Abstract
Background
Analysing dietary patterns (DP) evaluates overall dietary intake, taking account of its complexity, quality, variance and the interaction between different foods, providing an alternative approach for the evaluation of nutritional influences on age-related macular degeneration (AMD) risk.
Aims
To evaluate the relationship between DP and AMD in an older female population.
Methods
Data was analysed from the cross-sectional Irish Nun Eye Study involving 1233 older women with a restricted lifestyle (mean age 76.3 years [range, 56–100 years). The Wisconsin Age-related Maculopathy Grading System was used to classify digital colour macular fundus images and dietary intake was assessed using a food frequency questionnaire (n = 1033). A posteriori DP were derived using principal component analysis. Logistic regression models examined associations between DP and AMD risk with adjustment for confounders.
Results
Two DP were identified: a ‘healthy’ pattern characterised by a high intake of oily fish, wholegrains, vegetables and fruit; and an ‘unhealthy’ pattern characterised by high-fat dairy products, sugar, sweets and chips. Of the participants included within the analysis, AMD status were categorised as controls (n = 818, 86.9%), early AMD (n = 83, 8.8%) and late AMD (n = 21, 2.2%). Regression analysis failed to identify any significant associations between healthy or unhealthy DP and AMD risk, in unadjusted and adjusted models.
Conclusion
No evidence of an association between the DP identified and AMD risk was detected in this well-characterised population. Further research is required to determine the overall dietary influences on AMD risk in general population cohorts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Age-related macular degeneration (AMD) is the leading cause of vision impairment within the developed world in people aged > 50 years and treatment options for slowing the progression of visual loss due to late AMD are limited [1]. Nutritional influences and dietary interventions have been shown to modulate AMD associated risk [2,3,4,5,6,7,8]. Previous dietary based analyses have largely focused on the evaluation of individual nutrients, foods or groups of foods to identify independent nutritional influences affecting AMD risk, although the associations observed, have not always been consistent [9,10,11,12,13]. The analysis of dietary patterns (DP) evaluates overall dietary intake, taking account of its complexity, quality, variance and the interaction between different foods, providing an alternative approach for the evaluation of nutritional influences on AMD risk [14, 15].
Some studies have outlined the potential nutritional or dietary influences on AMD risk [7, 16,17,18,19]. Data emerging from the Age-Related Eye Disease Study (AREDS), advocates a healthy diet with supplementation of zinc, lutein/zeaxanthin and vitamins C and E, as an effective strategy for reducing AMD progression from its early to visually-disabling late stage [16]. Another study by Chiu et al. also noted the potential benefits of omega-3 fatty acids for reducing AMD progression [20]. In relation to dietary carbohydrates, other studies have highlighted that the quality of carbohydrates consumed as measured by the glycaemic index, modify AMD risk [17, 20,21,22,23]. However, quantification of the glycaemic index of a specific food can be problematic, as it varies with respect to the other foods consumed at the same time [24]. Overall, this cumulative evidence showing associations with different foods and nutrients could suggest that the overall diet may be important given that the consumption of one food may affect the nutritional value and bioavailability of others, and the nutritional benefits may also be influenced by the individual’s health status or severity of AMD.
To our knowledge, only two studies have previously reported on the associations between a posteriori-derived DP and AMD. In the US-based AREDS study, Chiu and colleagues identified two major DP, labelled as ‘Oriental’ and ‘Western’, which were strongly associated with reduced and increased risk of AMD (both early and late) respectively, accounting for <20% of the total dietary variance assessed [14, 15]. An Australian study (the Melbourne Collaborative Cohort study [MCCS]) identified a significant association between ‘processed foods’ and a higher prevalence of late AMD [25]. To date, we are unaware of any study that has examined associations between DP and AMD risk in European populations. As such, the aim of this study was to evaluate a posteriori-derived DP of nutritional intake and associated risk of AMD in a well-characterised cohort of older Irish women with a restricted lifestyle.
Methods
Study population
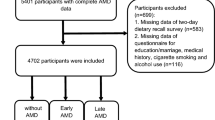
The study population was drawn from the Irish Nun Eye Study (INES), a cross-sectional study, in which DP were analysed in association with AMD prevalence. This unique population subscribes to a set of rules that governs behaviour in terms of material possessions, emotional and physical attachment, maintenance of a daily structured religious life of abstinence and prayer with dietary and lifestyle limitations and therefore represents a relatively homogenous population. A total of 1233 females were recruited between 2007 and 2009 from 123 convents across the island of Ireland; all participants were Irish, of white ethnicity, had been residing in a convent for 25 years or more and were older than 55 years. Prior to the commencement of research, ethical approval was obtained from the Office for Research Ethics Committee Northern Ireland and informed written consent was obtained from all participants. DP analysis was performed on 1033 participants who completed a food frequency questionnaire (FFQ) as part of the study and for whom retinal images were available. The study design and sampling procedures have been described previously [26, 27].
Age-related macular degeneration characterisation
Colour macular centred fundus images were graded independently by the Network of Ophthalmic Reading Centres UK (NetwORC UK). A standardised procedure adopted from definitions of the Wisconsin Age-related Maculopathy Grading System was used to classify features of early and late AMD [28] and the definitions were based on the International Classification for Age-related Macular Degeneration [29]. The presence of features within a 6000-μm circle centred on the fovea were recorded. Drusen were categorised according to size, characteristics of homogeneity of surface features and outline. Pigmentary changes were classified into two categories: hyperpigmentation and hypopigmentation. These features were used to assign each eye to a severity grade as follows: Controls: no features of AMD or the presence of soft distinct drusen (> 63 μm and ≤ 125 μm) or pigmentary abnormalities only. Cases were defined as early AMD: soft, indistinct (≥ 125 μm) or reticular drusen only or soft distinct drusen with pigmentary abnormalities; late AMD: either geographic atrophy (i.e. well-demarcated area of retinal pigment atrophy with visible choroidal vessels) or neovascular AMD (i.e. presence of any of the following: serous or hemorrhagic retinal or retinal pigment epithelial detachment, subretinal neovascular membrane, or periretinal fibrous scar).
Assessment of dietary intake
The semi-quantitative Scottish Collaborative Group FFQ was used to assess the dietary intake of each participant (http://www.foodfrequency.org/). This validated tool consisted of 19 sections comprised of 170 food items. Participants had to indicate the frequency of consumption of each food item during the previous 2–3 month period. Frequency was measured in terms of the number of standard portions per day/week/month/rarely. Reported food intakes were converted into a daily weight using food portion sizes [30].
Dietary pattern identification
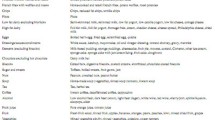
The individual food items within the FFQ were manually allocated to one of 38 food groups according to food type and macronutrient content (Table 1). Principal component analysis (PCA) with orthogonal rotation (varimax) was applied to this data to generate DP data and factor loadings for each individual food group. Examination of the scree plot was used to determine the number of factors to be retained. To aid data interpretation, food groups which had a factor loading > 0.2 were retained for describing the DP. The PCA produced factor scores for each participant which were categorised into quartiles for each DP with the fourth quartile indicating greatest conformity to the specific DP.
Demographic, lifestyle and anthropometric data
A structured questionnaire was used to assess alcohol and smoking status, disease status (presence or absence) and medication usage. Diastolic blood pressure (DBP) and systolic blood pressure (SBP) were measured in a resting seated position using an oscillometric blood pressure aneroid sphygmomanometer (Speider and Keller). Participant’s weight and height were measured and body mass index (BMI) computed as weight (kilograms)/height (metres) squared.
Statistical analysis
IBM SPSS version 21 (SPSS Inc., Chicago, IL, USA) was used for statistical analyses. For all analyses, statistical significance was considered P < 0.05. Descriptive statistics were obtained for all variables of interest. Categorical and continuous variables were summarised as n (%) and mean (SD), respectively. One-way analysis of variance (ANOVA) was used to assess the differences between the quartiles for continuous variables. Chi square tests were used to investigate the differences between quartiles for categorical variables. Post-hoc pairwise comparisons were performed using Mann-Whitney U tests between quartiles to determine the location of any differences (data not shown).
Logistic regression was used to explore the relationship between the DP identified (with each DP distributed into equal quartiles) and AMD status (controls versus AMD (i.e. early or late AMD)) in both unadjusted (model 1) and adjusted analyses (models 2 and 3). The reference category was the lowest quartile for each DP, indicating lowest conformity to the pattern. Within this analysis, the confounding factors were those identified from similar previously published studies and those commonly recognised to influence early or late AMD [14, 15, 25]. Model 1 was unadjusted; Model 2 was adjusted for age and BMI; Model 3 was adjusted for model 2 plus smoking, alcohol, hypertension, diabetes mellitus (DM), ischaemic heart disease (IHD) and cerebrovascular accident (CVA).
Results
The mean age of participants was 76.3 years (standard deviation [SD] 8.0; Table 2). Based on BMI classifications, 45% (n = 412) of participants were of normal weight, 12% (n = 111) were underweight, 28% (n = 258) were overweight and 15% (n = 138) were classified as clinically obese. Of the participants included within the analysis, AMD status was categorised as controls (n = 818, 86.9%), early AMD (n = 83, 8.8%) and late AMD (n = 21, 2.2%). Images from 19 participants (2%) were of insufficient quality for AMD phenotyping. The majority of participants were non-consumers of alcohol (n = 864, 92%), and had never smoked, (n = 905, 96%). Approximately 3% of participants (n = 30) had self-reported CVA, 11% had IHD (n = 107), 3% had DM (n = 31) and hypertension was reported in 41% of participants (n = 390).
PCA identified two major DP labelled ‘healthy’ and ‘unhealthy’. The factor loadings for both DP are presented in Table 3. The ‘healthy’ DP was characterised (in decreasing order of factor loadings) by lutein/zeaxanthin-rich vegetables, green leafy vegetables, alliums, vegetables, fruit, tomatoes, legumes, nuts, oily fish, low-fat dairy products, pizza, dressings/sauces/condiments, wholegrain breakfast cereal and red meat. The ‘unhealthy’ DP was characterised (in decreasing order of factor loadings) by chips, French fries, alcohol, high fat dairy products, soups, desserts, sugars and sweets, wholegrains, dressings/sauces/condiments, processed meat, potatoes, eggs, refined grains, refined breakfast cereal, chocolate, vegetables, red meat, white fish and shellfish. Together, the DP characterised accounted for approximately 16% of the total dietary variance within the population.
No significant differences in participant characteristics were observed across quartiles within the ‘healthy’ DP, with the exception of age (p < 0.001) and BMI (p = 0.05); females in the lowest quartile tended to be older and had a higher BMI compared to those in the other quartiles. Participants in the lowest quartile of the ‘unhealthy’ DP also tended to be older (p = 0.01) while those in the highest quartile were more likely to consume alcohol (p < 0.001) and smoke (p = 0.05) (data not shown).
There was no significant difference in AMD risk (i.e. controls versus AMD) across quartiles of either the ‘healthy’ or ‘unhealthy’ DP, in both the unadjusted and adjusted analyses (Table 4). Participants who adhered closely to an ‘unhealthy’ DP had a greater risk of AMD compared to those in the other quartiles, although this failed to reach significance. In the unadjusted analysis (model 1) the odds ratio for AMD in the highest fourth of the ‘healthy’ DP (i.e. most healthy) compared to the lowest fourth (i.e. least healthy) was 0.86 (95% CI 0.48–1.55; P = 0.62), while the odds ratio for AMD in the highest fourth of the ‘unhealthy’ DP (i.e. most unhealthy) compared to the lowest fourth (i.e. least unhealthy) was 1.50 (95% CI 0.82–2.75; P = 0.19). Model 2 was adjusted for age and BMI (‘healthy’ OR = 1.51 (95% CI 0.80–2.86; P = 0.20), ‘unhealthy’ OR = 1.34 (95% CI 0.71–2.54; P = 0.37). Model 3 was adjusted for model 2 covariates plus smoking and alcohol status, hypertension, DM, IHD and CVA (‘healthy’ OR = 1.50 (95% CI 0.79–2.86; P = 0.21), ‘unhealthy’ OR = 1.45 (95% CI 0.76–2.78; P = 0.26)).
Discussion
To our knowledge, this is the first study to examine associations between a posteriori-derived DP and AMD risk in a European population. Two major DP were identified using PCA and labelled as ‘healthy’ and ‘unhealthy’ with no evidence of association with AMD risk in this older population with a restricted lifestyle. Previous evidence of the association between DP and AMD risk is limited and somewhat conflicting [14, 15]. The MCCS found no association between DP and early AMD risk. However, they noted that a ‘grains and fish’ DP, characterised by frequent consumption of boiled rice, muesli, fish (not fried), chicken (not fried), vegetables, and avoidance of white bread was associated with 51% reduced odds of late AMD while a diet characterised by red and processed meats and fried foods was significantly associated with a higher prevalence of late AMD across quartiles of adherence [25]. In contrast to our findings, results from the US-based AREDS study by Chiu et al. identified strong associations between an ‘Oriental’ and ‘Western’ DP with decreased and increased risk for both early and late AMD, respectively [14]. However, comparing DP across the various studies is challenging given the complexity of the composition of the DP and the geographical variations in dietary intake. In addition, potential confounding from known or unknown variables such as genetic data (effect modification due to genotype) or variation in the classification of AMD definitions could limit detectable associations with dietary outcomes [10].
Other studies have considered various combinations of single foods and nutrients and AMD risk [11, 31]. A retrospective case-control study examining diet quality used the Alternate Healthy Eating Index (AHEI) to evaluate scores for the intake of five different food groups (grains, fruits, vegetables, meat and milk) and both total and saturated fat intake, identifying participants in the highest quartile of dietary quality with lower risk of late AMD (OR, 0.54; 95% CI, 0.30–0.99) compared to those in the lowest quartile. However, no association was found using a HEI score [31]. The Carotenoids in Age-Related Eye Disease Study (CAREDS) used FFQ data from 1313 female participants aged 55 to 74 years to generate a modified HEI (mHEI) which considered intake of whole grains, vegetables, fruits, meat, beans, eggs, fish and milk, total fat, saturated fat, sugar and alcohol [11, 32]. Their results showed a 46% lower odds for early AMD in the highest mHEI quintile compared to the lowest quintile (OR, 0.54; 95% CI, 0.33–0.88) [11]. Merle and colleagues assessed associations between progression to late AMD and adherence to a Mediterranean diet in 2525 AREDS participants [33]. An alternate Mediterranean diet (aMeDi) score was used to evaluate the intake of nine food groups (whole grains, fruit, vegetables, legumes, nuts, red and processed meats, fish, alcohol) as well as a mono-unsaturated to saturated fats ratio. A 26% lower risk of progression to late AMD was significantly associated with an elevated aMeDi score after adjusting for age, sex, AMD grade, total energy intake, AREDS treatment, smoking, BMI, educational level, supplement use and 10 genetic variants (hazard ratio [HR], 0.74; 95% CI, 0.61–0.91; P-trend = 0.007) [33].
This study had a number of key strengths: the standardised collection of data including potential confounding factors, the photographic grading of AMD from high-resolution digital macula-centred images and the use of phenotypic data from both eyes of each participant for analysis. AMD classification was undertaken in an independent retinal grading centre without any knowledge of the associated nutritional data. The use of DP to evaluate a broader overview of dietary intake as opposed to the assessment of individual nutrients or foods alone provides a more comprehensive understanding of the synergistic interactions between food consumption and disease risk and reduces the influences of residual confounding. The use of an a posteriori approach for DP analysis is also considered to be an approach that is free from the constraints of a priori hypotheses [34, 35].
Limitations of this study include the cross-sectional design which prevents determination of causal influences associated with dietary intake. An ‘unhealthy’ DP characterised by a range of ‘unhealthy’ foods may be associated with increased risk of AMD, however due to the low prevalence of AMD in this population (n = 83 for early AMD and n = 21 for late AMD), and the fact that this cohort were reasonably healthy given their age (96% non-smokers, 91% non-drinkers and 45% with a normal BMI) and consuming a restricted diet, statistical power to detect an association with AMD may have been limited. Although, we evaluated dietary intake as a whole and the analysis included known non-dietary confounders, it is possible that residual confounding still represents a concern. However, the homogeneous lifestyle of this cohort is likely to have limited residual confounding. There are also limitations associated with the assessment of dietary intake. Although FFQs are commonly used to infer DP and dietary intake in epidemiological studies, they are nonetheless prone to inherent error and are limited in their ability to assess all dietary components. Although 170 foods were considered within the FFQ, additional foods consumed may have been missed. There are also drawbacks of DP analysis including consolidation of the food items into groups, choosing the number of factors to be retained, selecting the method of rotation and naming the factors identified. As this was a cross-sectional study, AMD status and dietary intake were only assessed at a single time point and therefore we were unable to account for temporal changes over a period of time. However, other studies have used comparable methods and highlighted the reproducibility of DP over time [36, 37].
In conclusion, this study showed no evidence of an association between DP and AMD risk in an ageing and well-characterised cohort with a restricted lifestyle. Nevertheless, increasing evidence suggests unhealthier food choices may contribute to increased AMD risk [3, 11, 31]. Critically, further longitudinal assessment and intervention studies are necessary to evaluate dietary intake of specific foods and DP as a risk factor for AMD, particularly in older adults with late AMD.
References
Miller JW (2013) Age-related macular degeneration revisited–piecing the puzzle: the LXIX Edward Jackson memorial lecture. Am J Ophthalmol 155:1–35
Seddon JM, George S, Rosner B (2006) Cigarette smoking, fish consumption, omega-3 fatty acid intake, and associations with age-related macular degeneration: the US twin study of age-related macular degeneration. Arch Ophthalmol 124:995–1001
Chong EW, Simpson JA, Robman LD et al (2009) Red meat and chicken consumption and its association with age-related macular degeneration. Am J Epidemiol 169:867–876
Adams MK, Chong EW, Williamson E et al (2012) 20/20—alcohol and age-related macular degeneration: the Melbourne Collaborative Cohort Study. Am J Epidemiol 176:289–298
Chong EW, Robman LD, Simpson JA et al (2009) Fat consumption and its association with age-related macular degeneration. Arch Ophthalmol 127:674–680
Chong EW, Wong TY, Kreis AJ, Simpson JA, Guymer RH (2007) Dietary antioxidants and primary prevention of age related macular degeneration: systematic review and meta-analysis. BMJ 335:755
The Age-Related Eye Disease Study 2 (AREDS2) Research Group (2013) Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trials. JAMA 309:2005–2015
Seddon JM, Rosner B, Sperduto RD et al (2001) Dietary fat and risk for advanced age-related macular degeneration. Arch Ophthalmol 119:1191–1199
Smith W, Mitchell P, Leeder SR (2000) Dietary fat and fish intake and age-related maculopathy. Arch Ophthalmol 118:401–404
Wang JJ, Rochtchina E, Smith W et al (2009) Combined effects of complement factor H genotypes, fish consumption, and inflammatory markers on long-term risk for age-related macular degeneration in a cohort. Am J Epidemiol 169:633–641
Mares JA, Voland RP, Sondel SA et al (2011) Healthy lifestyles related to subsequent prevalence of age-related macular degeneration. Arch Ophthalmol 129:470–480
San Giovanni JP, Chew EY, Clemons TE et al (2007) The relationship of dietary carotenoid and vitamin A, E, and C intake with age-related macular degeneration in a case-control study: AREDS report no. 22. Arch Ophthalmol 125:1225–1232
Tan JS, Wang JJ, Flood V, Mitchell P (2009) Dietary fatty acids and the 10-year incidence of age-related macular degeneration: the Blue Mountains Eye Study. Arch Ophthalmol 127:656–665
Chiu CJ, Chang ML, Li T, Gensler G, Taylor A (2017) Visualization of dietary patterns and their associations with age-related macular degeneration. Invest Ophthalmol Vis Sci 58:1404–1410
Chiu CJ, Chang ML, Zhang FF, Li T, Gensler G, Schleicher M, Taylor A (2014) The relationship of major American dietary patterns to age-related macular degeneration. Am J Ophthalmol 158:118–127
Age-Related Eye Disease Study Research Group (2001) A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol 119:1417–1436
Chiu CJ, Milton RC, Klein R, Gensler G, Taylor A (2009) Dietary compound score and risk of age-related macular degeneration in the age-related eye disease study. Ophthalmology 116:939–946
Stevens R, Barlett H, Cooke R (2015) Dietary analysis and nutritional behaviour in people with and without age-related macular disease. Clin Nutr ESPEN 10(3):e112–e117
Braakhuis A, Raman R, Vaghefi E (2017) The association between dietary intake of antioxidants and ocular disease. Diseases 5:3
Chiu CJ, Klein R, Milton RC, Gensler G, Taylor A (2009) Does eating particular diets alter the risk of age-related macular degeneration in users of the age-related eye disease study supplements? Br J Ophthalmol 93:1241–1246
Chiu CJ, Hubbard LD, Armstrong J, Rogers G, Jacques PF, Chylack LT Jr, Hankinson SE, Willett WC, Taylor A (2006) Dietary glycemic index and carbohydrate in relation to early age-related macular degeneration. Am J Clin Nutr 83:880–886
Chiu CJ, Milton RC, Gensler G, Taylor A (2007) Association between dietary glycemic index and age-related macular degeneration in nondiabetic participants in the age-related eye disease study. Am J Clin Nutr 86:180–188
Chiu CJ, Milton RC, Klein R, Gensler G, Taylor A (2007) Dietary carbohydrate and progression of age-related macular degeneration, a prospective study from the age-related eye disease study. Am J Clin Nutr 86:1210–1218
Moghaddam E, Vogt JA, Wolever TM (2006) The effects of fat and protein on glycemic responses in nondiabetic humans vary with waist circumference, fasting plasma insulin, and dietary fiber intake. J Nutr 136:2506–2511
Amirul Islam FM, Chong EW, Hodge AM, Guymer RH, Aung KZ, Makeyeva GA, Baird PN, Hopper JL, English DR, Giles GG, Robman LD (2014) Dietary patterns and their associations with age-related macular degeneration: the Melbourne collaborative cohort study. Ophthalmology 121:1428–1434
McGowan A, Silvestri G, Moore E et al (2014) Retinal vascular caliber, iris color and age-related macular degeneration in the Irish Nun Eye Study. Invest Ophthalmol Vis Sci 56:382–387
McGowan A, Silvestri G, Moore E, Silvestri V, Patterson CC, Maxwell AP, McKay GJ (2015) Evaluation of the retinal vasculature in hypertension and chronic kidney disease in an elderly population of Irish Nuns. PLoS One 10:e0136434
Klein R, Davis MD, Magli YL, Segal P, Klein BEK, Hubbard L (1991) The Wisconsin age-related maculopathy grading system. Ophthalmology 98:1128–1134
Bird AC, Bressler NM, Bressler SB et al (1995) An international classification and grading system for age-related maculopathy and age-related macular degeneration. The International ARM Epidemiological Study Group. Surveys of Ophthalmology 39:367–374
Food Standards Agency (2006) Food portion sizes, 3rd edn. TSO, London
Montgomery MP, Kamel F, Pericak-Vance MA, Haines JL, Postel EA, Agarwal A, Richards M, Scott WK, Schmidt S (2010) Overall diet quality and age-related macular degeneration. Ophthalmic Epidemiol 17:58–65
Guenther PM, Reedy J, Krebs-Smith SM, Reeve BB, Basiotis PP (2008) Development of the healthy eating index-2005. J Am Diet Assoc 108:1896–1901
Merle BM, Silver RE, Rosner B, Seddon JM (2015) Adherence to a Mediterranean diet, genetic susceptibility, and progression to advanced macular degeneration: a prospective cohort study. Am J Clin Nutr 102:1196–1206
Kastorini CM, Papadakis G, Milionis HJ, Kalantzi K, Puddu PE, Nikolaou V, Vemmos KN, Goudevenos JA, Panagiotakos DB (2013) Comparative analysis of a-priori and a-posteriori dietary patterns using state-of-the-art classification algorithms: a case/case-control study. Artif Intell Med 59:175–183
Neville CE, Montgomery S, Silvestri G et al (2017) Dietary patterns and retinal vessel caliber in the Irish Nun Eye Study. J Nutr Health Aging:1–8
Hu FB, Rimm E, Smith-Warner SA, Feskanich D, Stampfer MJ, Ascherio A, Sampson L, Willett WC (1999) Reproducibility and validity of dietary patterns assessed with a food-frequency questionnaire. Am J Clin Nutr 69:243–249
Hu FB, Rimm EB, Stampfer MJ, Ascherio A, Spiegelman D, Willett WC (2000) Prospective study of major dietary patterns and risk of coronary heart disease in men. Am J Clin Nutr 72:912–921
Acknowledgements
The authors thank all study participants and also Janet Kyle for provision of the food frequency questionnaire and providing advice regarding the set up and design of the study. The project was funded by the Northern Ireland Health and Social Care Research and Development Office (grant number RRG project 4.41), and the Department for Education and Learning, Northern Ireland. The funding organisations had no role in the design or conduct of this research.
Funding
G. Silvestri reports grants from Northern Ireland Health and Personal Social Services Research and Development Office during this study, personal fees from Bayer and non-financial support from Allergan outside the submitted work. G. McKay reports personal fees from Boehringer Ingelheim.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
McCarter, R.V., Neville, C.E., Silvestri, G. et al. Dietary patterns were not associated with age-related macular degeneration: a cross-sectional analysis in the Irish Nun Eye Study. Ir J Med Sci 188, 1005–1012 (2019). https://doi.org/10.1007/s11845-018-1932-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11845-018-1932-9