Abstract
Background
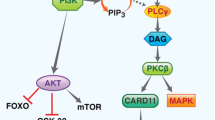
Malignant lymphomas are one of the most common cancers worldwide and with high biologic heterogeneity, while the phosphoinositide 3 kinase (PI3K)/mTOR pathway is crucial in maintaining cell growth and survival both in physiological and in pathological conditions (i.e., lymphoma). PI3K inhibitors have been proven to be effective in several subtypes of lymphomas. However, the high incidence of treatment-related adverse events as well as the special safety profile in PI3K inhibitors draws great attention. Thus, this meta-analysis was conducted to compare adverse events in PI3K inhibitors to conventional regimens in lymphoma patients.
Methods
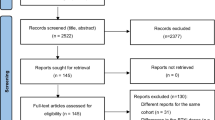
Articles were retrieved from PubMed, Cochrane, and Embase to identify randomized controlled trials and phase III clinical trials that used PI3K inhibitors comparing with non-PI3K inhibitors in lymphoma patients. To achieve the appropriate results, we calculated the risk ratio and 95% confidence intervals.
Results
Four trials with 1399 patients that met our criteria were included. The PI3K inhibitors group significantly increased the risk of all-grade adverse events (AEs) (RR 0.95, 95% CI: 0.92–0.98) and high-grade AEs (RR 0.63, 95% CI: 0.57–0.70), compared with the non-PI3K inhibitors group. Besides, the incidence of neutropenia (RR 0.81, 95% CI: 0.74–0.90), pneumonia (RR 0.62, 95% CI: 0.46–0.83), and diarrhea (RR 0.40, 95% CI: 0.32–0.49) were significantly high in the PI3Ki group, while the incidence of anemia (RR 0.78, 95% CI: 0.50–1.20) and thrombocytopenia (RR 0.85, 95% CI: 0.51–1.42) had no statistic significant.
Conclusion
PI3K inhibitors increased the risk of certain AEs in lymphoma patients.







Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Abate D et al (2019) Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: a systematic analysis for the Global Burden of Disease Study. JAMA Oncol 5:1749–1768.https://doi.org/10.1001/jamaoncol.2019.2996
(2015) Non-Hodgkin lymphoma statistics. In: Cancer Research UK. https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/non-hodgkin-lymphoma. Accessed 28 Sep 2021
SEER Cancer Stat Facts. In: SEER. https://seer.cancer.gov/statfacts/index.html. Accessed 28 Sep 2021
Vivanco I, Sawyers CL (2002) The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer 2:489–501. https://doi.org/10.1038/nrc839
Engelman JA, Luo J, Cantley LC (2006) The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet 7:606–619. https://doi.org/10.1038/nrg1879
Mishra R, Patel H, Alanazi S et al (2021) PI3K inhibitors in cancer: clinical implications and adverse effects. Int J Mol Sci 22:3464. https://doi.org/10.3390/ijms22073464
Cumpston M, Li T, Page MJ et al (2019) Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev 10:ED000142. https://doi.org/10.1002/14651858.ED000142
Gao S, Zhang M, Wu K et al (2020) Risk of adverse events in lymphoma patients treated with brentuximab vedotin: a systematic review and meta-analysis. Expert Opin Drug Saf 19:617–623. https://doi.org/10.1080/14740338.2020.1718103
Zelenetz AD, Barrientos JC, Brown JR et al (2017) Idelalisib or placebo in combination with bendamustine and rituximab in patients with relapsed or refractory chronic lymphocytic leukaemia: interim results from a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol 18:297–311. https://doi.org/10.1016/S1470-2045(16)30671-4
Flinn IW, Hillmen P, Montillo M et al (2018) The phase 3 DUO trial: duvelisib vs ofatumumab in relapsed and refractory CLL/SLL. Blood 132:2446–2455. https://doi.org/10.1182/blood-2018-05-850461
Matasar MJ, Capra M, Özcan M et al (2021) Copanlisib plus rituximab versus placebo plus rituximab in patients with relapsed indolent non-Hodgkin lymphoma (CHRONOS-3): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol 22:678–689. https://doi.org/10.1016/S1470-2045(21)00145-5
Furman RR, Sharman JP, Coutre SE et al (2014) Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med 370:997–1007. https://doi.org/10.1056/NEJMoa1315226
Curran E, Smith SM (2014) Phosphoinositide 3-kinase inhibitors in lymphoma. Curr Opin Oncol 26:469–475. https://doi.org/10.1097/CCO.0000000000000113
Bernal A, Pastore RD, Asgary Z et al (2001) Survival of leukemic B cells promoted by engagement of the antigen receptor. Blood 98:3050–3057. https://doi.org/10.1182/blood.v98.10.3050
Davis RE, Ngo VN, Lenz G et al (2010) Chronic active B-cell-receptor signalling in diffuse large B-cell lymphoma. Nature 463:88–92. https://doi.org/10.1038/nature08638
Munk Pedersen I, Reed J (2004) Microenvironmental interactions and survival of CLL B-cells. Leuk Lymphoma 45:2365–2372. https://doi.org/10.1080/10428190412331272703
Rosich L, Saborit-Villarroya I, Lopez-Guerra M et al (2013) The phosphatidylinositol-3-kinase inhibitor NVP-BKM120 overcomes resistance signals derived from microenvironment by regulating the Akt/FoxO3a/Bim axis in chronic lymphocytic leukemia cells. Haematologica 98:1739–1747. https://doi.org/10.3324/haematol.2013.088849
Schmitz R, Young RM, Ceribelli M et al (2012) Burkitt lymphoma pathogenesis and therapeutic targets from structural and functional genomics. Nature 490:116–120. https://doi.org/10.1038/nature11378
Okkenhaug K (2002) Impaired B and T cell antigen receptor signaling in p110delta PI 3-kinase mutant mice. Science.https://doi.org/10.1126/science.1073560
Durand CA, Hartvigsen K, Fogelstrand L et al (2009) Phosphoinositide 3-kinase p110δ regulates natural antibody production, marginal zone and B-1 B cell function, and autoantibody responses. J Immunol 183:5673–5684. https://doi.org/10.4049/jimmunol.0900432
Gopal AK, Kahl BS, de Vos S et al (2014) PI3Kδ inhibition by idelalisib in patients with relapsed indolent lymphoma. N Engl J Med 370:1008–1018. https://doi.org/10.1056/NEJMoa1314583
Flinn IW, Miller CB, Ardeshna KM et al (2019) DYNAMO: a phase II study of duvelisib (IPI-145) in patients with refractory indolent non-Hodgkin lymphoma. JCO 37:912–922. https://doi.org/10.1200/JCO.18.00915
Dreyling M, Santoro A, Mollica L et al (2017) Phosphatidylinositol 3-kinase inhibition by copanlisib in relapsed or refractory indolent lymphoma. JCO 35:3898–3905. https://doi.org/10.1200/JCO.2017.75.4648
Sharman JP, Coutre SE, Furman RR et al (2019) Final results of a randomized, phase III study of rituximab with or without idelalisib followed by open-label idelalisib in patients with relapsed chronic lymphocytic leukemia. J Clin Oncol 37:1391–1402. https://doi.org/10.1200/JCO.18.01460
Flinn IW, O’Brien S, Kahl B et al (2018) Duvelisib, a novel oral dual inhibitor of PI3K-δ, γ, is clinically active in advanced hematologic malignancies. Blood 131:877–887. https://doi.org/10.1182/blood-2017-05-786566
Davids MS, Fisher DC, Tyekucheva S et al (2021) A phase 1b/2 study of duvelisib in combination with FCR (DFCR) for frontline therapy for younger CLL patients. Leukemia 35:1064–1072. https://doi.org/10.1038/s41375-020-01010-6
Davids MS, Kuss BJ, Hillmen P et al (2020) Efficacy and safety of duvelisib following disease progression on ofatumumab in patients with relapsed/refractory CLL or SLL in the DUO crossover extension study. Clin Cancer Res 26:2096–2103. https://doi.org/10.1158/1078-0432.CCR-19-3061
Coutré SE, Barrientos JC, Brown JR et al (2015) Management of adverse events associated with idelalisib treatment: expert panel opinion. Leuk Lymphoma 56:2779–2786. https://doi.org/10.3109/10428194.2015.1022770
Benson AB, Ajani JA, Catalano RB et al (2004) Recommended guidelines for the treatment of cancer treatment-induced diarrhea. JCO 22:2918–2926. https://doi.org/10.1200/JCO.2004.04.132
Greenwell IB, Ip A, Cohen JB (2017) PI3K inhibitors: understanding toxicity mechanisms and management. Oncology (Williston Park) 31:821–828
Dreyling M, Morschhauser F, Bouabdallah K et al (2017) Phase II study of copanlisib, a PI3K inhibitor, in relapsed or refractory, indolent or aggressive lymphoma. Ann Oncol 28:2169–2178. https://doi.org/10.1093/annonc/mdx289
Esposito A, Viale G, Curigliano G (2019) Safety, tolerability, and management of toxic effects of phosphatidylinositol 3-kinase inhibitor treatment in patients with cancer: a review. JAMA Oncol 5:1347–1354. https://doi.org/10.1001/jamaoncol.2019.0034
Busaidy NL, Farooki A, Dowlati A et al (2012) Management of metabolic effects associated with anticancer agents targeting the PI3K-Akt-mTOR pathway. J Clin Oncol 30:2919–2928. https://doi.org/10.1200/JCO.2011.39.7356
Miller BW, Przepiorka D, de Claro RA et al (2015) FDA approval: idelalisib monotherapy for the treatment of patients with follicular lymphoma and small lymphocytic lymphoma. Clin Cancer Res 21:1525–1529. https://doi.org/10.1158/1078-0432.CCR-14-2522
Kienle DL, Stilgenbauer S (2020) Approved and emerging PI3K inhibitors for the treatment of chronic lymphocytic leukemia and non-Hodgkin lymphoma. Expert Opin Pharmacother 21:917–929. https://doi.org/10.1080/14656566.2020.1737010
Lynch RC, Gopal AK (2020) Phosphatidylinositol-3-kinase inhibition in follicular lymphoma. Hematol Oncol Clin North Am 34:727–741. https://doi.org/10.1016/j.hoc.2020.02.008
Funding
This study was supported by the National Natural Scientific Foundation of China (Nos. 82170180, 81770126, 82000218), Fujian Natural Science Foundation of China (No. 2020J011246, No. 2020J05303), and Xiamen Municipal Bureau of Science and Technology (No. 3502Z20209003).
Author information
Authors and Affiliations
Contributions
Conceptualization, Z. J. L., W. L. X., and B. X.; data curation, G. X. W. and Y. T. H.; formal analysis, W. H. S.; funding acquisition, W. L. X. and B. X.; methodology, Z. J. L., and H. Y. Z.; resources, W. L. X. and B. X.; software, W. H. S. and Z. J. L.; supervision, Z. J. L. and B. X.; validation, Z. J. L. and W. L. X.; writing—original draft, W. H. S.; writing—review and editing, H. Y. Z.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shan, W., Wu, G., Huang, Y. et al. Adverse events in lymphoma patients treated with phosphoinositide 3 kinase Inhibitor in clinical trials: a meta-analysis. Ann Hematol 101, 1741–1753 (2022). https://doi.org/10.1007/s00277-022-04876-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-022-04876-x