Abstract
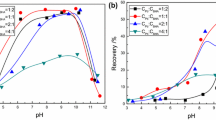
In this study, selective adsorption mechanism of zinc ions on the surfaces of galena and sphalerite in the flotation separation of Pb-Zn was comprehensively explored by flotation tests, Zeta potential measurements, Fourier transform interferometric radiometer (FTIR) spectroscopy, X-ray photoelectron spectroscopy (XPS) analysis, and density functional theory (DFT) calculations. Microflotation test showed that the use of ZnSO4 in alkaline pulp could selectively separate galena from sphalerite better than that without ZnSO4. Zeta potential test results showed that ZnSO4 could significantly increase the Zeta potential of sphalerite under alkaline conditions, but had little effect on galena. The results of FTIR test showed that xanthan characteristic peak appeared in xanthate-treated galena at pH = 10, while sphalerite treated with xanthate did not. XPS and DFT calculation results showed that sphalerite had stronger adsorption capacity for hydroxyl ions than galena. DFT calculation further confirmed that Zn(OH)2 could be adsorbed on the surface of hydroxylated sphalerite instead of hydroxylated galena and formed a new interface microstructure of sphalerite (Znsurf-O-Zn-O-H), resulting in the inhibition of sphalerite. This paper further deepened the selective adsorption mechanism of zinc ions on the surface of sphalerite in the flotation separation of Pb-Zn under alkaline conditions.










Similar content being viewed by others
Change history
18 December 2023
A Correction to this paper has been published: https://doi.org/10.1007/s11837-023-06313-4
References
M.Y. Jung, S.H. Oh, and J.W. Moon, Correlation between natural floatability and static contact angle of sulfide minerals, (2014).
H. Wang, S. Wen, G. Han, L. Xu, and Q. Feng, Activation mechanism of lead ions in the flotation of sphalerite depressed with zinc sulfate. Miner. Eng. 146, 106132 (2020).
C. Sui, D. Lee, A. Casuge, and J. Finch, Comparison of the activation of sphalerite by copper and lead. Min. Metall. Explorat. 16, 53–61 (1999).
A.R. Gerson, A.G. Lange, K.E. Prince, and R.S.C. Smart, The mechanism of copper activation of sphalerite. Appl. Surf. Sci. 137, 207–223 (1999).
G. Siva Reddy and C. Konda Reddy, The chemistry of activation of sphalerite—A review. Min. Process. Extract. Metall. Rev. 4, 1–38 (1988).
C. Xue and Z. Wei, Reaction mechanism and research progress of depressants in sphalerite flotation, Multipurp. Utilizat. Min. Resour., 38-43 (2017).
T. Qiu, Q. Nie, Y. He, and Q. Yuan, Density functional theory study of cyanide adsorption on the sphalerite (1 1 0) surface. Appl. Surf. Sci. 465, 678–685 (2019).
B. Guo, Y. Peng, and R. Espinosa-Gomez, Cyanide chemistry and its effect on mineral flotation. Miner. Eng. 66, 25–32 (2014).
S. Malghan, Role of sodium sulfide in the flotation of oxidized copper, lead, and zinc ores. Min. Metall. Explorat. 3, 158–163 (1986).
V. Bocharov, V. Ignatkina, and A. Kayumov, Rational separation of complex copper–zinc concentrates of sulfide ore. J. Min. Sci. 52, 793–801 (2016).
E.E. Öz, Evaluation of Kosovo-Artana concentrator tailings, In, Middle East Technical University, (2011).
L. Zhang, J. Gao, S.A. Khoso, L. Wang, Y. Liu, P. Ge, M. Tian, and W. Sun, A reagent scheme for galena/sphalerite flotation separation: insights from first-principles calculations. Miner. Eng. 167, 106885 (2021).
T. Khmeleva, J. Chapelet, W. Skinner, and D. Beattie, Depression mechanisms of sodium bisulphite in the xanthate-induced flotation of copper activated sphalerite. Int. J. Miner. Process. 79, 61–75 (2006).
Y. Mu, Y. Peng, and R.A. Lauten, The depression of pyrite in selective flotation by different reagent systems—a Literature review. Miner. Eng. 96–97, 143–156 (2016).
T. Pak, T.-C. Sun, C.-Y. Xu, and Y. Jo, Flotation and surface modification characteristics of galena, sphalerite and pyrite in collecting-depressing-reactivating system. J. Centr. South Univer. 19, 1702–1710 (2012).
Y. Cao, L. Sun, Z. Gao, W. Sun, and X. Cao, Activation mechanism of zinc ions in cassiterite flotation with benzohydroxamic acid as a collector. Miner. Eng. 156, 106523 (2020).
H. Hahne and W. Kroontje, Significance of pH and chloride concentration on behavior of heavy metal pollutants: mercury (II), cadmium (II), zinc (II), and lead (II), in, Wiley Online Library, (1973).
P. Clarke, P. Arora, D. Fornasiero, J. Ralston, and R.S.C. Smart, Separation of chalcopyrite or galena from sphalerite: a flotation and X-ray photoelectron spectroscopic study, In: Mineral Processing: recent Advances and Future Trends, Allied Publishers Limited, New Delhi, pp. 369-378, (1995).
M. Cao and Q. Liu, Reexamining the functions of zinc sulfate as a selective depressant in differential sulfide flotation—The role of coagulation. J. Colloid Interface Sci. 301, 523–531 (2006).
H. El-Shall, D. Elgillani, and N. Abdel-Khalek, Role of zinc sulfate in depression of lead-activated sphalerite. Int. J. Miner. Process. 58, 67–75 (2000).
M. Segall, P.J. Lindan, M.A. Probert, C.J. Pickard, P.J. Hasnip, S. Clark, and M. Payne, First-principles simulation: ideas, illustrations and the CASTEP code. J. Phys.: Condens. Matt. 14, 2717 (2002).
B.J. Skinner, Unit-cell edges of natural and synthetic sphalerites. Am. Mineral: J. Earth Planet. Mater. 46(11–12), 1399–1411 (1961).
R. Wyckoff, Rocksalt structure, in Crystal Structures, vol 1. (Interscience publishers, New York, 1963), pp. 85–237.
J.P. Perdew, K. Burke, and Y. Wang, Generalized gradient approximation for the exchange-correlation hole of a many-electron system. Phys. Rev. B 54, 16533 (1996).
Y.J. Luo, L.M. Ou, J.H. Chen, G.F. Zhang, Y.Q. Xia, B.H. Zhu, and H.Y. Zhou, Hydration mechanisms of smithsonite from DFT-D calculations and MD simulations. Int. J. Min. Sci. Technol. 32, 605–613 (2022).
T. Bucko, S. Lebegue, J. Hafner, and J.G. Angyan, Tkatchenko-Scheffler van der Waals correction method with and without self-consistent screening applied to solids. Phys Rev B. https://doi.org/10.1103/PhysRevB.87.064110 (2013).
K. Wright, G.W. Watson, S.C. Parker, et al., Simulation of the structure and stability of sphalerite (ZnS) surfaces. Am Mineral 83(1), 141–146 (1998).
J.H. Chen, Y. Chen, and Y.Q. Li, Effect of vacancy defects on electronic properties and activation of sphalerite (110) surface by first-principles. Trans. Nonferr. Metals Soci. China 20, 502–506 (2010).
H.M. Steele, K. Wright, and I.H. Hillier, A quantum-mechanical study of the (110) surface of sphalerite (ZnS) and its interaction with Pb2+ species. Phys. Chem. Miner. 30, 69–75 (2003).
J. Chen, X. Long, and Y. Chen, Comparison of multilayer water adsorption on the hydrophobic galena (PbS) and hydrophilic pyrite (FeS2) surfaces: a DFT study. J. Phys. Chem. C 118, 11657–11665 (2014).
J.A. Tossell and D.J. Vaughan, Electronic structure and the chemical reactivity of the surface of galena. Can. Mineral. 25, 381–392 (1987).
H. Zhang, W. Sun, C. Zhang, J. He, D. Chen, and Y. Zhu, Adsorption performance and mechanism of the commonly used collectors with Oxygen-containing functional group on the ilmenite surface: a DFT study. J Molec Liq 346, 117829 (2022).
H. Zhang, W. Sun, Y. Zhu, J. He, D. Chen, and C. Zhang, Effects of the goethite surface hydration microstructure on the adsorption of the collectors dodecylamine and sodium oleate. Langmuir 37, 10052–10060 (2021).
T.-S. Qiu, G.-D. Li, X.-B. Li, H.-S. Yan, and C. Liu, Influence of high concentration Zn2+ on floatability of sphalerite in acidic system. Trans. Nonferr. Metals Soci. China 31, 2128–2138 (2021).
Y.-F. Cui, F. Jiao, W.-Q. Qin, L.-Y. Dong, and X. Wang, Synergistic depression mechanism of zinc sulfate and sodium dimethyl dithiocarbamate on sphalerite in Pb−Zn flotation system. Trans. Nonferr. Metals Soci. China 30, 2547–2555 (2020).
H. Wang, S. Wen, G. Han, and Q. Feng, Effect of copper ions on surface properties of ZnSO4-depressed sphalerite and its response to flotation. Sep. Purif. Technol. 228, 115756 (2019).
D.R. Vučinić, P.M. Lazić, and A.A. Rosić, Ethyl xanthate adsorption and adsorption kinetics on lead-modified galena and sphalerite under flotation conditions. Colloids Surf., A 279, 96–104 (2006).
K. Venkateswaran and P. Ramachandran, Electroleaching of sulphides: a review. Bull. Electrochem. 1, 147–155 (1985).
H. Zhu, B. Yang, R. Martin, H. Zhang, D. He, and H. Luo, Flotation separation of galena from sphalerite using hyaluronic acid (HA) as an environmental-friendly sphalerite depressant. Miner. Eng. 187, 107771 (2022).
Y. Zhang, Z. Cao, Y. Cao, and C. Sun, FTIR studies of xanthate adsorption on chalcopyrite, pentlandite and pyrite surfaces. J. Mol. Struct. 1048, 434–440 (2013).
W. Huang, R. Liu, F. Jiang, H. Tang, L. Wang, and W. Sun, Adsorption mechanism of 3-mercaptopropionic acid as a chalcopyrite depressant in chalcopyrite and galena separation flotation. Colloids Surf., A 641, 128063 (2022).
Z. Wang, Y. Qian, L.-H. Xu, B. Dai, J.-H. Xiao, and K. Fu, Selective chalcopyrite flotation from pyrite with glycerine-xanthate as depressant. Miner. Eng. 74, 86–90 (2015).
H. Peng, D. Wu, M. Abdalla, W. Luo, W. Jiao, and X. Bie, Study of the effect of sodium sulfide as a selective depressor in the separation of chalcopyrite and molybdenite. Minerals 7, 51 (2017).
X. Wang, J. Liu, Y. Zhu, and Y. Han, Adsorption and depression mechanism of an eco-friendly depressant PCA onto chalcopyrite and pyrite for the efficiency flotation separation. Colloids Surf., A 620, 126574 (2021).
Q. Wei, L. Dong, F. Jiao, W. Qin, Z. Pan, and Y. Cui, The synergistic depression of lime and sodium humate on the flotation separation of sphalerite from pyrite. Miner. Eng. 163, 106779 (2021).
S.A. Khoso, Y. Hu, M. Tian, Z. Gao, and W. Sun, Evaluation of green synthetic depressants for sulfide flotation: synthesis, characterization and floatation performance to pyrite and chalcopyrite. Sep. Purif. Technol. 259, 118138 (2021).
S. Song, A. Lopez-Valdivieso, and M. Ojeda-Escamilla, Electrophoretic mobility study of the adsorption of alkyl xanthate ions on galena and sphalerite. J. Colloid Interface Sci. 237, 70–75 (2001).
T. Subrahmanyam, C. Prestidge, and J. Ralston, Contact angle and surface analysis studies of sphalerite particles. Miner. Eng. 9, 727–741 (1996).
X. Guo, L. Li, Z. Liu, D. Yu, J. He, R. Liu, B. Xu, Y. Tian, and H.-T. Wang, Hardness of covalent compounds: roles of metallic component and d valence electrons. J. Appl. Phys. 104, 023503 (2008).
Acknowledgements
This work was financially supported by the National Key Research and Development Program of China (No. 2022YFC2904503); the National Natural Science Foundation of China (Nos. 52074356, U20A20269); the Science and Technology Innovation Program of Hunan Province (No. 2022RC1183); the Natural Science Foundation of Hunan Province (No. 2021JJ20069); 2023 Innovation-driven Plan project of Central South University (No. 2023CXQD002); Changsha Science and Technology Project; National 111 Project (No. B14034); the Special Fund for Carbon Peak and Carbon Neutrality Science and Technology Innovation of Jiangsu Province in 2022 (No. BE2022601); the Fundamental Research Funds for the Central Universities of Central South University Project (No. 50621747). This work was carried out in part using hardware and/or software provided by the Computing Platform of Mineral Processing Computational Chemistry at School of Mineral Processing and Bioengineering of Central South University, the High-Performance Computing Centers of Central South University, and Tianhe II supercomputer at the National Supercomputing Center in Guangzhou.
Author information
Authors and Affiliations
Contributions
FZ Writing–original draft, Formal analysis, Software, Writing—review & editing. WS Visualization, Writing—review & editing, Validation. HZ Visualization, Writing—review & editing; DC Visualization, Writing—review & editing, Validation. SC Visualization, Writing—review & editing, Validation. JC Visualization, Writing—review & editing, Validation. CZ Writing—original draft, Supervision, Conceptualization, Methodology, Funding acquisition, Writing—review & editing.
Corresponding author
Ethics declarations
Conflict of interest
We certify that we have no financial or personal ties to individuals or groups that could unreasonably affect our work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article has been corrected. In the DFT calculations of the methodology section the sentence “The H2O, SBX, and Zn(OH)2 were pre-optimized in a cubic cell of 20 × 20 × 20 Å3 using the k-point of gamma and cutoff energy of 370 eV.” was changed to “The H2O, OH−, and Zn(OH)2 were pre-optimized in a cubic cell of 20 × 20 × 20 Å3 using the k-point of gamma and cutoff energy of 400 eV.”.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, F., Sun, W., Zhang, H. et al. Selective Adsorption Mechanism of Zinc Ions on the Surfaces of Galena and Sphalerite in the Flotation Separation of Pb-Zn. JOM 75, 4808–4818 (2023). https://doi.org/10.1007/s11837-023-06098-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-023-06098-6