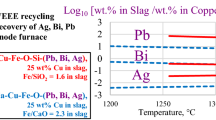
Abstract
The recycling of waste electronic and electrical equipment (WEEE) and other secondary copper-containing materials through the "black copper" process relies on the selective distribution of metals among the gas, slag, and copper-rich liquid phase. This distribution is controlled by the effective oxidation/reduction potential, often expressed in terms of oxygen partial pressure. Separation of Ni and Sn presents a certain challenge in recycling though black copper route due to similar distribution coefficients over a wide range of oxygen partial pressures and possibly can be improved by optimizing the slag chemistry. This study provides experimental information on the distribution of Ni, Sn, and Zn between fayalite slags or calcium ferrite slags and copper-rich metal at 1250°C. The study uses high-temperature equilibration, quenching, and electron probe X-ray microanalysis (EPMA) techniques along with laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) for selected measurements of low concentrations. The oxygen partial pressure is controlled by the CO/CO2/Ar gas flow or by measuring the concentration of copper oxide in the slag. The effect of slag composition in terms of Fe/SiO2 or Fe/CaO ratio is studied by using different holding materials, such as silica (SiO2) ampoule, solid spinel (Fe3O4), wüstite (FeO), or dicalcium ferrite (Ca2Fe2O5). The experimental results are compared with literature data and used to optimize thermodynamic models for FactSage® software. The results demonstrate an opportunity for more effective separation of Ni and Sn using at oxidizing stage by using calcium ferrite slag promoting the formation of Sn4+.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recycling of secondary materials such as copper scrap and waste electronic and electrical equipment (WEEE) has been proven to be efficient and economical using high-temperature processing,1,2,3,4,5 as demonstrated by largest copper recycling companies. Aurubis Lünen uses a combination of submerged lance furnace and top blown rotary converter,6 Umicore Hoboken employs ISAsmelt-type furnace in its integrated recycling plant,7 Boliden Rönnskär plant processes WEEE in E-Kaldo furnace,8 and Dowa Kosaka uses top-submerged lance (TSL) furnace.9 The commercial solutions for new smelters are offered by Metso®.10 In all these processes, the separation of metals is achieved by selective partitioning of target metals among gas, slag, and copper-rich metallic phase; the latter is often referred to as “black copper”. The recovery of precious metals such as Au, Ag, and PGM largely drives the economics of these processes,2 but increasing demand for Ni and supply constraints of Sn create incentives to increase the throughput and recovery of these metals. An example flowsheet of the black copper process developed by Metso is shown in Fig. 1.11
Example flowsheet of WEEE and copper scrap processing using the black copper route from Närhi et al.10 The figure has been modified from the cited source with the authors’ permission.
The reduction smelting stage (Step 1) is designed to burn out organic components, fume metallic Zn into the gas phase, and oxidize and separate into the slag phase the following elements in oxidic form: SiO2, Al2O3, FeO, and CaO, with minor concentrations of TiO2, Na2O, and K2O. The resulting black copper contains significant amounts of Pb, Ni, Sn, Sb, Au, Au, and PGMs.12 The minimal operating temperature and fluxing are determined by the formation of solid phases in the slag, in particular FeAl2O4-based spinel or CaAl2Si2O8-based feldspar. Jak et al.12 demonstrated that operation at 1250°C is possible and can be achieved without significant additional energy input. The oxygen partial pressure must be as low as possible to provide “clean slag”. It is limited by the formation of metallic iron. Jak et al.12 calculated that P(O2) = 10−10 atm is sufficient to keep the concentrations Pb and Cu in slag below 1.5 wt.% and 0.5 wt.%, respectively. The exact concentrations depend on the slag chemistry and are affected by the entrainment of metal droplets. At these conditions, the concentration of Fe in black copper is expected to be about 0.1 wt.%–0.2 wt.%.12
The purpose of Step 2 is to selectively oxidize Pb, Sn, and Sb out of black copper into second slag. In literature, this step is called oxidative smelting or converting.1 It is not possible to prevent the undesirable partial oxidation of Cu and Ni into the slag during converting. Therefore, an additional step is required for slag cleaning (Step 3), aimed at recovering Cu and Ni, while keeping Pb, Sn, and Sb in the slag. Further reduction at Step 3 allows recovering Pb, Sn, and Sb in the form of metallic alloy. Steps 2 and 3 require fine control of the P(O2) in the system and can be done in stages. For the temperature of 1250°C, Step 2 requires P(O2) of about 10−6–10−5 atm, while the estimation for Step 3 is P(O2) = 10−8 atm, followed by P(O2) = 10−10 atm. Two recycling streams are introduced because of undesirable reactions: recycled Cu-Ni alloy stream from Step 3 to Step 2 and “circulated slag” stream from Step 3 to Step 1. Thus, it becomes important that the possible slag chemistry at the converting stage (Step 2) should promote the effective separation of Cu and Ni from Pb and Sn as well as having low liquidus at both high and low concentrations of Cu2O and PbO.
The optimization of the recycling process, as outlined in Fig. 1, requires computational models linked to thermodynamics.13 Increasing the selectivity of separation of Sn and Ni at Step 2 and Step 3 would reduce the mass of recycling streams. The activity coefficient of ZnO in slag at Step 1 directly affects the recovery of this element in the overall process. The present study provides the fundamental experimental information on the distribution of Ni, Sn, and Zn between two types of slag and metallic copper as a function of oxidation/reduction potential in the system. The two slags are fayalite-based and calcium ferrite-based slag. The experimental results are critically assessed together with literature data on distribution and on phase equilibria involving these elements. An indirect comparison of different types of data is possible through thermodynamic modelling. Parameters of thermodynamic models are then optimized using the combined dataset and become a part of a larger database, which is capable of predictions not only with fayalite and calcium ferrite slags but also other slag systems.
The fundamental research on minor elements distribution between various slags and copper has been published by Takeda et al.14,15 and Yazawa et al.16 Their pioneering studies laid the foundation for high-temperatures equilibration techniques at fixed conditions. This approach has been further developed and applied to the measurement of distribution coefficients of minor elements between different types of slags and copper.17,18,19,20,21
The distribution coefficients of Ni between fayalite-based slag and copper were measured experimentally by Wang et al.,22 Danczak et al.23 Klemettinen et al.,24 and Sukhomlinov et al.25 Silica-saturated slag was used by Wang et al.22 and Sukhomlinov et al.25 In the experiments of Danczak et al.23 and Klemettinen et al.,24 slags contained around 15-18 wt.% Al2O3. For calcium ferrite slag, the distribution of Ni was measured by Takeda et al.,14 and Eerola et al.26 They used CaO or MgO crucibles. In most of these studies,22,23,25 the oxygen partial pressure was controlled directly by using CO-CO2, CO2-N2 gas mixtures, but Eerola et al.26 calculated the P(O2) from the measured concentration of oxygen in copper alloy after the equilibration experiment. The composition of slag was measured by dissolution and gravimetric methods,22 atomic absorption,14,26 ICP,26 EPMA,23,24,25 and LA-ISP-MS23,25
The distribution coefficients of Sn between fayalite slag and copper were measured by Van den Bulck et al.,27 Nagamori and Mackey,28 Hidayat et al.,19 Anindya,29 Avarmaa et al.,30 Takeda et al.,14 Danczak et al.,23 and Klemettinen et al.31 Takeda et al.14 and Van den Bulck et al.27 equilibrated slags with both metal and tridymite (SiO2). In the experiments of Van den Bulck,27 slags contained 3-10 wt.% CaO; in the experiments of Nagamori and Mackey,28 slags contained up to 15 wt.% Al2O3. Avarmaa et al.29 performed experiments in magnesia crucibles; the slag contained up to 11 wt.% MgO and up to 24 wt.% CaO. Avarmaa et al.30 investigated the distribution of Sn between CaO-free/CaO-containing alumina iron silicate slags and metallic copper.30 Slags were in equilibrium with spinel. In the experiments of Danczak et al.23 and Klemettinen et al.,31 slags were held in alumina crucibles and contained around 15 wt.% Al2O3 and 0–6 wt.% MgO. They were in equilibrium with FeAl2O4-based spinel. Only one study by Takeda et al.14 is available for the distribution of Sn between calcium ferrite-based slag and copper. It corresponded to the conditions close to converting process.
The distribution coefficients of Zn between fayalite-based slag and copper were measured by Takeda et al.,14 Yazawa et al.,32 and Hidayat et al.19 In these experiments, slags were in equilibrium with tridymite (SiO2). However, the literature data are not in agreement, indicating the need for further experimental studies to clarify the discrepancies. Takeda et al.14 and Yazawa et al.32 reported results of laboratory experiments on the distribution of Zn between calcium ferrite slag and copper, and Ohshima and Hayashi33 examined them in industrial process. Literature data are mostly in agreement, except for the results of Ohshima and Hayashi,33 who reported higher distribution coefficient, wt.% Zn in slag/wt.% Zn in copper, compared to other studies.14,32
The need to verify relatively old results for Sn and Zn in calcium ferrite slags,14,32,33 the discrepancy of data for Zn in fayalite slags,19,32 and the presence of Al2O3 and MgO in most experimental results for Ni and Sn prompted the present experimental study. For the purpose of thermodynamic database development, it is desirable to have a reliable dataset within the Cu-Fe-O-Si-(Ni, Sn, Zn) and Ca-Cu-Fe-O-(Ni, Sn, Zn) systems, free of Al2O3 and MgO. Once the results are established and model parameters optimized, the effects of Al2O3 and MgO can be introduced in targeted experiments. In all the above-mentioned studies, the concentrations of Ni and Sn in copper were relatively small, typically < 1 wt.%, and Zn < 0.1 wt.%. Using the datasets with small and large concentrations together during the thermodynamic modelling would result in more reliable predictions. Many publications14,16,23,32 do not report the original compositional data, making it more difficult to use them for thermodynamic modelling. Furthermore, the experimental results of the present study are assessed using the thermodynamic calculations proving insights into the accuracy.
Experimental Methodology
The general experimental approach used in the present study involves high temperature equilibration at controlled conditions, rapid cooling (quenching), and direct measurement of the compositions of the equilibrium phases using electron probe X-ray microanalysis (EPMA).
The following oxides and metal powders were used to prepare the mixtures: pre-calcined SiO2, Fe2O3, Fe, Cu, Ni, Sn, Zn, ZnO, and SnO2 (all ≥ 99.9 wt.% purity) supplied by Alfa Aesar, MA, USA. For the experiments with calcium ferrite slag, a master slag with the CaO:FeO 1:1 mass ratio was preliminarily prepared. The compositions of the initial mixtures were selected following the calculation using FactSage® and current version of the thermodynamic database to produce one solid crystalline phase (tridymite, wüstite, spinel, or dicalcium ferrite) in equilibrium with liquid slag and copper metal. Phase assemblages were targeted to contain no more than 10% solids and to result in achieving concentrations of Ni, Sn, and Zn above EPMA detection limits at fixed measurement conditions, in both slag and metal phase. The detection limits are discussed further.
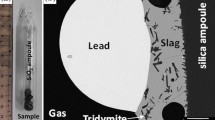
Substrates were prepared in advance from high-purity Fe3O4 (spinel), FeO (wüstite), Ca2Fe2O5 (dicalcium ferrite), and SiO2 (quartz). They were used to support the mixtures for equilibration. Figure 2 shows different setups for using these substrates. The spinel substrate was made from the 99.5 wt.% pure iron foil (supplied by Goodfellow Cambridge Ltd., Huntingdon, England) folded into the required shape and then oxidized at 1200°C (1473 K) for 2 h in pure CO2 gas corresponding to P(O2) = 10−3.9 atm. Based on the previous assessment of the Fe–O system,34 the resulting magnetite composition was deemed to be close to stoichiometric Fe3O4. Several shapes of spinel substrates were tested. The final substrate shape adopted was in the form of an envelope with open ends (Fig. 2d). The wüstite substrate had the same shape and was prepared through oxidation of 99.5 wt.% pure iron foil at 1200°C (1473 K) for 4 h in Ar/CO/CO2 gas ratio corresponding to P(O2) = 10−8 atm. The Ca2Fe2O5 substrate was prepared from calcined CaO and Fe2O3 powders, pressed into a shape of a cone, and sintered in a muffle furnace at approximately 1220°C. This technique did not allow to avoid completely the micro-pores in the Ca2Fe2O5 substrate. The spinel, wüstite, or Ca2Fe2O5 substrates with the samples were placed into silica ampoules and then sealed under vacuum. The closed system equilibration was used to prevent the evaporation of volatile elements, i.e., Zn and Sn. To prevent a contact between the silica ampoule and the substrate, the latter was suspended within the ampoule using iridium wire fixed by an alumina ring, as shown in Fig. 2b. The experiments aimed at study of Ni distribution were carried out at closed and open system setups. Figure 2a shows the closed system setup. Figure 2c represents the open system setup, where the Ar/CO/CO2 gas flow was used to fix the target oxygen partial pressure as described in earlier publications.35,36 The equilibration time was 6 h, which was determined by the separate experimental series.
Experimental setups used in the present study for the equilibration with primary phase substrates: (a) sealed SiO2 ampoule with pelletised sample, vacuum inside, and Ar flow outside; (b) sealed SiO2 ampoule with suspended spinel, wüstite, or Ca2Fe2O5 substrate, vacuum inside, and Ar flow outside; (c) open SiO2 bucket in CO/CO2/Ar gas flow; (d) spinel, wüstite or Ca2Fe2O5 substrate in CO/CO2/Ar flow; (e) photo of broken SiO2 ampoule after experiment; (f) photo of block with mounted samples on spinel substrates; (g) photo of block with mounted samples on tridymite (SiO2) substrates; (h) photo of spinel bucket after oxidation.
A JEOL JXA8200L (trademark of Japan Electron Optics Ltd., Tokyo) electron probe X-ray microanalyser (EPMA) with wavelength-dispersive detectors (WDD) was used to measure the composition of different phases including slag, copper, spinel, wüstite, and Ca2Fe2O5. The EPMA was operated at an acceleration voltage of 15 kV and a probe current of 15 nA. First, standard calibration was performed on hematite, wollastanite, and quartz. Secondary standards of Cu metal, Ni metal, ZnO, SnO2, and Ni2SiO4 were used to measure the compositions of copper, spinel, wüstite, Ca2Fe2O5, and slag phases. All reference materials were provided by Charles M. Taylor, Stanford, CA (SPI Supplies®). The detection limits for Ni, Sn, and Zn were estimated to be 0.08 wt.% (800 ppm), 0.05 wt.% (500 ppm), and 0.09 wt.% (900 ppm), respectively. The concentration of oxygen in the slag and solid oxide phases was not measured but calculated based on the measured cation concentration with assigned oxidation states, i.e., Cu, Si, and Ca were expressed as Cu2O, SiO2, and CaO for both types of slags, while Fe was expressed as FeO for fayalite slag and Fe2O3 for calcium ferrite slag. Compositions of slag, metal and solid phases in each sample were measured in 10-50 different points to ensure the absence of compositional gradients, which was one of the indicators of the achievement of equilibrium.
In the present study, the concentration of Zn in the metal phase was below the detection limit of EPMA. Therefore, a method was developed to measure Zn concentration in the metal phase by laser ablation ICP-MS (LA-ICP-MS). The main factor affecting the accuracy of quantitative analysis of metallic phase by LA-ICP-MS was the choice of external standard. It has been well documented that metal and sulphides ablate very differently from oxide minerals/glasses during laser ablation; thus, the use of commercially available silicate standards such as NIST 610 and 612 glass could cause significant systematic errors in sulphide analyses. In the present study, matrix-matching Cu standards doped with known concentration of Zn were prepared and used as the external standards to ensure the accuracy of the measurement. The measurement was conducted using an Agilent Technologies 7700 ICP-MS coupled to an ANU HelEX laser-ablation system with a 193-nm-wavelength EXCIMER laser (110 (ArF) COMPex, Lambda Physik) at the Research School of Earth Sciences, Australian National University. The analysis condition (laser fluence, spot-size and repetition rate) was fine tuned to ensure the stable output of ICP-MS signal when ablating Cu metal phase. Cu concentration determined by EPMA was used as internal standard for quantification of the Zn concentration.
Thermodynamic Modelling
Thermodynamic calculations and optimization of the model parameters for the present study were performed using FactSage 8.237 thermodynamic package. The phases and solutions are given in Table I. Most of the model parameters were adopted from assessments of the chemical sub-systems of the Ca–Cu–Fe–O–Si–(Ni, Sn, Zn) system. A massive re-assessment of these systems starting from the properties of CaO, SiO2, and FeO1.5 was performed in 2022; the results have not yet been published. Systematic experimental phase equilibria study and thermodynamic modelling are under way and model parameters are constantly being updated. The calculations in the present study are performed with the internal PYROSEARCH thermodynamic database, version May 2023. The experimental data of the present study were used as a part of a large dataset. Quantitatively, the agreement is being monitored using the sum of squared deviations between the experimental and calculated distribution values.
Experimental Results
Typical micrographs of the experimental samples are shown in Figs. 3 and 4. The measured compositions of coexisted phases are given are given in Tables II and III.
Discussion
The experimental results plotted on corresponding graphs are presented in Figs. 5 and 6 and compared with available literature data. Two types of calculations were performed. To compare the experimental results of the present study and model predictions, 1 g slag and 1 g metal, with phase compositions taken from Table II, were combined with 0.1 g respective solid phase (SiO2, FeO, Fe3O4, or Ca2Fe2O5) and the resulting bulk compositions were used as an input for FactSage® Equilib module. The amount of oxygen in the calculation was adjusted to obtain the same distribution of copper L Cu (slag/metal) as in the experiment after equilibration or fixed directly for open system experiments. In the calculations, large concentrations of Ni, Sn, or Zn in either metal or slag phase are used, like in the experiments. These elements are not “minor”, so the assumption of Henrian behaviour of activity coefficient in either phase cannot not be applied, but instead the activity coefficients are calculated by the model. The calculations also provide the effective P(O2) in the system for the closed series of experiments, where P(O2) is unknown. In Figs. 5 and 6, the results of these calculations are shown using empty square symbols connected to the filled symbols representing experimental data. A second type of calculation provides the distribution coefficient at infinitely small concentrations of minor elements (< 0.01 wt.% in any phase), where Henrian behaviour of activity coefficient can be assumed. The limiting conditions for the slag composition are determined by the phase equilibrium with one of the solid phases at 1250°C. These calculation results are shown using solid lines in Figs. 5 and 6. Thus, the distance between the calculated lines and calculated positions of empty squares demonstrates the effect of high concentrations of Ni, Sn, or Zn elements on the distribution coefficients. The distance between the lines shows the effect of Fe/SiO2 ratio of Fe/CaO ratio in the slag on the distribution coefficients, as predicted by the model.
Phase equilibria and distribution coefficients for the fayalite-based slag/copper in the Cu-Fe-O-Si-(Ni, Sn, Zn) system at 1250°C: (a) Projection of the slag compositions onto the Cu2O-FeO-SiO2 plane. (b) Distribution coefficients of Ni.22,23,25 (c) Distribution coefficients of Sn.19,23,27,28,29,30,31 (d) Distribution coefficients of Zn.14,32 Filled squares are the experimental results of the present study. Empty squares correspond to the thermodynamic calculation exactly at the experimental conditions. Other symbols represent experimental literature data with references given above. Lines are thermodynamic calculation for the infinite dilution of minor element (< 0.1 wt.% in any phase).
Phase equilibria and distribution coefficients for the calcium ferrite-based slag/copper in the Ca-Cu-Fe-O-(Ni, Sn, Zn ) system at 1250°C: (a) Projection of the slag composition onto the Cu2O-CaO-FeO plane. (b) Distribution coefficients of Ni.14,26 (c) Distribution coefficients of Sn.14 (d) Distribution coefficients of Zn.14,16,33 Filled squares are the experimental results of the present study. Empty squares correspond to the thermodynamic calculation exactly at the experimental conditions. Other symbols represent experimental literature data with references given above. Lines are thermodynamic calculation for the infinite dilution of minor element (< 0.1 wt.% in any phase).
Figure 5a illustrates the compositions of the fayalite-based slags in the experiments. Experimental points cover the low-Cu2O range of slag composition limited by the formation of tridymite, spinel, or wüstite. The “jump” into the high-Cu2O slag region was not investigated experimentally in the present study but can be important for the oxidizing smelting steps.
Distribution coefficient of Ni between fayalite slag and copper is shown in Fig. 5b. Obtained experimental data are consistent with literature results.23,25 Model predictions at P(O2) = 10−7...10−5 atm are higher than in the experiments of Wang et al.22 but supported by the results of the present study and by Sukhomlinov et al.25 Thus, the experimental and modelling results of the present study resolved the disagreement among the data by Sukhomlinov et al.25 and Wang et al.22 At P(O2) = 10−10...10−9 atm, all experimental points were done in equilibrium with wüstite. The overall accuracy of the present study does not allow to resolve the effect of the Fe/SiO2 ratio in slag on the Ni distribution, indicating the difference < 0.3 in log10L values. When plotted on the figure, the results of the “open” and “closed” experiments are consistent, indicating the method of calculation of P(O2) from L Cu (slag/metal) is valid.
The literature data on Sn distribution between fayalite-based slag and copper are plotted in Fig. 5c and show some scatter due to changes in slag composition, temperatures, and differences in equilibration and analytical techniques. Experimental results of the present study contribute to the dataset which is being used in thermodynamic modelling. At present, reasonable agreement is observed between the model predictions and experimental results of the present study, with small systematic deviation towards lower L Sn predicted by the model. Fe/SiO2 ratio was found to influence Sn distribution between phases at lower oxygen partial pressure values (< 10−7 atm) according to calculation results. However, experimental data did not confirm this difference as all experiments for Sn distribution have been carried out on only spinel or wüstite substrate. Further improvements of the model are now conducted simultaneously with phase equilibrium studies in the Cu-Sn-Si-O42 and Fe-Sn-Si-O systems; the latter is not yet published.
Experimental results for the Zn distribution between fayalite slag and copper are shown in Fig. 5d. At P(O2) < 10−7 atm, the results are consistent with earlier publications14,32 and demonstrate that lower values of distribution coefficient reported by Hidayat et al.19 were likely incorrect because of very small concentrations of Zn in copper metal phase. The results of the present study between P(O2) = 10−7…10−5 atm are probably incorrect. Apparently, neither EPMA nor LA-ICP-MS techniques can provide accurate measurement for such low concentrations of Zn in slag. In case of Zn, the effect of Fe/SiO2 ratio on Zn distribution is observed in the experiment at P(O2) = 10−8.3 atm: higher Fe/SiO2 corresponding to equilibrium with wüstite results in lower distribution coefficient compared to slags with lower Fe/SiO2 corresponding to equilibrium with tridymite. These experimental results are supported by model predictions based on the thermodynamic assessment of the Fe-Zn-Si-O system.43
Figure 6a illustrates the compositions of the calcium ferrite-based slags in the experiments. Experimental points cover the low-Cu2O range of slag composition limited by the formation of Ca2Fe2O5, spinel, or wüstite. Unlike fayalite slag, the calcium ferrite-based slags at 1250°C have a continuous region of liquid from low to high concentrations of Cu2O.
Experimental results on Ni distribution between calcium-ferrite slag and copper are shown in Fig. 6b. The data obtained in 1983-198414,26 are confirmed by the present study, which gives more confidence in studying the calcium ferrite slags using the quenching method. The quenching of calcium-ferrite slags is challenging because of low viscosity of liquid phase. As a result, the precipitation of secondary phases during the quenching is very hard to avoid, as can be seen in microstructures in Fig. 4. Based on experience, small size and unclear boundaries of crystalline phases indicate their origin during the quenching. To measure the slag composition corresponding to higher temperature, before the precipitation of the secondary phases, larger probe diameter of 50 µm was used. Very small Fe/CaO ratio on the distribution coefficient is predicted by the model, and the accuracy of experiments does not allow establishing it. The experimental data on Ni distribution for “closed” and “open” systems are consistent with each other, giving the confidence in using the measured L Cu (slag/metal) values for the estimation of the P(O2) in the system by the model.
The distribution of Sn between calcium-ferrite slag and copper is shown in Fig. 6c. Experiments of the present study confirm the results of the only available study by Takeda et al.14 at P(O2) = 10−9…10−7 atm, but deviations are observed at higher P(O2), with lower values obtained in the present study. The disagreement between model prediction and the results of by Takeda et al.14 at P(O2) < 10−9 atm indicate further need of experiments in this region. Currently, the study of phase equilibria in the Ca-Fe-Sn-O system is being conducted, which will be used together with distribution data to further understand the balance between Sn2+ and Sn4+ in slags.
The distribution coefficient of Zn between calcium ferrite-based slag and copper metal is shown in Fig. 6d. Good agreement was obtained in the study of Takeda et al.14 at P(O2) = 10−9…10−6 atm, but the points above 10–6 atm do not help to resolve the inconsistency between Takeda et al.14 and Ohshima and Hayashi.33 These points are not considered accurate.
Industrial Application
The difference of Ni, Sn, and Zn distribution between the fayalite and calcium ferrite slags in equilibrium with coper is demonstrated by the experiments of the present study and model calculations. In industrial practice, routine direct P(O2) measurement is difficult to undertake. However, increasing oxygen pressure is closely related to the increase in dissolved copper in slag. The processes are controlled by the measurement of composition, i.e., wt.% Cu in slag, which is similar to the closed experiments of this study. Figure 7 shows the distribution coefficients as a function of wt.% Cu in slag, calculated by FactSage 8.2 and the internal thermodynamic database. Like in earlier diagrams, the fully liquid region of slag in terms of Fe/SiO2 or Fe/CaO ratio is limited by the formation of solid phases: tridymite or Ca2Fe2O5, spinel, and wüstite. For the same wt.% Cu in slag, the value of the distribution coefficient will somewhat depend on the Fe/SiO2 or Fe/CaO ratio, which is shown by two limiting lines.
In terms of distribution coefficient, fayalite slags are more suitable to remove Zn, but calcium ferrite slags have superior removal of Ni and Sn across the range of oxygen partial pressures tested. Also, the disappearance of fayalite-based liquid slag region at 1250 ° above 10 wt.% Cu can present a certain challenge to the batch-type converting and slag reduction processes. Therefore, the choice of slag composition can impact the efficiency of removing specific elements from a copper-containing system, and this can be controlled by monitoring the composition of the slag in industrial practice. The results of the present study are currently being implemented in designing an additional refining step for the removal of Ni, Sn, and Zn from copper in anode furnace, and diagrams in Fig. 7 are being used to explain the results of the process simulation to metallurgical engineers.
Conclusion
The integrated experimental and modelling approach has been used to establish the distribution coefficients of Ni, Sn, and Zn between fayalite slag and copper and between calcium ferrite slag and copper. It is demonstrated that calcium ferrite slags have higher selectivity for the separation of Ni and Sn compared to fayalite-based slags, which may create an opportunity for improving the e-scrap recycling process. In real processes, many elements are present together, and experimental results on distribution coefficients alone do not provide the necessary information on phase equilibria and energy balance. Thus, the dataset of the present study is a part of a larger programme, aimed at complete characterization of the gas-slag-matte-speiss-metal-solids equilibria in the Cu-Pb-Zn-Fe-O-S-SiO2-(Al2O3-CaO-MgO-Cr2O3)-(As-Sn-Sb-Bi-Sn-Au-Ni-Co) system.
References
M. Ghodrat, M.A. Rhamdhani, A. Khaliq, G. Brooks, and B. Samali, J. Mater. Cycles Waste Manage. 20, 386 https://doi.org/10.1007/s10163-017-0590-8 (2018).
M. Ghodrat, M.A. Rhamdhani, G. Brooks, S. Masood, and G. Corder, Journal Cleaner. Prod. 126, 178 https://doi.org/10.1016/j.jclepro.2016.03.033 (2016).
J. Wood, S. Creedy, R. Matusewicz, M. Reuter, Australians Ins.t Min. Metall. 7, 460 (2011)
G.A. Flores, C. Risopatron, and J. Pease, JOM 72(10), 3447 https://doi.org/10.1007/s11837-020-04255-9 (2020).
N. Borowski, T. Lux, R. Degel, F. Kaußen, and M. Reuter, Proc. EMC 2021, 219 (2021).
F.J. Westhoff, Lünen. Metall. (Isernhagen, Germany) 65, 375 (2011)
B. De Cooman, Proceedings of EMC 2007, 545 (2007).
A. Lennartsson, F. Engström, C. Samuelsson, B. Björkman, and J. Pettersson, J. Sustain. Metall. https://doi.org/10.1007/s40831-018-0157-5 (2018).
T. Morise, M. Naka, and T. Shiratori, J. MMIJ 123, 758 https://doi.org/10.2473/journalofmmij.123.758 (2007).
L. Närhi, S. Hughes, H. Holmgren, Metso: Outotec Webinar (2021)
L. Närhi, S. Hughes, H. Holmgren. Metso: Outotec Webinar (2022)
E. Jak, T. Hidayat, V. Prostakova, D. Shishin, M. Shevchenko, and P.C. Hayes, Proceedings of EMC 2019, 587 (2019).
M.A. Reuter, Metall. Mater. Trans. B 47, 3194 https://doi.org/10.1007/s11663-016-0735-5 (2016).
Y. Takeda, S. Ishiwata, and A. Yazawa, Trans. Japan Inst. Met. 24, 518 (1983).
Y. Takeda, S. Ishiwata, and A. Yazawa, Nippon Kogyo Kaishi 100, 103 (1984).
A. Yazawa, S. Nakazawa, Y. Takeda, Proc. Int. Sulfide Smelt. Symp. Metall. Soc. AIME, 99 (1983)
S. Surapunt, Y. Takeda, and K. Itagaki, Metall. Rev. MMIJ 13, 3 (1996).
J. Hino, Y. Takeda, Mine. Metals Mater. Soc. 545 (1983)
T. Hidayat, J. Chen, P.C. Hayes, and E. Jak, Int J Mater Res. 112, 178 https://doi.org/10.1515/ijmr-2020-7857 (2021).
S. Sineva, D. Shishin, R. Starykh, P.C. Hayes, and E. Jak, J. Sustain. Metall. 7, 569 https://doi.org/10.1007/s40831-021-00360-4 (2021).
M.K. Islam, M. Somerville, M.I. Pownceby, J. Tardio, N. Haque, and S. Bhargava, JOM. https://doi.org/10.1007/s11837-021-04683-1 (2021).
S.S. Wang, A.J. Kurtis, and J.M. Toguri, Can Metall Q. 12, 383 (1973).
A. Dańczak, L. Klemettinen, H. O’Brien, P. Taskinen, D. Lindberg, and A. Jokilaakso, J. Sustain. Metall. 7(1), 1 https://doi.org/10.1007/s40831-020-00318-y (2020).
L. Klemettinen, K. Avarmaa, P. Taskinen, and A. Jokilaakso, Proc. Extract. Conf. https://doi.org/10.1007/978-3-319-95022-8_86 (2018).
D. Sukhomlinov, L. Klemettinen, K. Avarmaa, H. O’Brien, P. Taskinen, and A. Jokilaakso, Metall. Mater. Trans. B 50, 1752 https://doi.org/10.1007/s11663-019-01576-2 (2019).
H. Eerola, K. Jylha, and P. Taskinen, Trans. Inst. Min. Metall. C 93, 193 (1984).
A. Van den Bulck, S. Turner, M. Guo, A. Malfliet, and B. Blanpain, Proc. Extract. Conf. https://doi.org/10.1007/978-3-319-95022-8_86 (2018).
M. Nagamori, and P.J. Mackey, Metall Trans B 8B, 39 (1977).
A. Anindya, PhD thesis, RMIT University (2012)
K. Avarmaa, S. Yliaho, and P. Taskinen, Waste Manage. 71, 400 https://doi.org/10.1016/j.wasman.2017.09.037 (2018).
L. Klemettinen, K. Avarmaa, H. O’Brien, and P. Taskinen, Minerals. https://doi.org/10.3390/min9010039 (2019).
A. Yazawa, S. Nakazawa, Y. Takeda, Proc. Int. Sulfide Smelt. Symp. Metall. Soc. AIME, 99 (1985)
E. Ohshima, and M. Hayashi, Metall. Rev. MMIJ 3, 113 (1986).
T. Hidayat, D. Shishin, E. Jak, and S. Decterov, Calphad 48, 131 https://doi.org/10.1016/j.calphad.2014.12.005 (2015).
S. Sineva, M. Shevchenko, D. Shishin, T. Hidayat, J. Chen, P.C. Hayes, and E. Jak, JOM. https://doi.org/10.1007/s11837-020-04326-x (2020).
S. Sineva, D. Shishin, R. Starykh, M. Shevchenko, and E. Jak, Met. Mater. Trans. B. https://doi.org/10.1007/s11663-022-02529-y (2022).
C.W. Bale, E. Bélisle, P. Chartrand, S.A. Decterov, G. Eriksson, A.E. Gheribi, K. Hack, I.H. Jung, Y.B. Kang, J. Melançon, and A.D. Pelton, Calphad 55, 1–9 https://doi.org/10.1016/j.calphad.2016.05.002 (2016).
A.D. Pelton, S.A. Decterov, G. Eriksson, C. Robelin, and Y. Dessureault, Metall. Mater. Trans. B 31, 651 https://doi.org/10.1007/s11663-000-0103-2 (2000).
A.D. Pelton, and P. Chartrand, Metall. Mater. Trans. A 32, 1355 https://doi.org/10.1007/s11661-001-0226-3 (2001).
M. Hillert, J. Alloys Compd. 320, 161 (2001).
O. Redlich, and A.T. Kister, Ind. Ing. Chem. 40, 345 (1948).
R.V. Starykh, M. Shevchenko, D. Shishin, and E. Jak, Calphad 74, 1023 (2021).
M. Shevchenko, and E. Jak, Calphad 68, 1017 https://doi.org/10.1016/j.calphad.2019.101735 (2020).
Acknowledgements
The research funding and technical support are provided by the consortium of copper producing companies: Anglo American Platinum (South Africa), Aurubis AG (Germany), BHP Billiton Olympic Dam Operation (Australia), Boliden (Sweden), Glencore Technology (Australia), Metso Oy (Finland), Peñoles (Mexico), RHI Magnesita (Austria), Rio Tinto Kennecott (USA) and Umicore NV (Belgium), as well Australian Research Council Linkage program LP190101020 “Future copper metallurgy for the age of e-mobility and the circular economy”. The authors are grateful to Suping Huang, Marina Chernishova and Samaneh Ashjaa for assistance with conducting experiments. Present study would not be possible without the facilities and technical assistance of the staff of the Australian Microscopy & Microanalysis Research Facility at the Centre for Microscopy and Microanalysis in The University of Queensland.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sineva, S., Shishin, D., Prostakova, V. et al. Experimental Study and Thermodynamic Modelling of Equilibrium Distributions of Ni, Sn and Zn Between Slag and Black Copper for E-Scrap Recycling Applications. JOM 75, 4254–4268 (2023). https://doi.org/10.1007/s11837-023-06059-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-023-06059-z