Opinion statement
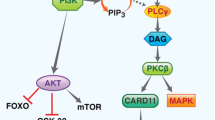
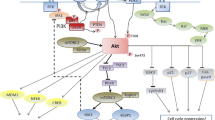
Phosphatidylinositol 3-kinase (PI3K) inhibitors represent a novel class of agents targeting the key cellular regulatory PI3K/AKT/mTOR pathway involved in crucial functions such as cellular proliferation, cell cycle regulation, protein synthesis, and cell motility. This review starts with an overview of the PI3K pathway and the rationale for its targeting in lymphoma and potential on-target side effects of PI3K inhibition. With three agents now FDA approved for the treatment of relapsed and refractory (R/R) indolent non-Hodgkin lymphoma (iNHL), idelalisib, copanlisib, and duvelisib, we aim to review the pivotal trials leading to their approval as well as their clinical applications according to lymphoma subtypes. Important treatment-related adverse events are also reviewed and a perspective on the clinical role of these agents is provided, as well as some practical guidance on how to prevent, monitor, and manage potential adverse events in the clinic. PI3K inhibitors have an established role in the management of R/R iNHL, but their use and development are hampered by adverse events, particularly when used in combination with other anti-lymphoma therapies. Finally, this review highlights areas in need of more research in order to optimally use these agents in the care of patients with lymphoma.

Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance
Young RM, Staudt LM. Targeting pathological B cell receptor signalling in lymphoid malignancies. Nat Rev Drug Discov. 2013;12(3):229–43.
Luongo F, Colonna F, Calapa F, Vitale S, Fiori ME, De Maria R. PTEN tumor-suppressor: the dam of stemness in cancer. Cancers. 2019;11(8).
Okkenhaug K, Vanhaesebroeck B. PI3K in lymphocyte development, differentiation and activation. Nat Rev Immunol. 2003;3(4):317–30.
Janku F. Phosphoinositide 3-kinase (PI3K) pathway inhibitors in solid tumors: from laboratory to patients. Cancer Treat Rev. 2017;59:93–101.
Andre F, Ciruelos E, Rubovszky G, et al. Alpelisib for PIK3CA-mutated, hormone receptor-positive advanced breast cancer. N Engl J Med. 2019;380(20):1929–40.
Puente XS, Pinyol M, Quesada V, Conde L, Ordóñez GR, Villamor N, et al. Whole-genome sequencing identifies recurrent mutations in chronic lymphocytic leukaemia. Nature. 2011;475(7354):101–5.
Landau DA, Tausch E, Taylor-Weiner AN, Stewart C, Reiter JG, Bahlo J, et al. Mutations driving CLL and their evolution in progression and relapse. Nature. 2015;526(7574):525–30.
Reddy A, Zhang J, Davis NS, Moffitt AB, Love CL, Waldrop A, et al. Genetic and functional drivers of diffuse large B cell lymphoma. Cell. 2017;171(2):481–94 e415.
Bea S, Valdes-Mas R, Navarro A, et al. Landscape of somatic mutations and clonal evolution in mantle cell lymphoma. Proc Natl Acad Sci U S A. 2013;110(45):18250–5.
• Dreyling M, Santoro A, Mollica L, et al. Phosphatidylinositol 3-kinase inhibition by copanlisib in relapsed or refractory indolent lymphoma. J Clin Oncol. 2017;35(35):3898–905 The results of this phase 2 trial led to the FDA approval of copanlisib in patients with R/R FL.
Amrein L, Shawi M, Grenier J, Aloyz R, Panasci L. The phosphatidylinositol-3 kinase I inhibitor BKM120 induces cell death in B-chronic lymphocytic leukemia cells in vitro. Int J Cancer. 2013;133(1):247–52.
Scheffold A, Jebaraj BMC, Tausch E, Bloehdorn J, Ghia P, Yahiaoui A, et al. IGF1R as druggable target mediating PI3K-delta inhibitor resistance in a murine model of chronic lymphocytic leukemia. Blood. 2019;134(6):534–47.
• Flinn IW, Miller CB, Ardeshna KM, et al. DYNAMO: a phase II study of duvelisib (IPI-145) in patients with refractory indolent non-Hodgkin lymphoma. J Clin Oncol. 2019;37(11):912–22 The result of this phase 2 trial led to the FDA approval of duvelisib in patients with R/R FL.
Sehn LH, Chua N, Mayer J, Dueck G, Trněný M, Bouabdallah K, et al. Obinutuzumab plus bendamustine versus bendamustine monotherapy in patients with rituximab-refractory indolent non-Hodgkin lymphoma (GADOLIN): a randomised, controlled, open-label, multicentre, phase 3 trial. Lancet Oncol. 2016;17(8):1081–93.
• Gopal AK, Kahl BS, de Vos S, et al. PI3Kdelta inhibition by idelalisib in patients with relapsed indolent lymphoma. N Engl J Med. 2014;370(11):1008–18 The results of this phase 2 trial led to the FDA approval of idelalisib in patients with R/R FL and SLL.
Kahl BS, Yang DT. Follicular lymphoma: evolving therapeutic strategies. Blood. 2016;127(17):2055–63.
Conconi A, Lobetti-Bodoni C, Montoto S, et al. Life expectancy of young adults with follicular lymphoma. Ann Oncol. 2015;26(11):2317–22.
Pulte D, Gondos A, Brenner H. Expected long-term survival of older patients diagnosed with non-Hodgkin lymphoma in 2008-2012. Cancer Epidemiol. 2012;36(1):e19–25.
Olszewski AJ, Castillo JJ. Survival of patients with marginal zone lymphoma: analysis of the Surveillance, Epidemiology, and End Results database. Cancer. 2013;119(3):629–38.
Somoza JR, Koditek D, Villasenor AG, et al. Structural, biochemical, and biophysical characterization of idelalisib binding to phosphoinositide 3-kinase delta. J Biol Chem. 2015;290(13):8439–46.
Flinn IW, Kahl BS, Leonard JP, Furman RR, Brown JR, Byrd JC, et al. Idelalisib, a selective inhibitor of phosphatidylinositol 3-kinase-delta, as therapy for previously treated indolent non-Hodgkin lymphoma. Blood. 2014;123(22):3406–13.
Kahl BS, Spurgeon SE, Furman RR, Flinn IW, Coutre SE, Brown JR, et al. A phase 1 study of the PI3Kdelta inhibitor idelalisib in patients with relapsed/refractory mantle cell lymphoma (MCL). Blood. 2014;123(22):3398–405.
Paul J, Soujon M, Wengner AM, Zitzmann-Kolbe S, Sturz A, Haike K, et al. Simultaneous inhibition of PI3Kdelta and PI3Kalpha induces ABC-DLBCL regression by blocking BCR-dependent and -independent activation of NF-kappaB and AKT. Cancer Cell. 2017;31(1):64–78.
Kaneda MM, Messer KS, Ralainirina N, et al. PI3Kgamma is a molecular switch that controls immune suppression. Nature. 2016;539(7629):437–42.
De Henau O, Rausch M, Winkler D, et al. Overcoming resistance to checkpoint blockade therapy by targeting PI3Kgamma in myeloid cells. Nature. 2016;539(7629):443–7.
Horwitz SM, Koch R, Porcu P, Oki Y, Moskowitz A, Perez M, et al. Activity of the PI3K-delta,gamma inhibitor duvelisib in a phase 1 trial and preclinical models of T-cell lymphoma. Blood. 2018;131(8):888–98.
Lannutti BJ, Meadows SA, Herman SE, et al. CAL-101, a p110delta selective phosphatidylinositol-3-kinase inhibitor for the treatment of B-cell malignancies, inhibits PI3K signaling and cellular viability. Blood. 2011;117(2):591–4.
Liu N, Rowley BR, Bull CO, Schneider C, Haegebarth A, Schatz CA, et al. BAY 80-6946 is a highly selective intravenous PI3K inhibitor with potent p110alpha and p110delta activities in tumor cell lines and xenograft models. Mol Cancer Ther. 2013;12(11):2319–30.
Winkler DG, Faia KL, DiNitto JP, Ali JA, White KF, Brophy EE, et al. PI3K-delta and PI3K-gamma inhibition by IPI-145 abrogates immune responses and suppresses activity in autoimmune and inflammatory disease models. Chem Biol. 2013;20(11):1364–74.
Garcia-Echeverria C, Sellers WR. Drug discovery approaches targeting the PI3K/Akt pathway in cancer. Oncogene. 2008;27(41):5511–26.
Burger MT, Pecchi S, Wagman A, Ni ZJ, Knapp M, Hendrickson T, et al. Identification of NVP-BKM120 as a potent, selective, orally bioavailable class I PI3 kinase inhibitor for treating cancer. ACS Med Chem Lett. 2011;2(10):774–9.
Qian C, Lai CJ, Bao R, Wang DG, Wang J, Xu GX, et al. Cancer network disruption by a single molecule inhibitor targeting both histone deacetylase activity and phosphatidylinositol 3-kinase signaling. Clin Cancer Res. 2012;18(15):4104–13.
Foster P, Yamaguchi K, Hsu PP, Qian F, du X, Wu J, et al. The selective PI3K inhibitor XL147 (SAR245408) inhibits tumor growth and survival and potentiates the activity of chemotherapeutic agents in preclinical tumor models. Mol Cancer Ther. 2015;14(4):931–40.
Wang KF, Yang H, Jiang WQ, Li S, Cai YC. Puquitinib mesylate (XC-302) induces autophagy via inhibiting the PI3K/AKT/mTOR signaling pathway in nasopharyngeal cancer cells. Int J Mol Med. 2015;36(6):1556–62.
Vakkalanka S. Inhibition of PI3Kd kinase by a selective, small molecular inhibitor suppresses B-cell proliferation and leukemic cell growth. AACR. 2012:Poster 3741.
Moreno O, et al. Safety, pharmacokinetics, and pharmacodynamics of ME-401, an oral, potent, and selective inhibitor of phosphatidylinositol 3-kinase P110δ, following single ascending dose administration to healthy volunteers. Clin Ther. 2018;40(11):0149–2918.
Wagner-Johnston ND, Schuster SJ, de Vos S, et al. Long-term follow-up of idelalisib monotherapy in patients with double-refractory marginal zone lymphoma or lymphoplasmacytic lymphoma/Waldenstrom’s macroglobulinemia. Blood. 2019;134(Supplement_1):4006–4006.
Wagner-Johnston ND, Gopal AK, Kahl BS, et al. Patient-reported outcomes data from a phase 2 study of idelalisib in patients with refractory indolent B-cell non-Hodgkin lymphoma (iNHL). J Clin Oncol. 2014;32(15_suppl):e19554-e19554.
• Eyre TA, Osborne WL, Gallop-Evans E, et al. Results of a multicentre UK-wide compassionate use programme evaluating the efficacy of idelalisib monotherapy in relapsed, refractory follicular lymphoma. Br J Haematol. 2018;181(4):555–9 This retrospective cohort study represents the only real-world series outlining the efficacy of idelalisib in patients with R/R FL.
Gopal AK, Fanale MA, Moskowitz CH, Shustov AR, Mitra S, Ye W, et al. Phase II study of idelalisib, a selective inhibitor of PI3Kdelta, for relapsed/refractory classical Hodgkin lymphoma. Ann Oncol. 2017;28(5):1057–63.
de Vos S, Wagner-Johnston ND, Coutre SE, Flinn IW, Schreeder MT, Fowler NH, et al. Combinations of idelalisib with rituximab and/or bendamustine in patients with recurrent indolent non-Hodgkin lymphoma. Blood Adv. 2016;1(2):122–31.
Smith SM, Pitcher BN, Jung SH, Bartlett NL, Wagner-Johnston N, Park SI, et al. Safety and tolerability of idelalisib, lenalidomide, and rituximab in relapsed and refractory lymphoma: the Alliance for Clinical Trials in Oncology A051201 and A051202 phase 1 trials. Lancet Haematol. 2017;4(4):e176–82.
Barr PM, Saylors GB, Spurgeon SE, Cheson BD, Greenwald DR, O’Brien SM, et al. Phase 2 study of idelalisib and entospletinib: pneumonitis limits combination therapy in relapsed refractory CLL and NHL. Blood. 2016;127(20):2411–5.
Ma S, Chan RJ, Gu L, et al. Impact of idelalisib treatment interruption with or without dose reduction on outcomes in relapsed / refractory indolent non-Hodgkin’s lymphoma and chronic lymphocytic leukemia. Blood. 2019;134(Supplement_1):5468–5468.
Ma S, Chan RJ, Ye W, et al. Survival outcomes following idelalisib interruption in the treatment of relapsed or refractory indolent non-Hodgkin’s lymphoma and chronic lymphocytic leukemia. Blood. 2018;132(Suppl 1):3149–3149.
Dreyling M, Morschhauser F, Bouabdallah K, Bron D, Cunningham D, Assouline SE, et al. Phase II study of copanlisib, a PI3K inhibitor, in relapsed or refractory, indolent or aggressive lymphoma. Ann Oncol. 2017;28(9):2169–78.
Knight ZA, Gonzalez B, Feldman ME, et al. A pharmacological map of the PI3-K family defines a role for p110alpha in insulin signaling. Cell. 2006;125(4):733–47.
Symons JD, McMillin SL, Riehle C, et al. Contribution of insulin and Akt1 signaling to endothelial nitric oxide synthase in the regulation of endothelial function and blood pressure. Circ Res. 2009;104(9):1085–94.
Mensah FA, Blaize JP, Bryan LJ. Spotlight on copanlisib and its potential in the treatment of relapsed/refractory follicular lymphoma: evidence to date. OncoTargets Ther. 2018;11:4817–27.
Zinzani PL, Santoro A, Mollica L, et al. Copanlisib, a PI3K inhibitor, demonstrates a favorable long-term safety profile in a pooled analysis of patients with hematologic malignancies. Blood. 2019;134(Supplement_1):4009–4009.
Keating KN, Hiemeyer F, Garcia-Vargas JE, Childs BH, Dreyling MH, Zinzani PL. Patient-reported outcomes from a phase 2 study of copanlisib in patients with relapsed/refractory indolent B-cell non-Hodgkin lymphoma (iNHL). J Clin Oncol. 2017;35(15_suppl):e18123-e18123.
Burris HA 3rd, Flinn IW, Patel MR, et al. Umbralisib, a novel PI3Kdelta and casein kinase-1epsilon inhibitor, in relapsed or refractory chronic lymphocytic leukaemia and lymphoma: an open-label, phase 1, dose-escalation, first-in-human study. Lancet Oncol. 2018;19(4):486–96.
Davids MS, Kim HT, Nicotra A, Savell A, Francoeur K, Hellman JM, et al. Umbralisib in combination with ibrutinib in patients with relapsed or refractory chronic lymphocytic leukaemia or mantle cell lymphoma: a multicentre phase 1-1b study. Lancet Haematol. 2019;6(1):e38–47.
Nastoupil LJ, Lunning MA, Vose JM, Schreeder MT, Siddiqi T, Flowers CR, et al. Tolerability and activity of ublituximab, umbralisib, and ibrutinib in patients with chronic lymphocytic leukaemia and non-Hodgkin lymphoma: a phase 1 dose escalation and expansion trial. Lancet Haematol. 2019;6(2):e100–9.
Brown JR, Hamadani M, Hayslip J, et al. Voxtalisib (XL765) in patients with relapsed or refractory non-Hodgkin lymphoma or chronic lymphocytic leukaemia: an open-label, phase 2 trial. Lancet Haematol. 2018;5(4):e170–80.
Soumerai JD, Pagel JM, Jagadeesh D, et al. Initial results of a dose escalation study of a selective and structurally differentiated PI3Kδ inhibitor, ME-401, in relapsed/refractory (R/R) follicular lymphoma (FL) and chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL). J Clin Oncol. 2018;36(15_suppl):7519–7519.
Horwitz SM, Moskowitz AJ, Jacobsen ED, et al. The combination of duvelisib, a PI3K-δ,γ inhibitor, and romidepsin is highly active in relapsed/refractory peripheral T-cell lymphoma with low rates of transaminitis: results of parallel multicenter, phase 1 combination studies with expansion cohorts. Blood. 2018;132(Suppl 1):683–683.
Zinzani P, Samaniego F, Jurczak W, et al. Umbralisib monotherapy demonstrates efficacy and safety in patients with relapsed/refractory marginal zone lymphoma: a multicenter, open-label, registration directed phase 2 study. Hematol Oncol. 2019;37(S2):182–3.
Flinn IW, Hillmen P, Montillo M, Nagy Z, Illés Á, Etienne G, et al. The phase 3 DUO trial: duvelisib vs ofatumumab in relapsed and refractory CLL/SLL. Blood. 2018;132(23):2446–55.
Jones JA, Robak T, Brown JR, Awan FT, Badoux X, Coutre S, et al. Efficacy and safety of idelalisib in combination with ofatumumab for previously treated chronic lymphocytic leukaemia: an open-label, randomised phase 3 trial. Lancet Haematol. 2017;4(3):e114–26.
• Cuneo A, Barosi G, Danesi R, et al. Management of adverse events associated with idelalisib treatment in chronic lymphocytic leukemia and follicular lymphoma: a multidisciplinary position paper. Hematol Oncol. 2019;37(1):3–14 Useful review addressing the management of adverse events encountered with idelalisib in CLL and FL.
• Cheson BD, O’Brien S, Ewer MS, et al. Optimal management of adverse events from copanlisib in the treatment of patients with non-Hodgkin lymphomas. Clin Lymphoma Myeloma Leuk. 2019;19(3):135–41 Useful review addressing the management of adverse events encountered with copanlisib in NHL.
Philip AZ. Idelalisib and rituximab in 17p deletion-positive splenic marginal zone lymphoma. J Natl Compr Cancer Netw. 2018;16(3):230–3.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Vladimir Sapon-Cousineau declares that he has no conflict of interest.
Sasha Sapon-Cousineau declares that she has no conflict of interest.
Sarit Assouline has received speaker’s honoraria from Janssen and AbbVie, and has received compensation from Janssen, Roche Canada, and Pfizer for participation on advisory boards.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical collection on Lymphoma
Rights and permissions
About this article
Cite this article
Sapon-Cousineau, V., Sapon-Cousineau, S. & Assouline, S. PI3K Inhibitors and Their Role as Novel Agents for Targeted Therapy in Lymphoma. Curr. Treat. Options in Oncol. 21, 51 (2020). https://doi.org/10.1007/s11864-020-00746-8
Published:
DOI: https://doi.org/10.1007/s11864-020-00746-8