Abstract
Background
The genomic knowledge on urothelial carcinoma is expanding. It is recognised that urothelial carcinoma is a disease with a high somatic mutation rate and a high prevalence of genetic alterations, as discussed by Thomas and Sonpavde (2022). In the context of a disease rich with somatic alterations, continuing efforts to better identify which patients may benefit most from targeted therapy, immunotherapy and combination therapy may ultimately lead to improved outcomes for patients with this disease.
Aims
We aimed to ascertain the frequency of next-generation sequencing (NGS) and the prevalence of genomic alterations amongst patients with metastatic urothelial cancer (mUC) in Ireland. We studied patients who received a targeted therapy following the detection of an oncogenic alteration on NGS and assessed their outcomes.
Methods
Patients with a diagnosis of mUC between 2017 and 2022 were identified from Urology MDT databases as well as pharmacy databases across three Irish cancer centres. A retrospective review of patient notes including a comprehensive review of histopathology, radiology data, prior therapies and NGS reports was carried out for each patient.
Results
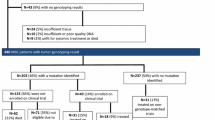
111 patients diagnosed with mUC between 2017 and 2022 were identified for inclusion across three hospital sites. NGS was carried out on the tumour specimens of 66 patients (59%). Thirty-six potentially therapeutically targetable alterations were identified amongst thirty-five patients. The most frequent alterations identified were PIK3CA mutations, FGFR3 mutations or fusions and ERBB2 somatic mutations. Fifteen patients (13.5%) received therapy directed at a genetic alteration. The most common targeted therapy received was erdafitinib (60%) followed by trastuzumab (33%) with one patient receiving alpelisib monotherapy. The median duration of treatment with targeted therapy was 3 months (range 1–34 months). Two patients were observed to have durable responses to erdafitinib approaching 3 years duration.
Conclusions
This study provides an understanding of the use of NGS and prevalence of genomic alterations in an Irish patient population.




Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author, KR, upon reasonable request.
References
Saginala K, Barsouk A, Aluru JS et al (2020) A Epidemiology of bladder cancer. Med Sci 8:15
Von der Maase H, Sengelov L, Roberts JT et al (2005) Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol 23:4602–4608
De Santis M, Bellmunt J, Mead G et al (2012) Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: EORTC study 30986. J Clin Oncol 30:191–199
Bellmunt J, De Wit R, Vaughn DJ et al (2017) Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med 376:1015–1026
Massard C, Gordon et al (2016) Safety and efficacy of durvalumab (MEDI4736), an anti-programmed cell death ligand-1 immune checkpoint inhibitor, in patients with advanced urothelial bladder cancer. J Clin Oncol 34:3119–3125
Patel MR, Ellerton J et al (2018) Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN Solid Tumor): pooled results from two expansion cohorts of an open-label, phase 1 trial. Lancet Oncol 19:51–64
Sharma P, Retz M, Siefker-Radtke A et al (2017) Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol 18(3):312–322
Powles T, Duran I et al (2018) Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. The Lancet 391(10122):748–757
Rosenberg JE, Hoffman-Censits J et al (2016) Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet 387:1909–1920
Vuky J, Balar AV, Castellano D et al (2020) Long-term outcomes in KEYNOTE-052: phase II study investigating first-line pembrolizumab in cisplatin-ineligible patients with locally advanced or metastatic urothelial cancer. J Clin Oncol 38(23):2658–2666
Bamias A, Davis I et al (2023) Final overall survival (OS) analysis of atezolizumab (atezo) monotherapy vs chemotherapy (chemo) in untreated locally advanced or metastatic urothelial carcinoma (mUC) from the phase 3 IMvigor130 study. J Clin Oncol 41(6_suppl). LBA441-LBA441. Published online February 21, 2023
Powles T, Park SH, Voog E et al (2020) Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N Engl J Med 383:1218–1230
Thomas J, Sonpavde G (2022) Molecularly targeted therapy towards genetic alterations in advanced bladder cancer. Cancers 14:1795. https://doi.org/10.3390/cancers14071795
The Cancer Genome Atlas Research Network (2014) Comprehensive molecular characterization of urothelial bladder carcinoma. Nature 507:315–322
Loriot Y, Necchi A, Park SH et al (2019) Erdafitinib in locally advanced or metastatic urothelial carcinoma. N Engl J Med 381:338–348
Hussain MHA, MacVicar GR, Petrylak DP et al (2007) Trastuzumab, paclitaxel, carboplatin, and gemcitabine in advanced human epidermal growth factor receptor-2/neu—positive urothelial carcinoma: results of a multicenter phase II National Cancer Institute trial. J Clin Oncol 25:2218–2224
Meric-Bernstam F, Hainsworth J, Bose R et al (2021) MyPathway HER2 basket study: pertuzumab (P) + trastuzumab (H) treatment of a large, tissue-agnostic cohort of patients with HER2-positive advanced solid tumors. J Clin Oncol 39((Suppl. 15)):3004
Wülfing C, Machiels JP, Richel DJ et al (2009) A single-arm, multicenter, open-label phase 2 study of lapatinib as the second-line treatment of patients with locally advanced or metastatic transitional cell carcinoma. Cancer 115(13):2881–90
de Vries EGE, Rüschoff J, Lolkema M et al (2023) Phase II study (KAMELEON) of single-agent T-DM1 in patients with HER2-positive advanced urothelial bladder cancer or pancreatic cancer/cholangiocarcinoma. Cancer Med. https://doi.org/10.1002/cam4.5893. Epub ahead of print. PMID: 37119523
Galsky MD, Del Conte G, Foti S et al (2022) Primary analysis from DS8201-A-U105: a phase 1b, two-part, open-label study of trastuzumab deruxtecan (T-DXd) with nivolumab (nivo) in patients (pts) with HER2-expressing urothelial carcinoma (UC). J Clin Oncol 40(6):438–438. https://doi.org/10.1200/JCO.2022.40.6_suppl.438
Milowsky MI, Iyer G, Regazzi AM et al (2013) Phase II study of everolimus in metastatic urothelial cancer. BJU Int 112(4):462–470. https://doi.org/10.1111/j.1464-410X.2012.11720.x. Epub 2013 Apr 3
Bellmunt J, Lalani A-KA, Jacobus S et al (2018) Everolimus and pazopanib (E/P) benefit genomically selected patients with metastatic urothelial carcinoma. Br J Cancer 119:707–712
McPherson V, Reardon B, Bhayankara A et al (2020) (2020) A phase 2 trial of buparlisib in patients with platinum-resistant metastatic urothelial carcinoma. Cancer 126:4532–4544
Grivas P, Loriot Y, Feyerabend S et al (2020) Rucaparib for recurrent, locally advanced, or metastatic urothelial carcinoma (mUC): results from ATLAS, a phase II open-label trial. J Clin Oncol 38:440
Rosenberg JE, Park SH, Kozlov V et al (2023) Durvalumab plus olaparib in previously untreated, platinum-ineligible patients with metastatic urothelial carcinoma: a multicenter, randomized, phase II trial (BAYOU). J Clin Oncol 41(1):43–53
Sanguedolce F, Zanelli M, Palicelli A et al (2023) HER2 expression in bladder cancer: a focused view on its diagnostic, prognostic, and predictive role. Int J Mol Sci 13;24(4):3720
Albarrán V, Rosero DI et al (2022) Her-2 targeted therapy in advanced urothelial cancer: from monoclonal antibodies to antibody-drug conjugates. Int J Mol Sci 23:12659
Zhao J, Xu W, Zhang Z et al (2015) Prognostic role of HER2 expression in bladder cancer: a systematic review and meta-analysis. Int Urol Nephrol 47:87–94
Meric-Bernstam F, Hainsworth J, Bose R et al (2021) MyPathway HER2 basket study: pertuzumab (P) + trastuzumab (H) treatment of a large, tissue-agnostic cohort of patients with HER2-positive advanced solid tumors. J Clin Oncol 39(Suppl. 15):3004
Oudard S, Culine S, Vano YA et al (2015) Multicentre randomised phase II trial of gemcitabine+platinum, with or without trastuzumab, in advanced or metastatic urothelial carcinoma overexpressing Her2. Eur J Cancer 51:45–54
Cerbone L, Sternberg CN, Sengeløv L et al (2016) Results from a phase I study of lapatinib with gemcitabine and cisplatin in advanced or metastatic bladder cancer: EORTC trial 30061. Oncology 90:21–28
Choudhury NJ, Campanile A, Antic T et al (2016) Afatinib activity in platinum-refractory metastatic urothelial carcinoma in patients with ERBB alterations. J Clin Oncol 34:2165–2171
Sheng X, He Z, Shi Y et al (2022) RC48-ADC for metastatic urothelial carcinoma with HER2-positive: combined analysis of RC48-C005 and RC48-C009 trials. J Clin Onco 40(Suppl. 16):4520
Robertson AG, Kim J, Al-Ahmadie H et al (2017) Comprehensive molecular characterization of muscle-invasive bladder cancer. Cell 171:540–556.e25
Loriot Y et al (2019) Erdafitinib in locally advanced or metastatic urothelial carcinoma. N Engl J Med 381:338–348
Loriot Y, Matsubara N et al (2023) Phase 3 THOR study: results of erdafitinib (erda) versus chemotherapy (chemo) in patients (pts) with advanced or metastatic urothelial cancer (mUC) with select fibroblast growth factor receptor alterations (FGFRalt). J Clin Oncol 41(17_suppl). LBA4619-LBA4619. Published online June 07, 2023
Powles T, Bellmunt J, Comperat E et al (2021) Bladder cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol 33(3):244–258
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
This study was granted approval by the Research Ethics Committee HSE South Eastern Area. This study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ronan, K., Jordan, E., Leonard, C. et al. Frequency of next-generation sequencing, prevalence of targetable mutations and response to targeted therapies amongst patients with metastatic urothelial cancer in Ireland: a multi-centre retrospective study of real-world data. Ir J Med Sci 193, 1155–1161 (2024). https://doi.org/10.1007/s11845-023-03569-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11845-023-03569-2