstanding importance because it describes the largest whole-genome analysis of acral melanoma to date. The use of mutational signatures including substitution, indel, structural rearrangement, and copy number signatures provides important insights into the mutational processes at play in AM.
Liang WS, Hendricks W, Kiefer J, Schmidt J, Sekar S, Carpten J, Craig DW, Adkins J, Cuyugan L, Manojlovic Z, Halperin RF, Helland A, Nasser S, Legendre C, Hurley LH, Sivaprakasam K, Johnson DB, Crandall H, Busam KJ, et al. Integrated genomic analyses reveal frequent TERT aberrations in acral melanoma. Genome Res. 2017;27(4):524–32.
Yu J, Yu J, Wu X, Guo Q, Yin T, Cheng Z, Dai J, Kong Y, Guo J. The TERT copy number gain is sensitive to telomerase inhibitors in human melanoma. Clin Sci (Lond). 2020;134(2):193–205.
Liu J, Yu W, Gao F, Qi S, Du J, Ma X, Zhang Y, Zheng J, Su J. CCND1 copy number increase and cyclin D1 expression in acral melanoma: a comparative study of fluorescence in situ hybridization and immunohistochemistry in a Chinese cohort. Diagn Pathol. 2021;16(1):60.
Namiki T, Tanemura A, Valencia JC, Coelho SG, Passeron T, Kawaguchi M, Vieira WD, Ishikawa M, Nishijima W, Izumo T, Kaneko Y, Katayama I, Yamaguchi Y, Yin L, Polley EC, Liu H, Kawakami Y, Eishi Y, Takahashi E, et al. AMP kinase-related kinase NUAK2 affects tumor growth, migration, and clinical outcome of human melanoma. Proc Natl Acad Sci U S A. 2011;108(16):6597–602.
Deng J, Wang ES, Jenkins RW, Li S, Dries R, Yates K, Chhabra S, Huang W, Liu H, Aref AR, Ivanova E, Paweletz CP, Bowden M, Zhou CW, Herter-Sprie GS, Sorrentino JA, Bisi JE, Lizotte PH, Merlino AA, et al. CDK4/6 inhibition augments antitumor immunity by enhancing T-cell activation. Cancer Discov. 2018;8(2):216–33.
Schaer DA, Beckmann RP, Dempsey JA, Huber L, Forest A, Amaladas N, Li Y, Wang YC, Rasmussen ER, Chin D, Capen A, Carpenito C, Staschke KA, Chung LA, Litchfield LM, Merzoug FF, Gong X, Iversen PW, Buchanan S, et al. The CDK4/6 inhibitor abemaciclib induces a T cell inflamed tumor microenvironment and enhances the efficacy of PD-L1 checkpoint blockade. Cell Rep. 2018;22(11):2978–94.
Jin X, Ding D, Yan Y, Li H, Wang B, Ma L, Ye Z, Ma T, Wu Q, Rodrigues DN, Kohli M, Jimenez R, Wang L, Goodrich DW, de Bono J, Dong H, Wu H, Zhu R, Huang H. Phosphorylated RB promotes cancer immunity by inhibiting NF-κB activation and PD-L1 expression. Mol Cell. 2019;73(1):22–35.e6.
• Yu J, Yan J, Guo Q, Chi Z, Tang B, Zheng B, Yu J, Yin T, Cheng Z, Wu X, Yu H, Dai J, Sheng X, Si L, Cui C, Bai X, Mao L, Lian B, Wang X, et al. Genetic aberrations in the CDK4 pathway are associated with innate resistance to PD-1 blockade in Chinese patients with non-cutaneous melanoma. Clin Cancer Res. 2019;25(21):6511–23. This reference is of importance because it discovers that genetic aberrations in the CDK4 pathway are associated with innate resistance to anti-PD-1 therapy in patients with advanced melanoma. Moreover, this reference provides a strong rationale for combining CDK4/6 inhibitors with anti-PD-1 antibody for the treatment of advanced melanomas.
Zhou C, Lin A, Cao M, Ding W, Mou W, Guo N, Chen Z, Zhang J, Luo P. Activation of the DDR pathway leads to the down-regulation of the TGFβ pathway and a better response to ICIs in patients with metastatic urothelial carcinoma. Front Immunol. 2021;12:634741.
Teo MY, Seier K, Ostrovnaya I, Regazzi AM, Kania BE, Moran MM, Cipolla CK, Bluth MJ, Chaim J, Al-Ahmadie H, Snyder A, Carlo MI, Solit DB, Berger MF, Funt S, Wolchok JD, Iyer G, Bajorin DF, Callahan MK, Rosenberg JE. Alterations in DNA damage response and repair genes as potential marker of clinical benefit from PD-1/PD-L1 blockade in advanced urothelial cancers. J Clin Oncol. 2018;36(17):1685–94.
Maréchal A, Zou L. DNA damage sensing by the ATM and ATR kinases. Cold Spring Harb Perspect Biol. 2013;5(9):a012716.
• Kim R, Kwon M, An M, Kim ST, Smith SA, Loembé AB, PGS M, Armenia J, Lukashchuk N, Shah N, Dean E, Park WY, Lee J. Phase II study of ceralasertib (AZD6738) in combination with durvalumab in patients with advanced/metastatic melanoma who have failed prior anti-PD-1 therapy. Ann Oncol. 2022;33(2):193–203. This reference is of importance because it is the phase II trial that demonstrated ceralasertib in combination with durvalumab has promising antitumor activity among patients with metastatic melanoma who have failed anti-programmed cell death protein 1 therapy.
Kim ST, Smith SA, Mortimer P, Loembé AB, Cho H, Kim KM, Smith C, Willis S, Irurzun-Arana I, Berges A, Hong JY, Park SH, Park JO, Park YS, Lim HY, Kang WK, Kozarewa I, Pierce AJ, Dean E, Lee J. Phase I study of ceralasertib (AZD6738), a novel DNA damage repair agent, in combination with weekly paclitaxel in refractory cancer. Clin Cancer Res. 2021;27(17):4700–9.
Li A, Yi M, Qin S, Chu Q, Luo S, Wu K. Prospects for combining immune checkpoint blockade with PARP inhibition. J Hematol Oncol. 2019;12(1):98.
Vikas P, Borcherding N, Chennamadhavuni A, Garje R. Therapeutic potential of combining PARP inhibitor and immunotherapy in solid tumors. Front Oncol. 2020;10:570.
Loureiro JB, Raimundo L, Calheiros J, Carvalho C, Barcherini V, Lima NR, Gomes C, Almeida MI, Alves MG, Costa JL, Santos MMM, Saraiva L. Targeting p53 for melanoma treatment: counteracting tumour proliferation, dissemination and therapeutic resistance. Cancers (Basel). 2021;13(7):1648.
Forschner A, Hilke FJ, Bonzheim I, Gschwind A, Demidov G, Amaral T, Ossowski S, Riess O, Schroeder C, Martus P, Klumpp B, Gonzalez-Menendez I, Garbe C, Niessner H, Sinnberg T. MDM2, MDM4 and EGFR amplifications and hyperprogression in metastatic acral and mucosal melanoma. Cancers (Basel). 2020;12(3):540.
Kim E, Zucconi BE, Wu M, Nocco SE, Meyers DJ, McGee JS, Venkatesh S, Cohen DL, Gonzalez EC, Ryu B, Cole PA, Alani RM. MITF expression predicts therapeutic vulnerability to p300 inhibition in human melanoma. Cancer Res. 2019;79(10):2649–61.
Shi Q, Lin L, Chen J, Zhang W, Guo W, Wang X, Wang H, Guo S, Yue Q, Ma J, Yu L, Zhu G, Zhao T, Zhao J, Liu Y, Gao T. Chunying Li. Clin Cancer Res: Integrative genomic profiling uncovers therapeutic targets of acral melanoma in Asian populations; 2022. https://doi.org/10.1158/1078-0432.CCR-21-3344.
Zhang F, Tang X, Fan S, Liu X, Sun J, Ju C, Liang Y, Liu R, Zhou R, Yu B, Zhang C, Zhang Z, Kang T, Huang G, Lv XB. Targeting the p300/NONO axis sensitizes melanoma cells to BRAF inhibitors. Oncogene. 2021;40(24):4137–50.
Maertens O, Kuzmickas R, Manchester HE, Emerson CE, Gavin AG, Guild CJ, Wong TC, De Raedt T, Bowman-Colin C, Hatchi E, Garraway LA, Flaherty KT, Pathania S, Elledge SJ, Cichowski K. MAPK Pathway suppression unmasks latent DNA repair defects and confers a chemical synthetic vulnerability in BRAF-, NRAS-, and NF1-mutant melanomas. Cancer Discov. 2019;9(4):526–45.
Khushalani N, Brohl A, Markowitz J, et al. 797 Significant anti-tumor activity of HBI-8000, a class I histone deacetylase inhibitor (HDACi) in combination with nivolumab (NIVO) in anti-PD1 therapy-naïve advanced melanoma (TN-Mel). Journal for ImmunoTherapy of Cancer. 2020;8(Suppl 3):A476–7.
Yu J, Xie Y, Wu X, et al. Targeting cancer-associated fibroblasts synergizes with anti-PD-1 immunotherapy in advanced acral melanoma[J]. Pigment Cell Melanoma Res. 2020;33(1):196–7.
Wu X, Yu J, Yan J, Dai J, Si L, Chi Z, Sheng X, Cui C, Ma M, Tang H, Xu T, Yu H, Kong Y, Guo J. PI3K/AKT/mTOR pathway inhibitors inhibit the growth of melanoma cells with mTOR H2189Y mutations in vitro. Cancer Biol Ther. 2018;19(7):584–9.
Savoia P, Fava P, Casoni F, Cremona O. Targeting the ERK signaling pathway in melanoma. Int J Mol Sci. 2019;20(6):1483.
Goldsberry WN, Londoño A, Randall TD, Norian LA, Arend RC. A review of the role of Wnt in cancer immunomodulation. Cancers (Basel). 2019;11(6):771.
Author information
Authors and Affiliations
Contributions
Writing and editing by Yiqun Zhang; supervision by Shijie Lan and Di Wu. All the authors have read and agreed to the version of the manuscript.
Corresponding author
Ethics declarations
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Conflict of interest
No conflict of interest exists in the submission of this manuscript, and manuscript is approved by all authors for publication.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Skin Cancer
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, Y., Lan, S. & Wu, D. Advanced Acral Melanoma Therapies: Current Status and Future Directions. Curr. Treat. Options in Oncol. 23, 1405–1427 (2022). https://doi.org/10.1007/s11864-022-01007-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11864-022-01007-6