Abstract
Background
Cell division cycle 42 (CDC42) regulates macrophage polarization, vascular inflammation, atherosclerosis progression, and modifies differentiation of T helper (Th) cells, while its potential as a biomarker in coronary heart disease (CHD) patients is still lacking. This study aimed to evaluate CDC42 expression, its correlation with Th1, Th2, and Th17 cells, adhesion molecules, and biochemical indexes in CHD patients.
Methods
One hundred two CHD patients and 50 controls were enrolled. CDC42 expression in peripheral blood mononuclear cells was assessed by reverse transcription quantitative polymerase chain reaction in all participants. In CHD patients, Th1, Th2, and Th17 cells were detected by flow cytometric analysis; meanwhile, serum levels of inflammatory cytokines and adhesion molecules were detected by enzyme-linked immunosorbent assay.
Results
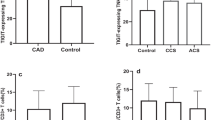
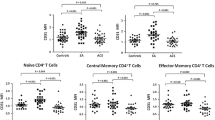
CDC42 was lower in CHD patients (median (interquartile range (IQR)) = 0.431 (0.304–0.722)) than in controls (median (IQR) = 0.985 (0.572–1.760)) (p < 0.001). CDC42 was positively associated with Th2 cells (p = 0.016) and interleukin (IL)-10 (p = 0.034), but negatively correlated with Th17 cells (p < 0.001) and IL-17A (p < 0.001) in CHD patients. However, no association was found in CDC42 with Th1 cells (p = 0.199) or interferon-γ (p = 0.367) in CHD patients. Besides, CDC42 was negatively correlated with vascular cell adhesion molecule-1 (p = 0.013) and intercellular cell adhesion molecule-1 (p = 0.001) in CHD patients. Additionally, CDC42 negatively associated with C-reactive protein (p < 0.001), Gensini score (p < 0.001), total cholesterol (p = 0.039), and low-density lipoprotein cholesterol (p = 0.014), but not with other biochemical indexes (p > 0.05) in CHD patients.
Conclusion
CDC42 correlates with Th2 cells, Th17 cells, and adhesion molecules, also reflects inflammation, coronary stenosis degree, and cholesterol level in CHD patients.





Similar content being viewed by others
References
Malakar AK, Choudhury D, Halder B et al (2019) A review on coronary artery disease, its risk factors, and therapeutics. J Cell Physiol 234(10):16812–16823. https://doi.org/10.1002/jcp.28350
Bauersachs R, Zeymer U, Briere JB et al (2019) Burden of coronary artery disease and peripheral artery disease: a literature review. Cardiovasc Ther 2019:8295054. https://doi.org/10.1155/2019/8295054
Matsuzawa Y, Lerman A (2014) Endothelial dysfunction and coronary artery disease: assessment, prognosis, and treatment. Coron Artery Dis 25(8):713–724. https://doi.org/10.1097/MCA.0000000000000178
Wong D, Turner AW, Miller CL (2019) Genetic insights into smooth muscle cell contributions to coronary artery disease. Arterioscler Thromb Vasc Biol 39(6):1006–1017. https://doi.org/10.1161/ATVBAHA.119.312141
Houston M (2018) The role of noninvasive cardiovascular testing, applied clinical nutrition and nutritional supplements in the prevention and treatment of coronary heart disease. Ther Adv Cardiovasc Dis 12(3):85–108. https://doi.org/10.1177/1753944717743920
Behnes M, Fastner C, Ansari U et al (2015) New oral anticoagulants in coronary artery disease. Cardiovasc Hematol Disord Drug Targets 15(2):101–105. https://doi.org/10.2174/1871529x1502151209111429
Leigh JA, Alvarez M, Rodriguez CJ (2016) Ethnic minorities and coronary heart disease: an update and future directions. Curr Atheroscler Rep 18(2):9. https://doi.org/10.1007/s11883-016-0559-4
Wirtz PH, von Kanel R (2017) Psychological stress, inflammation, and coronary heart disease. Curr Cardiol Rep 19(11):111. https://doi.org/10.1007/s11886-017-0919-x
De P, Aske JC, Dey N (2019) RAC1 takes the lead in solid tumors. Cells 8(5). https://doi.org/10.3390/cells8050382
Harris KP, Tepass U (2010) Cdc42 and vesicle trafficking in polarized cells. Traffic 11(10):1272–1279. https://doi.org/10.1111/j.1600-0854.2010.01102.x
Ito TK, Yokoyama M, Yoshida Y et al (2014) A crucial role for CDC42 in senescence-associated inflammation and atherosclerosis. PLoS One 9(7):e102186. https://doi.org/10.1371/journal.pone.0102186
Li J, Liu Y, Jin Y et al (2017) Essential role of Cdc42 in cardiomyocyte proliferation and cell-cell adhesion during heart development. Dev Biol 421(2):271–283. https://doi.org/10.1016/j.ydbio.2016.12.012
AlBadri A, Wei J, Quesada O et al (2020) Coronary vascular function and cardiomyocyte injury: a report from the WISE-CVD. Arterioscler Thromb Vasc Biol 40(12):3015–3021. https://doi.org/10.1161/ATVBAHA.120.314260
Tskvitaria-Fuller I, Seth A, Mistry N et al (2006) Specific patterns of Cdc42 activity are related to distinct elements of T cell polarization. J Immunol 177(3):1708–1720. https://doi.org/10.4049/jimmunol.177.3.1708
Guo F (2021) RhoA and Cdc42 in T cells: are they targetable for T cell-mediated inflammatory diseases? Precis Clin Med 4(1):56–61. https://doi.org/10.1093/pcmedi/pbaa039
Kalim KW, Yang JQ, Li Y et al (2018) Reciprocal regulation of glycolysis-driven Th17 pathogenicity and regulatory T cell stability by Cdc42. J Immunol 200(7):2313–2326. https://doi.org/10.4049/jimmunol.1601765
Herner M, Agasthi P (2021) Cardiac stress imaging, StatPearls, Treasure Island (FL)
Gensini GG (1983) A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol 51(3):606. https://doi.org/10.1016/s0002-9149(83)80105-2
Sun N, Ye L, Chang T et al (2014) microRNA-195-Cdc42 axis acts as a prognostic factor of esophageal squamous cell carcinoma. Int J Clin Exp Pathol 7(10):6871–6879
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods 25(4):402–408. https://doi.org/10.1006/meth.2001.1262
Mo XY, Li T, Hu ZP (2013) Decreased levels of cell-division cycle 42 (Cdc42) protein in peripheral lymphocytes from ischaemic stroke patients are associated with Golgi apparatus function. J Int Med Res 41(3):642–653. https://doi.org/10.1177/0300060513480093
Yang JQ, Kalim KW, Li Y et al (2019) Rational targeting Cdc42 restrains Th2 cell differentiation and prevents allergic airway inflammation. Clin Exp Allergy 49(1):92–107. https://doi.org/10.1111/cea.13293
Guo F, Zhang S, Tripathi P et al (2011) Distinct roles of Cdc42 in thymopoiesis and effector and memory T cell differentiation. PLoS One 6(3):e18002. https://doi.org/10.1371/journal.pone.0018002
Zhong L, Simard MJ, Huot J (2018) Endothelial microRNAs regulating the NF-kappaB pathway and cell adhesion molecules during inflammation. FASEB J 32(8):4070–4084. https://doi.org/10.1096/fj.201701536R
Dong LM, Chen XW, He XX et al (2019) Cell division cycle protein 42 regulates the inflammatory response in mice bearing inflammatory bowel disease. Artif Cells Nanomed Biotechnol 47(1):1833–1838. https://doi.org/10.1080/21691401.2019.1596936
Harjunpaa H, Llort Asens M, Guenther C et al (2019) Cell adhesion molecules and their roles and regulation in the immune and tumor microenvironment. Front Immunol 10:1078. https://doi.org/10.3389/fimmu.2019.01078
Bui TM, Wiesolek HL, Sumagin R (2020) ICAM-1: a master regulator of cellular responses in inflammation, injury resolution, and tumorigenesis. J Leukoc Biol 108(3):787–799. https://doi.org/10.1002/JLB.2MR0220-549R
Price DT, Loscalzo J (1999) Cellular adhesion molecules and atherogenesis. Am J Med 107(1):85–97. https://doi.org/10.1016/s0002-9343(99)00153-9
Lv J, Zeng J, Guo F et al (2018) Endothelial Cdc42 deficiency impairs endothelial regeneration and vascular repair after inflammatory vascular injury. Respir Res 19(1):27. https://doi.org/10.1186/s12931-018-0729-8
Li Q, Zhang Z, Du R et al (2012) Association analysis between endothelial function related factors and coronary artery stenosis degree in coronary heart disease patients with type 2 diabetes mellitus. J Pediatr Endocrinol Metab 25(7–8):711–716. https://doi.org/10.1515/jpem-2012-0159
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
The study was approved by the Institutional Review Board, and all written informed consent forms were obtained from participants.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhou, M., Wu, J. & Tan, G. The relation of circulating cell division cycle 42 expression with Th1, Th2, and Th17 cells, adhesion molecules, and biochemical indexes in coronary heart disease patients. Ir J Med Sci 191, 2085–2090 (2022). https://doi.org/10.1007/s11845-021-02836-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11845-021-02836-4