Abstract
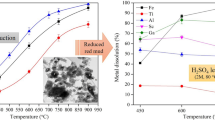
Red mud is an alkaline solid residue produced from the extraction of alumina from bauxite. However, the remaining alumina in red mud will cause secondary loss of aluminum resources. In the Bayer process, high alkalinity leads to severe environmental pollution issues and safety hazards. In this study, alkali and alumina were simultaneously recovered by using NaFeO2 (NF) as an additive in a hydrothermal process. Harmless secondary red mud with low Na2O content was obtained, avoiding the alkalinity pollution compared with conventional alumina extraction process. The influence of hydrothermal conditions on the extraction rate of alkali and alumina in red mud was also systematically studied. The chemical composition and microstructure of red mud before and after the reaction were studied using various characterization techniques such as XRF, XRD, and SEM. The results show that the A/S (molar ratio of Al2O3 to SiO2) in the secondary red mud is reduced to 0.21, the Na2O content is 0.43%, and the recovery rate of alumina is 80% with NF as additive. The product is hydroandradite (Ca3(FexAl1−x)2(SiO4)y(OH)9−y) with less aluminum content and hematite. This work will provide a theoretical basis for achieving secondary resource recovery and handling the environmental pollution issues.









Similar content being viewed by others
References
M.A. Khairul, J. Zanganeh, and B. Moghtaderi, Resour. Conserv. Recycl. 141, 483 https://doi.org/10.1016/j.resconrec.2018.11.006 (2019).
Y. Zhao, J. Wang, Z.K. Luan, X.J. Peng, Z. Liang, and L. Shi, J. Hazard. Mater. 165, 1193 https://doi.org/10.1016/j.jhazmat.2008.10.114 (2009).
N. Deihimi, M. Irannajad, and B. Rezai, J. Environ. Manag. 206, 266 https://doi.org/10.1016/j.jenvman.2017.10.037 (2018).
C.R. Borra, Y. Pontikes, K. Binnemans, and T.V. Gerven, Miner. Eng. 76, 20 https://doi.org/10.1016/j.mineng.2015.01.005 (2015).
X.Y. Liu, K.L. Feilberg, W. Yan, E.H. Stenby, and E. Thormann, J. Dispers. Sci. Technol. 40, 1611 https://doi.org/10.1080/01932691.2018.1523012 (2019).
X. Liu, P. Gao, S. Yuan, Y. Lv, and Y.X. Han, Miner. Eng. 157, 106553 https://doi.org/10.1016/j.mineng.2020.106553 (2020).
M. Anirudh, K.S. Rekha, C. Venkatesh, and R. Nerella, Mater. Today Proc. 43, 1587 https://doi.org/10.1016/j.matpr.2020.09.504 (2021).
M. Gautam, B. Pandey, and M. Agrawal, Ecol. Ind. 88, 88 https://doi.org/10.1016/j.ecolind.2017.12.062 (2018).
S.G. Xue, Z. Liu, J.R. Fan, R. Xue, Y. Guo, W. Chen, W. Hartley, and F. Zhu, Environ. Pollut. 292, 118326 https://doi.org/10.1016/j.envpol.2021.118326 (2022).
S. Song, N. Zhang, J.B. Yuan, and Y.H. Zhang, J. Build. Eng. 43, 103222 https://doi.org/10.1016/j.jobe.2021.103222 (2021).
T. Hertel, A. Van Den Bulck, S. Onisei, P.P. Sivakumar, and Y. Pontikes, Cem. Concr. Res. 145, 106463 https://doi.org/10.1016/j.cemconres.2021.106463 (2021).
N. Ye, J.K. Yang, X.Y. Ke, J. Zhu, Y.L. Li, C. Xiang, H.B. Wang, L. Li, and B. Xiao, J. Am. Ceram. Soc. 97, 1652 https://doi.org/10.1111/jace.12840 (2014).
J. Carneiro, D.M. Tobaldi, M.N. Capela, R.M. Navais, M.P. Seabra, and J.A. Labrincha, Waste Manag. 80, 371 https://doi.org/10.1016/j.wasman.2018.09.032 (2018).
T.Y. Liu, X.Y. Li, L.M. Guan, P. Liu, T. Wu, Z. Li, and A.X. Lu, Ceram. Int. 42, 1733 https://doi.org/10.1016/j.ceramint.2015.09.131 (2016).
K. Kaya and S. Soyer-Uzun, Ceram. Int. 42, 7406 https://doi.org/10.1016/j.ceramint.2016.01.144 (2016).
G.T. Wei, L.H. Shao, J.H. Mo, Z.M. Li, and L.Y. Zhang, Environ. Sci. Pollut. Res. 24, 15067 https://doi.org/10.1007/s11356-017-9126-y (2017).
Y.J. Liu and R. Naidu, Waste Manag. 34, 2662 https://doi.org/10.1016/j.wasman.2014.09.003 (2014).
Z.B. Liu and H.X. Li, Hydrometallurgy 155, 29 https://doi.org/10.1016/j.hydromet.2015.03.018 (2015).
S. Rai, M.T. Nimje, M.J. Chaddha, S. Modak, K.R. Rao, and A. Agnihotri, Miner. Eng. 134, 222 https://doi.org/10.1016/j.mineng.2019.02.018 (2019).
X.B. Zhu, W. Li, and X.M. Guan, Trans. Nonferrous Met. Soc. China 25, 3139 https://doi.org/10.1016/S1003-6326(15)63944-9 (2015).
B. Xue, B.T. Wei, L.X. Ruan, F.F. Li, Y.S. Jiang, W.J. Tian, B. Su, and L.M. Zhou, Hydrometallurgy 186, 91 https://doi.org/10.1016/j.hydromet.2019.04.005 (2019).
D.R. Zhang, H.R. Chen, Z.Y. Nie, J.L. Xia, E.P. Li, X.L. Fan, and L. Zheng, Chem. Eng. J. 401, 125914 https://doi.org/10.1016/j.cej.2020.125914 (2020).
X.B. Zhu, W. Li, and X.M. Guan, J. Hazard. Mater. 286, 85 https://doi.org/10.1016/j.jhazmat.2014.12.048 (2015).
H. Peng, T. Kim, and J. Vaughan, Ind. Eng. Chem. Res. 59, 8174 https://doi.org/10.1021/acs.iecr.0c00423 (2020).
L.W. Cheng, Y.L.B. Wang, T.G. Qi, G.H. Hua, Q.S. Zhou, Z.H. Peng, and X.B. Li, J. Sustain. Metall. 8, 541 https://doi.org/10.1007/s40831-022-00515-x (2022).
X.L. Pan, H.F. Wu, J.L. Liu, Q.W. Liu, and H.Y. Yu, Hydrometallurgy 203, 105695 https://doi.org/10.1016/j.hydromet.2021.105695 (2021).
X.J. Wang, Y.Y. Wang, H. Jin, J.D. Li, and X.M. Wang, Bull. Environ. Contam. Toxicol. 109, 186 https://doi.org/10.1007/s00128-022-03492-9 (2022).
B.G. Xu and P. Smith, Hydrometallurgy 129, 26 https://doi.org/10.1016/j.hydromet.2012.08.013 (2012).
R. Zhang, S.L. Zheng, S.H. Ma, and Y. Zhang, J. Hazard. Mater. 189, 827 https://doi.org/10.1016/j.jhazmat.2011.03.004 (2011).
R.B. Li, X.L. Li, D.X. Wang, Y. Liu, and T.A. Zhang, Powder Technol. 333, 277 https://doi.org/10.1016/j.powtec.2018.04.031 (2018).
R.B. Li, T.A. Zhang, Y. Liu, G.Z. Lv, and L.Q. Xie, J. Hazard. Mater. 316, 94 https://doi.org/10.1016/j.jhazmat.2016.04.072 (2016).
B. Whittington and T. Fallows, Hydrometallurgy 45, 289 https://doi.org/10.1016/S0304-386X(96)00085-0 (1997).
J.M. Rivas-Mercury, P. Pena, A.H. de Aza, X. Turrillas, I. Sobrados, and J. Sanz, Acta Mater. 55, 1183 https://doi.org/10.1016/j.actamat.2006.09.032 (2007).
D.E. Giles, I.M. Ritchie, and B.A. Xu, Hydrometallurgy 32, 119 https://doi.org/10.1016/0304-386X(93)90061-H (1993).
R.B. Li, T.A. Zhang, Y. Liu, and S.B. Kuang, Metall. Mater. Trans. B 48, 1123 https://doi.org/10.1007/s11663-016-0911-7 (2017).
Acknowledgements
This work is supported by the Liaoning Province Applied Basic Research Program Project 2023JH2/101300245, the National Natural Science Foundation of China (grant no. 51974188) and the Liaoning Revitalization Talents Program (no. XLYC2008014).
Funding
Liaoning Province Applied Basic Research Program Project (2023JH2/101300245), Liaoning Revitalization Talents Program (No. XLYC2008014), National Natural Science Foundation of China, Grant (No. 51974188).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shao, J., Li, L., Wu, Y. et al. Recovery of Alumina and Alkali from Red Mud Using NaFeO2 (NF) as an Additive in the Hydrothermal Process. JOM 76, 1420–1428 (2024). https://doi.org/10.1007/s11837-023-06286-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-023-06286-4