Abstract
Background
Candidaemia is an important nosocomial infection, seen frequently in immunocompromised and critically ill patients and increasingly recognised in cystic fibrosis (CF) patients with totally implantable venous access devices (TIVADs). This study aims to investigate the incidence and risk factors for the development of TIVAD-associated candidaemia and to assess the rate of TIVAD-related complications in CF patients.
Methods
A 10-year retrospective study was carried out on adult CF patients attending a single centre. Complications were recorded including the incidence of candidaemia and correlated to clinical parameters. Complication rates were calculated based on incidence per 1000 catheter days. Statistical analysis was performed using Mann-Whitney U test and Fisher’s exact test.
Results
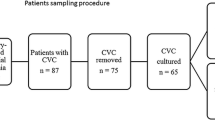
Fourteen cases of candidaemia were observed in the CF cohort, primarily caused by Candida parapsilosis and Candida albicans. Candidaemia was associated with lower FEV1 (p = 0.0117) and higher frequency of pulmonary exacerbation (p < 0.0001). A TIVAD complication rate of 0.337/1000 catheter days was observed in the CF cohort. Complications included venous thrombosis, stenosis, and port extrusion; complications were independently associated with more frequent pulmonary exacerbations (p = 0.04).
Conclusions
TIVAD complications are observed more commonly in those with lower FEV1 and frequent pulmonary exacerbations, suggesting that candidaemia may be related to antibiotic use and furthermore can occur following invasive procedures causing translocation of fungal species allowing transformation from colonisation to pathogenic infection.



Similar content being viewed by others
References
Amadori A, Antonelli A, Balteri I et al (2009) Recurrent exacerbations affect FEV(1) decline in adult patients with cystic fibrosis. Respir Med 103(3):407–413
Au L, Guduru K, Lipscomb G et al (2007) Candida endophthalmitis: a critical diagnosis in the critically ill. Clin Ophthalmol 1(4):551–554
Baddley JW, Benjamin DK Jr, Patel M et al (2008) Candida infective endocarditis. Eur J Clin Microbiol Infect Dis 27(7):519–529
Chotirmall SH, O'Donoghue E, Bennett K et al (2010) Sputum Candida albicans presages FEV(1) decline and hospital-treated exacerbations in cystic fibrosis. Chest 138(5):1186–1195
Deerojanawong J, Sawyer SM, Fink AM et al (1998) Totally implantable venous access devices in children with cystic fibrosis: incidence and type of complications. Thorax 53(4):285–289
di Costanzo J, Sastre B, Choux R et al (1988) Mechanism of thrombogenesis during total parenteral nutrition: role of catheter composition. JPEN J Parenter Enteral Nutr 12(2):190–194
Dimick JB, Pelz RK, Consunji R et al (2001) Increased resource use associated with catheter-related bloodstream infection in the surgical intensive care unit. Arch Surg 136(2):229–234
Hassan T, Chotirmall SH, Low TB et al (2010) Thrombolysis for indwelling catheter related thrombosis and superior vena cava obstruction in cystic fibrosis: a case series. Ir J Med Sci 179(3):469–470
McCarthy C, Dimitrov BD, Meurling IJ et al (2013) The CF-ABLE score: a novel clinical prediction rule for prognosis in patients with cystic fibrosis. Chest 143(5):1358–1364
Mermel LA (2000) Prevention of intravascular catheter-related infections. Ann Intern Med 132(5):391–402
Pappas PG, Kauffman CA, Andes DR et al (2016) Executive Summary: Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis 62(4):409–417
Ramsey BW (1996) Management of pulmonary disease in patients with cystic fibrosis. N Engl J Med 335(3):179–188
Royle TJ, Davies RE, Gannon MX (2008) Totally implantable venous access devices—20 years’ experience of implantation in cystic fibrosis patients. Ann R Coll Surg Engl 90(8):679–684
Sanders DB, Bittner RC, Rosenfeld M et al (2011) Pulmonary exacerbations are associated with subsequent FEV1 decline in both adults and children with cystic fibrosis. Pediatr Pulmonol 46(4):393–400
Stenbit AE, Flume PA (2011) Pulmonary exacerbations in cystic fibrosis. Curr Opin Pulm Med 17(6):442–447
Warren DK, Quadir WW, Hollenbeak CS et al (2006) Attributable cost of catheter-associated bloodstream infections among intensive care patients in a nonteaching hospital. Crit Care Med 34(8):2084–2089
Wenzel RP (1995) Nosocomial candidemia: risk factors and attributable mortality. Clin Infect Dis 20(6):1531–1534
Yapar N (2014) Epidemiology and risk factors for invasive candidiasis. Ther Clin Risk Manag 10:95–105
Authors’ contributions
CMcC, OO’C, CG and NGMcE designed the study. The statistical analysis was carried out by CMcC, OO’C and MEO’B. The data collection was conducted by CMcC, OO’C, TMcE and AF. The manuscript was written by CMcC, OO’C, MEO’B, TMcE, AF, CG and NGMcE. CMcC, CG and NGMcE take responsibility for the integrity of the work as a whole, from the inception to the published article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
McCarthy, C., O’Carroll, O., O’Brien, M.E. et al. Risk factors for totally implantable venous access device-associated complications in cystic fibrosis. Ir J Med Sci 187, 429–434 (2018). https://doi.org/10.1007/s11845-017-1672-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11845-017-1672-2