Abstract
Background
Next-generation sequencing (NGS) has been introduced to many Korean institutions to support molecular diagnostics in cancer since 2017, when it became eligible for reimbursement by the National Health Insurance Service. However, the uptake of molecularly guided treatment (MGT) based on NGS results has been limited because of stringent regulations regarding prescriptions outside of approved indications, a lack of clinical trial opportunities, and limited access to molecular tumor boards (MTB) at most institutions. The KOSMOS-II study was designed to demonstrate the feasibility and effectiveness of MGT, informed by MTBs, using a nationwide precision medicine platform.
Methods
The KOSMOS-II trial is a large-scale nationwide master observational study. It involves a framework for screening patients with metastatic solid tumors for actionable genetic alterations based on local NGS testing. It recommends MGT through a remote and centralized MTB meeting held biweekly. MGT can include one of the following options: Tier 1, the therapeutic use of investigational drugs targeting genetic alterations such as ALK, EGFR, ERBB2, BRAF, FH, ROS1, and RET, or those with high tumor mutational burden; Tier 2, comprising drugs with approved indications or those permitted for treatment outside of the indications approved by the Health Insurance Review and Assessment Service of Korea; Tier 3, involving clinical trials matching the genetic alterations recommended by the MTB. Given the anticipated proportion of patients receiving MGT in the range of 50% ± 3.25%, this study aims to enroll 1,000 patients. Patients must have progressed to one or more lines of therapy and undergone NGS before enrollment.
Discussion
This pragmatic master protocol provides a mass-screening platform for rare genetic alterations and high-quality real-world data. Collateral clinical trials, translational studies, and clinico-genomic databases will contribute to generating evidence for drug repositioning and the development of new biomarkers.
Trial registration
NCT05525858.
Similar content being viewed by others
Background
The rapid development of molecularly targeted agents and immunotherapies, coupled with high-throughput tumor profiling using next-generation sequencing (NGS), is steering medical oncology towards “precision medicine.” Several precision medicine clinical trials in the United States and some European countries have adopted pragmatic platform trial designs [1,2,3,4,5]. Some studies have demonstrated that molecular profiling-guided therapy (MGT), determined by the genomic profile of a tumor and assessed by a molecular tumor board (MTB), may improve clinical outcomes in patients with refractory solid tumors [2, 6].
However, challenges remain with the clinical implementation of MGTs [7]. Molecular profiling remains expensive and is very intricate in terms of the number of target genes, variant calling procedures, and sequencing techniques [8]. Meanwhile, the expertise in interpreting and matching MGTs often varies among oncologists, especially those who work at community hospitals. Regulatory approval for MGTs is limited to very narrow indications (e.g., trastuzumab is approved for HER2-amplified breast or gastric cancer but not for HER2-amplified biliary or salivary gland cancer), and it is difficult to prescribe MGTs outside of regulatory approval for patients with rare actionable genomic alterations. In Korea, where prescriptions outside the approved indications are strictly controlled, access to MGT is limited unless patients participate in clinical trials using MGTs. However, the opportunity to enroll in clinical trials is restricted in Korea, especially outside the Seoul Metropolitan area [9].
We have previously conducted a pragmatic precision medicine trial, the KOSMOS-I pilot study, designed as a nationwide, prospective, multicenter, multi-cohort study of MGT within local clinical practice. Local NGS reports from patients with refractory metastatic solid tumors were assessed by a central molecular tumor board (cMTB), which convened twice weekly on a virtual platform. MGT options were provided in the form of the Therapeutic Use of Investigational Drugs (TUID) program, approved for individual patients by the Ministry of Food and Drug Safety (MFDS). MGT was found to be feasible for 51.3% (99/193) of the patients enrolled over just one year, from February 2021 to February 2022. This finding underscores the significant need for MGT in Korea and demonstrates the feasibility of MGT supported by nationwide cMTB [10].
Based on these results, we expanded the KOSMOS-I platform to enroll a larger number of patients. We designed a master observational trial (MOT), KOSMOS-II, which offers more MGT options, including TUID and investigator-initiated clinical trials (IITs), as part of the entire MOT framework. In this context, we describe the rationale and design of the KOSMOS-II trial, the current progress of this project, and discuss the operational issues and perspectives concerning this pragmatic platform.
Methods/design
Study design
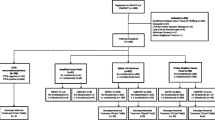
The KOSMOS-II trial comprises a framework for screening patients with actionable targets, operating a cMTB that recommends and provides MGT, and developing a clinico-genomic database (CGDB). MGT options include TUID with targeted and/or immunotherapies (Tier 1); local practice involving therapy within or outside the approved indications (Tier 2); or participation in clinical trials (Tier 3) (Fig. 1).
Study Scheme for KOSMOS-II trial. Abbreviations: NGS, next-generation sequencing; eCRF, electronic case report form; MTB, molecular tumor board; KSMO, Korean Society of Medical Oncology; KCSG, Korean Cancer Study Group; IP, investigational products; MGT, molecular profile guided therapy; IIT, investigator initiated clinical trials; Pt, patient
Study objectives
The primary objective of this study is to evaluate the feasibility of MGT in terms of the proportion of participants who received the treatment (MGT rate). The receipt of MGT is defined as receiving at least one dose of MGT targeting the genetic alterations (GAs) detected by NGS, regardless of the tier assigned by the cMTB. The second primary objective is to evaluate the effectiveness of MGT in terms of clinical benefit rate (CBR: the percentage of patients with complete response, partial response, or stable disease according to Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 [11] beyond 16 ± 2 weeks of treatment) in Tier 1.
The secondary objectives include the following:
-
1.
To evaluate the effectiveness of MGT in terms of the objective response rate, progression-free survival, treatment duration, and 1-year overall survival in Tier 1 and Tier 3A (investigator-initiated trials by KOSMOS-II study team), where the clinical outcomes of MGT are being captured.
-
2.
To evaluate the safety of MGT according to Common Terminology Criteria for Adverse Events (CTCAE) version 5.0
-
3.
To assess the operational feasibility of delivering MGT.
-
4.
To correlate the molecular profile with the clinical outcomes of MGT.
In this study, the explorative objectives are divided into translational and clinical objectives. The translational objectives are presented in the following section. The clinical explorative objective is to evaluate the extension of the MGT beyond the KOSMOS-I. In the KOSMOS-I trial, MGT was provided for up to 12 months. Therefore, the KOSMOS-II trial will enroll the patients after the completion of study treatment of KOSMOS-I if they do not experience disease progression to provide extended MGT. The clinical and molecular characteristics of the patients (prior KOSMOS-I participants) will be described separately from the whole study population.
Eligibility criteria
Patients with locally advanced or metastatic solid tumors are eligible for the study based on the following criteria: (1) progression on standard treatments or exhaustion of available treatment options; (2) patients have an available NGS report for the tumors, ideally obtained within the last 3 years, and provided by laboratories accredited by MFDS or regulatory bodies compatible with MFDS, such as Clinical Laboratory Improvement Amendments; (3) a life expectancy of at least 12 weeks; (4) observable adequate recovery from the most recent systemic or local treatment.
Study treatment
Table 1 presents the options available for Tier 1. These options are provided through TUID, which is available only for drugs that have already been approved for certain indications by MFDS, and it does not include investigational products without any approved indications. The KOSMOS-II centralized and standardized the TUID approval process for study participants. The protocol specifies the actionable GAs that match each drug on the Tier 1 list; however, the cMTB panel can recommend Tier 1 options if there is evidence that GAs other than the pre-specified ones can predict the benefit from the drug.
Tier 2 options include treatment according to local practice, such as chemotherapy for approved indications, if any, treatment outside approved indications that has been allowed by the Health Insurance Review and Assessment Service (HIRA), or best supportive care.
Tier 3 options, which involve participation in clinical trials, are always prioritized over Tier 1 or Tier 2 options, provided they are available. Clinical trials that assess MGT and are accessible in Korea are recommended. In particular, the opportunity to participate in IITs, supported by the KOSMOS-industry consortium (Table 2), is recommended if the patient is involved in the KOSMOS-II trial and the cMTB panel determines that the GAs from the patient’s tumor match the eligibility criteria of the IITs. These IITs, referred to as Tier 3A options, are designed in alignment with the master protocol of the KOSMOS-II trial to expedite the screening procedure within the KOSMOS-II platform and offer additional MGT options for KOSMOS-II participants while allowing them to explore the possibility of repurposing existing drugs.
Study process
After a participant signs an informed consent, their clinical information is entered into the electronic case report form, which automatically sends the information towards the virtual cMTB platform, NAVIFY®. The participants’ NGS report is separately uploaded to NAVIFY®. Seven cMTB panels are organized, each comprised of three to four medical oncologists and at least one or more pathologists or bioinformaticians. The virtual cMTB meetings are held twice a week. To ensure the participants’ personal information protection, the clinical information on NAVIFY® is pseudo-anonymized, and all members of the cMTB panels sign confidentiality agreements.
Overall, cMTB provides the following information after discussing each case:
-
1)
Actionable genomic alteration from the subject’s NGS report
-
2)
Preferred recommendations: (Tier 1, Tier 2, or Tier 3) (Fig. 1)
-
3)
Level of evidence per recommendation.
The level of evidence is assigned according to the Korean Precision Medicine Networking Group scale of clinical actionability of molecular targets (K-CAT) [12]; supporting references regarding the assigned K-CAT levels should also be provided. The cMTB can suggest a maximum of three treatment options in a hierarchy, and the investigator who submits the case can choose among the provided options.
The cMTB process is illustrated in Fig. 2. The NGS report is reviewed and commented on by the panel pathologist and/or bioinformatician prior to the meeting. If a patient exhibit only one actionable GA that matches one of the treatment options of Tier 1, the case can be assigned to Tier 1 treatment through an expedited review by the panel chair (a medical oncologist), pathologist, and/or bioinformatician, without being fully reviewed by all cMTB members on an online forum. If a case is not recommended for expedited review, the medical oncologists on the panel search for clinical trials available in Korea that are relevant to the submitted case. This process involves checking the MFDS website (https://nedrug.mfds.go.kr/index), Korea Disease Control and Prevention Agency Clinical Research Information Service (https://cris.nih.go.kr/cris/index/index.do), and the Korean Cancer Study Group (KCSG) website (https://www.kcsg.org). Treatment options are determined after a discussion on the online forum regarding the interpretation of GAs and the available options obtained through the search.
The status of various clinical trials of MGT in multiple institutions is monitored and curated by the KOSMOS-II study team and regularly provided to the cMTB panel members. Online forums and educational workshops for cMTB are organized and supported by the Korean Society of Medical Oncology.
Study assessments
Pretreatment evaluations require clinically appropriate radiographic studies to assess target or nontarget lesions for each participant, according to RECIST v1.1 criteria. For those who participate in Tier 1, tumor assessments are performed every 8 ± 2 weeks, according to RECIST v1.1 or iRECIST (for those who were treated with atezolizumab). For those who participate in Tier 3A trials (Table 2), tumor assessment is performed according to the protocol of each study, and the intervals between assessments range from 6 to 8 weeks.
Tier 1 serious adverse events are collected according to CTCAE v5.0. In Tier 3A studies, adverse events are identified and assessed according to each protocol.
Translational projects and the master protocol
Several translational projects utilizing the KOSMOS-II trial are ongoing. The translational explorative objectives are as follows:
-
1.
To correlate the response to immune checkpoint inhibitors with tumor mutational burden confirmed by local NGS testing.
-
2.
To correlate the response to immune checkpoint inhibitors with the Lunit SCOPE IO, an artificial intelligence-powered spatial tumor-infiltrating lymphocyte analysis of digitized data from qualified scanned images of hematoxylin and eosin-stained slides. This analysis demonstrates its predictive role in various types of tumors, including non-small cell lung cancer [13].
-
3.
To illustrate the genomic landscape of Korean patients with solid tumors through whole-genome sequencing (WGS) of participants who provided recently obtained tumor tissue (within 3 months from enrollment) and to explore the possibility of using WGS to identify the appropriate MGT.
Establishment of a clinico-genomic database
A specific collateral project of this study is the establishment of a nationwide CGDB in collaboration with the National Cancer Center of Korea (NCCK), designated the National Cancer Data Center (NCDC). The clinical characteristics of the participants, their genomic profiles, and outcomes of the MGT (Tier 1 and Tier 3A) are stored as anonymized codes and curated in the CGDB for further research and development. The resident registration numbers of the study participants are collected and transferred to the NCDC, where they are linked to the data by the Korean Statistical Information Service to update the survival status of the participants after the study ends. Efforts, such as collecting the metadata of each local NGS panel and transforming heterogeneous genomic variant call format (VCF) files into a standardized format, are ongoing to build the CGDB (Fig. 3).
Representation of the flow of integration and distribution of clinico-genomic data in the KOSMOS-II trial. Abbreviations: eCRF, electronic case report form; RRN, resident registration number; VCF, variant call format; KOSIS, Korean Statistical Information Service; NGS, next-generation sequencing; KCSG, Korean Cancer Study Group
KOSMOS-II investigators and companies participating in the KOSMOS Industry Consortium will have access to the CGDB, which will be transferred to the data center of the KCSG, the sponsor of this study. The KCSG has a partnership agreement with the NCCK to ensure and promote broad data sharing among academic societies, governmental organizations, and biopharmaceutical companies. The CGDB housed in the NCDC will be made available to the public 3 years after the completion of this study and distributed according to the pre-specified governance rules for the data.
Statistical considerations
Given that MGT was available for 51.3% (99/193) of patients in the KOSMOS-I trial [14], it is expected that the MGT rate in this study will be approximately 50%. To estimate the proportion of patients with a 95% confidence interval of 0.065, a total of 950 patients need to be included. Considering a 5% dropout rate, the plan is to enroll 1,000 patients in this study.
Considering the CBRs or disease control rates in previous genomically guided basket trials have ranged from 20 to 50% [4, 15, 16], the expected CBR of Tier 1 patients is 30%. Assuming the null hypothesis that the CBR is 20% or less, as might be expected from studies on investigational products reported in the early 2000s [17], 221 patients are needed to demonstrate that the true CBR is 30%, with a type I error of 5% (2-sided) and 90% power. Considering a 20% dropout rate, we need to enroll 265 patients in Tier 1. In addition, we will enroll approximately 35 exceptional responders from KOSMOS-I trial, resulting in a total of 300 patients being enrolled in Tier 1 for this study. The scope of data collection according to the endpoints in each tier is presented in Table 3.
Current status
This study enrolled its first participant in September 2022 and 418 patients (42% of the projected number) as of October 2023. A total of 31 institutions across Korea participated in this trial, with 29 of the 31 institutions having started to enroll patients. The virtual cMTB held its 110th meeting in October 2023. The expected duration of this study is 3 years, including 2 years of enrollment and 1 year of follow-up.
Discussion
This study is a type of MOT, a prospective observational trial that enrolls patients based on a precise molecular biomarker testing algorithm and incorporates interventional trials or real-world data (RWD). MOT is a new class of master clinical protocols proposed to bridge the gap between interventional trials and retrospective RWD in data collection for precision medicine [18].
MOT, such as the KOSMOS-II trial, can provide a common screening platform for various collateral studies. Identifying rare actionable GAs is challenging without comprehensive molecular testing in a large number of patients. The opportunity to offer patients innovative treatment options hinges on the seamless integration of the interpretation of molecular test results and drug-access programs, including clinical trials. The KOSMOS-II trial employed various NGS panels certified by healthcare authorities, such as MFDS, to efficiently screen participants and reduce the time and cost associated with testing and logistics.
In Korea, NGS for clinical diagnosis can only be performed by clinical laboratories certified according to the laboratory guidelines of MFDS. Most laboratories perform comprehensive genomic profiling, testing hundreds of genes, and follow the good laboratory standards of the Korean Society of Pathologists [8]. However, the heterogeneity of the panels in terms of sequencing methods, covered gene regions, and variant calling pipelines poses challenges in constructing a molecular matrix for MOT. To address these issues, the KOSMOS-II study team collects metadata for each submitted NGS panel, including the list and number of targeted genes, sequencer type, reference sequence, target capture kit, and variant-calling software. Even in the absence of a unified testing platform, this standardized curation of detected variants for each participant helps to provide reliable information about the actionability of the GAs. The Targeted Agent and Profiling Utilization Registry (TAPUR™) trial has already demonstrated that heterogeneous molecular testing methods can serve as platforms for precision medicine trials [3]. Our trial is anticipated to provide evidence supporting an efficient and pragmatic framework for nationwide screening in the realm of drug development and repositioning.
RWD is increasingly used to assess the impact of drugs in daily practice and to support the drug approval process, helping to address the limitations of clinical trial data. It can provide information about underserved populations or individuals with underlying diseases or organ dysfunction who are ineligible for clinical trials [19]. However, the accuracy of data, such as molecular annotations or clinical events (disease progression, recurrence, or adverse events from treatments), for cancer patients is often questioned in RWD because of the heterogeneity of testing methods and the result reporting, as well as non-standardized reports regarding efficacy and safety outcomes [18]. MOT can mitigate the disadvantages of RWD by prospectively collecting clinical data using a common case report form, while treatment and evaluation schedules are more flexible compared with those of traditional clinical trials [20]. In the KOSMOS-II trial, clinicopathologic information for all registered patients is being collected prospectively, and the response to MGT in Tier 1 and Tier 3A patients is being recorded according to the RECIST 1.1 criteria (also iRECIST for patients receiving immune checkpoint inhibitors).
Many precision medicine trials have shown that one or more potentially actionable GAs can be identified in approximately 30%–50% of patients, with approximately 10–20% of them being eligible for MGT [1, 2, 4, 6, 16, 21]. Some studies have demonstrated improved clinical outcomes with MGT compared with conventional therapy [2, 6]. The proportion of patients eligible for MGT can vary depending on the type of molecular profiling platform, the availability of MGT options, and the investigators’ treatment intentions. As demonstrated by our previous study, KOSMOS-I, we achieved a matching rate of 51.3% by implementing the cMTB combined with the TUID program, along with efficient communication for clinical trial enrollment among cMTB members and investigators. This rationale led us to hypothesize that the MGT rate is 50% in KOSMOS-II. The expected CBR in Tier 1 is 30%, which is a significant improvement for heavily treated and refractory populations. However, achieving desired efficacy levels may be challenging because in many cases, the genomic profiles for MGT are derived from the sequencing results of tumor samples obtained significantly earlier than the treatment, which may not accurately reflect the genomic profile at the time of MGT implementation. To address these concerns, we encourage investigators to submit NGS results from the most recently obtained tumor samples and to enroll patients in a WGS translational project using fresh tissue samples.
To the best of our knowledge, this is the first multicenter, nationwide precision medicine study that utilizes cMTB for all participants. While discussions on MTBs may positively affect treatment decisions [22], increase the likelihood of enrollment in clinical trials, and potentially improve clinical outcomes [23], the associated turnaround time and cost might prevent the adoption of MTB in clinical practice [24]. Additionally, MTBs have not been actively employed in many Korean institutions because of disparities in human resources, infrastructure for NGS interpretation, and clinical trial access [9]. The KOSMOS-II trial involves 31 institutions from all over South Korea, and 12 of them are located outside the Seoul metropolitan area. To facilitate cMTB in this large-scale study, we use a video-conferencing platform and clinicopathologic information curating software, NAVIFY®. Our study aims to measure the operational feasibility of a nationwide cMTB by assessing the turnaround time from the site’s request to the cMTB’s decision, and by examining the agreement rate between the cMTB’s recommendation and the actual treatment administered to patients. Furthermore, we aim to gauge the consensus among different panels on similar cases, particularly in assigning a level of clinical actionability to individual cases.
Through the KOSMOS-II trial, we also aim to develop a CGDB that includes participants’ clinical characteristics and genomic profiles by processing VCF files from various platforms, which will be linked to reliable survival data provided by national mortality statistics. Several collaborative efforts have been made to develop real-world CGDBs to assess the effects of genomic profiling and MGT on patient care [25, 26]. The CGDB for the KOSMOS-II study will contribute significantly to precision medicine by providing high-quality clinical data and accurate genomic information.
In conclusion, the KOSMOS-II trial is designed to test the hypothesis that cMTB-based MGT approach is both feasible and effective for treating refractory solid tumors on a nationwide scale. This MOT, incorporating RWD and IITs, is expected to provide an efficient platform for identifying new indications or biomarkers for existing drugs as well as investigational agents. In addition, the CGDB developed through the KOSMOS-II trial may contribute to collaborative efforts aimed at data-informed clinical decision-making.
Availability of data and materials
No datasets were generated or analysed during the current study.
Abbreviations
- NGS:
-
Next-Generation Sequencing
- MGT:
-
Molecularly Guided Treatment
- MTB:
-
Molecular Tumor Board
- cMTB:
-
Central Molecular Tumor Board
- TUID:
-
Therapeutic Use of Investigational Drugs
- MFDS:
-
The Ministry of Food and Drug Safety
- MOT:
-
Master observational trial
- IIT:
-
Investigator-initiated trial
- CGDB:
-
Clinico-Genomic Database
- GAs:
-
Genetic Alterations
- CBR:
-
Clinical Benefit Rate
- RECIST:
-
Response Evaluation Criteria in Solid Tumors
- CTCAE:
-
Common Terminology Criteria for Adverse Events
- HIRA:
-
The Health Insurance Review and Assessment Service
- K-CAT:
-
Korean Precision Medicine Networking Group scale of clinical actionability of molecular target
- KCSG:
-
Korean Cancer Study Group
- WGS:
-
Whole genome sequencing
- NCCK:
-
National Cancer Center of Korea
- NCDC:
-
National Cancer Data Center
- VCF:
-
Variant Call Format
- RWD:
-
Real-World Data
- TAPUR:
-
Targeted Agent and Profiling Utilization Registry trial
References
Le Tourneau C, Delord JP, Gonçalves A, Gavoille C, Dubot C, Isambert N, Campone M, Trédan O, Massiani MA, Mauborgne C, et al. Molecularly targeted therapy based on tumour molecular profiling versus conventional therapy for advanced cancer (SHIVA): a multicentre, open-label, proof-of-concept, randomised, controlled phase 2 trial. Lancet Oncol. 2015;16(13):1324–34.
Massard C, Michiels S, Ferté C, Le Deley M-C, Lacroix L, Hollebecque A, Verlingue L, Ileana E, Rosellini S, Ammari S, et al. High-throughput genomics and clinical outcome in hard-to-treat advanced cancers: results of the MOSCATO 01 trial. Cancer Discov. 2017;7(6):586–95.
Mangat PK, Halabi S, Bruinooge SS, Garrett-Mayer E, Alva A, Janeway KA, et al. Rationale and design of the Targeted Agent and Profiling Utilization Registry (TAPUR) study. JCO Precis Oncol. 2018;2:1–14.
van der Velden DL, Hoes LR, van der Wijngaart H, van Berge Henegouwen JM, van Werkhoven E, Roepman P, Schilsky RL, de Leng WWJ, Huitema ADR, Nuijen B, et al. The drug rediscovery protocol facilitates the expanded use of existing anticancer drugs. Nature. 2019;574(7776):127–31.
Flaherty KT, Gray RJ, Chen AP, Li S, McShane LM, Patton D, Hamilton SR, Williams PM, Iafrate AJ, Sklar J, et al. Molecular landscape and actionable alterations in a genomically guided cancer clinical trial: National Cancer Institute Molecular Analysis for Therapy Choice (NCI-MATCH). J Clin Oncol. 2020;38(33):3883–94.
Tsimberidou A-M, Hong DS, Ye Y, Cartwright C, Wheler JJ, Falchook GS, Naing A, Fu S, Piha-Paul S, Janku F, et al. Initiative for Molecular Profiling and Advanced Cancer Therapy (IMPACT): an MD Anderson precision medicine study. JCO Precis Oncol. 2017;1:1–18.
Mateo J, Steuten L, Aftimos P, André F, Davies M, Garralda E, Geissler J, Husereau D, Martinez-Lopez I, Normanno N, et al. Delivering precision oncology to patients with cancer. Nat Med. 2022;28(4):658–65.
Kim J, Park W-Y, Kim NKD, Jang SJ, Chun S-M, Sung C-O, Choi J, Ko Y-H, Choi Y-L, Shim HS, et al. Good laboratory standards for clinical next-generation sequencing cancer panel tests. J Pathol Transl Med. 2017;51(3):191–204.
Kim W, Jang S, Chang YJ. Regional differences in access to clinical trials for cancer in Korea. Qual Improv Health Care. 2021;27(1):20–5.
Kim TY, Kim SY, Kim JH, Jung HA, Choi YJ, Hwang IG, Cha Y, Lee GW, Lee YG, Kim TM, et al. 708P Final clinical outcomes of nationwide precision oncology pilot study; KOrean Precision Medicine Networking Group Study of MOlecular profiling guided therapy based on genomic alterations in advanced Solid tumors (KOSMOS) KCSG AL-20-05. Ann Oncol. 2023;34:S490–1.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47.
Yoon S, Kim M, Hong YS, Kim HS, Kim ST, Kim J, Yun H, Yoo C, Ahn HK, Kim HS, et al. Recommendations for the use of next-generation sequencing and the molecular tumor board for patients with advanced cancer: a report from KSMO and KCSG Precision Medicine Networking Group. Cancer Res Treat. 2022;54(1):1–9.
Park S, Ock C-Y, Kim H, Pereira S, Park S, Ma M, Choi S, Kim S, Shin S, Aum BJ, et al. Artificial intelligence-powered spatial analysis of tumor-infiltrating lymphocytes as complementary biomarker for immune checkpoint inhibition in non–small-cell lung cancer. J Clin Oncol. 2022;40(17):1916–28.
Kim TY, Kim JH, Jung HA, Choi YJ, Hwang IG, Cha Y, Lee GW, Lee YG, Ahn MS, Kim TM, et al. OD1–1 Preliminary results from the KOrean precision medicine networking group study of MOlecular profiling guided therapy based on genomic alterations in advanced solid tumors (KOSMOS): KCSG AL-20–05. ESMO Open. 2022;7(6).
Sicklick JK, Kato S, Okamura R, Schwaederle M, Hahn ME, Williams CB, De P, Krie A, Piccioni DE, Miller VA, et al. Molecular profiling of cancer patients enables personalized combination therapy: the I-PREDICT study. Nat Med. 2019;25(5):744–50.
Rodon J, Soria J-C, Berger R, Miller WH, Rubin E, Kugel A, Tsimberidou A, Saintigny P, Ackerstein A, Braña I, et al. Genomic and transcriptomic profiling expands precision cancer medicine: the WINTHER trial. Nat Med. 2019;25(5):751–8.
Italiano A, Massard C, Bahleda R, Vataire AL, Deutsch E, Magné N, Pignon JP, Vassal G, Armand JP, Soria JC. Treatment outcome and survival in participants of phase I oncology trials carried out from 2003 to 2006 at Institut Gustave Roussy. Ann Oncol. 2008;19(4):787–92.
Dickson D, Johnson J, Bergan R, Owens R, Subbiah V, Kurzrock R. The master observational trial: a new class of master protocol to advance precision medicine. Cell. 2020;180(1):9–14.
Sherman RE, Anderson SA, Dal Pan GJ, Gray GW, Gross T, Hunter NL, LaVange L, Marinac-Dabic D, Marks PW, Robb MA, et al. Real-world evidence — what is it and what can it tell us? N Engl J Med. 2016;375(23):2293–7.
Dickson D, Johnson J, Bergan R, Owens R, Subbiah V, Kurzrock R. Snapshot: trial types in precision medicine. Cell. 2020;181(1):208–208.e201.
Trédan O, Wang Q, Pissaloux D, Cassier P, de la Fouchardière A, Fayette J, Desseigne F, Ray-Coquard I, de la Fouchardière C, Frappaz D, et al. Molecular screening program to select molecular-based recommended therapies for metastatic cancer patients: analysis from the ProfiLER trial. Ann Oncol. 2019;30(5):757–65.
Behel V, Noronha V, Choughule A, Shetty O, Chandrani P, Kapoor A, Bondili SK, Bajpai J, Kumar R, Pai T, et al. Impact of molecular tumor board on the clinical management of patients with cancer. JCO Glob Oncol. 2022;8:e2200030.
Larson KL, Huang B, Weiss HL, Hull P, Westgate PM, Miller RW, Arnold SM, Kolesar JM. Clinical outcomes of molecular tumor boards: a systematic review. JCO Precis Oncol. 2021;5:1122–32.
Luchini C, Lawlor RT, Milella M, Scarpa A. Molecular tumor boards in clinical practice. Trends in Cancer. 2020;6(9):738–44.
AACR Project GENIE. Powering precision medicine through an international consortium. Cancer Discov. 2017;7(8):818–31.
Liu R, Rizzo S, Waliany S, Garmhausen MR, Pal N, Huang Z, Chaudhary N, Wang L, Harbron C, Neal J, et al. Systematic pan-cancer analysis of mutation–treatment interactions using large real-world clinicogenomics data. Nat Med. 2022;28(8):1656–61.
Acknowledgements
We gratefully acknowledge the support for this project and the manuscript by the staff of Korean Cancer Study Group (Hye-Yeon Jeon, Minju Lee, Sungsoo Kim, and Jinyeong Baek) and Korean Society of Medical Oncology (MiMinkyung Song). We also thank to our research coordinators, (Mijung Kwak, Na An) for their devoted contribution on this project.
Funding
The National R&D Program for Cancer Control, Ministry of Health and Welfare, Republic of Korea (Grant # HA22C0052) awarded support for this study through scientific peer review as part of the grant application for the Investigator-Initiated Multi-Center Clinical Trial Support Program.
KOSMOS—Industry Consortium including Roche (Basel, Switzerland) and Lunit (Seoul, Republic of Korea) also provided funding for this study.
The funders of the study have no role in the study design, data collection, data analysis, or data interpretation. The funding bodies will be informed of any planned publications.
Author information
Authors and Affiliations
Contributions
All authors (SYK, JHK, TK, SRP, SY, SL, SL, TMK, SH, HRK, HY, SL, JK, YC, KSC, HC, HR, GL, DYZ, and JBA) reviewed and approved the final version of the manuscript. SYK, JK, TK, HY, SL (Sejoon Lee), KSC, HC, HR, and GL conceptualized this study. SRP, SY, SL (Soohyeon Lee), SL (Se-Hoon Lee), TMK, SH, HRK, HY, SL (Sejoon Lee), JK, and YC participated in research activity planning and execution. DYZ and JBA supervised the study. Funding was obtained by JHK. SYK contributed to the writing of the original draft, and all authors contributed to revising the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Institutional Review Board and Protocol Review Committee of the Korean Cancer Study Group (protocol number AL22-09) and by the review boards of all 31 institutions. Informed consent will be obtained from all patients and/or their legal guardian(s) in compliance with the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
SYK receives research funding from Roche/Genentech. JHK receives research funding from Eisai, Ono Pharmaceutical, and Roche. SRP has receives research funding from ImmuneOncia Therapeutics, ONO Pharma Korea, and Incyte. SL (Soohyeon Lee) has stock and other ownership interests of Celgene/Bristol-Myers Squibb and Pfizer and receives research funding from Jeil Pharmaceutical Co. SL (Se-Hoon Lee) receives research funding from AstraZeneca, Lunit, and Merck. TMK has consulting or advisory roles outside this work, related to Amgen, AstraZeneca/MedImmune, Boryung, Daiichi-Sankyo, HK inno.N, IMBDx. Inc., Janssen, Novartis, Regeneron, Roche/Genentech, Samsung Bioepis, Takeda, and Yuhan. HRK received honoraria from AstraZeneca, Bristol Myers Squibb, Genentech/Roche, stock ownership in Bridgebio Therapeutics; served as a consultation or advisory role for Bayer, AstraZeneca, Bristol Myers Squibb, Takeda, and Yuhan; and received research funding from the Yonsei Lee Youn Jae Fellowship outside of the current work. YC is employed and has stock and other ownership interests with Abion. JBA receives research funding from Sanofi. The remaining authors declare no conflict of interest that are relevant to the content of this article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kim, S.Y., Kim, J.H., Kim, TY. et al. Pragmatic nationwide master observational trial based on genomic alterations in advanced solid tumors: KOrean Precision Medicine Networking Group Study of MOlecular profiling guided therapy based on genomic alterations in advanced Solid tumors (KOSMOS)-II study protocol KCSG AL-22–09. BMC Cancer 24, 574 (2024). https://doi.org/10.1186/s12885-024-12338-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-024-12338-y