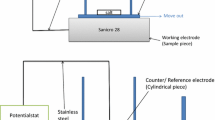
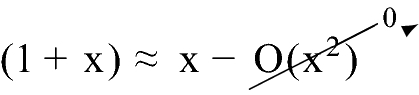Abstract
We have developed a new real-time framework for calculating the simultaneous accumulation of oxidation-induced and the internal/external fluid stresses during the corrosion of the zirconium metal, Zr. In order to track such interfacial stress when the zirconium metal turns oxide, we quantify the hypothetical real-time infiltration of the oxygen within the metal matrix in the curved boundary, leading to the augmentation in the volume, and we stoichiometrically compute the resulted equivalent oxide thickness. Subsequently, we calculate the accumulated compressive stress in real-time from both irreversible (plastic) and reversible (elastic) events which could be used for anticipation of the onset of failure. The developed analytical framework could quantify the design parameters for the safe operation of high-pressure pipes in corrosive environments.







Similar content being viewed by others
Data availability
The row data for producing the results in this manuscript are freely available upon request from the corresponding author at aryanfar@caltech.edu.
Notes
For Zr, \(R_{\mathrm{PB}}=1.56\).73
In
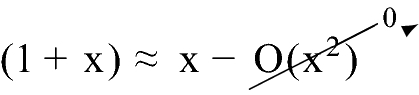
when x is small.
Abbreviations
- D :
-
Diffusion coefficient (\(\mathrm{m}^{2}\,\mathrm{s}^{-1}\))
- f :
-
Dimensionless stress factor (\([\text { }]\))
- \(\Omega \) :
-
Molar volume (\(\mathrm{m}^{3}\,\mathrm{mol}^{-1}\))
- T :
-
Temperature (K)
- \(R_{u}\) :
-
Gas constant (\(8.3\,\mathrm{J}\,\mathrm{mol}^{-1}\,\mathrm{K}^{-1})\)
- \(R_{\mathrm{I}}\) :
-
Inner radius (m)
- \(R_{\mathrm{O}}\) :
-
Outer radius (m)
- \(\sigma _{\mathrm{EL}}\) :
-
Elastic stress (Pa)
- \(\sigma _{\mathrm{PL}}\) :
-
Plastic stress (Pa)
- \(\sigma _{\mathrm{r}}\) :
-
Radial stress (Pa)
- \(\sigma _{\theta }\) :
-
Hoop stress (Pa)
- \(\sigma _{\mathrm{Tot}}\) :
-
Total stress (Pa)
- \(P_{\mathrm{I}}\) :
-
Internal pressure (Pa)
- \(P_{\mathrm{O}}\) :
-
External pressure (Pa)
- r :
-
Radial variable (m)
- E :
-
Elastic modulus (Pa)
- \(\nu \) :
-
Poisson’s ratio (\([\text { }]\))
- \(\delta V_{\mathrm{M}}\), \(\delta r_{\mathrm{M}}\) :
-
Infinitesimal increase in metallic volume and thickness (\(\mathrm{m}^{3}\), m)
- \(s_{c,I}\) :
-
Internal failure thickness (m)
- \(s_{c,O}\) :
-
External failure thickness (m)
- u :
-
Infinitesimal radial displacement (m)
- (\(\delta V\)) , V :
-
(Incremental) volume (\(\mathrm{m}^{3}\))
- \(\delta r_{\mathrm{PL}}\), \(\delta V_{\mathrm{PL}}\) :
-
Vertical (radial) swelling and volume change due to oxidation (\(\mathrm{m},\mathrm{m}^{3}\))
- \(\delta r_{\mathrm{EL}}\) :
-
Vertical (radial) swelling due to Poisson’s effect (m)
- \(\delta r\) :
-
Total infinitesimal swelling (m)
- \(\delta x_{\mathrm{PL}}\) :
-
Horizontal swelling due to oxidation (m)
- K :
-
Bulk modulus (Pa)
- (dt), t :
-
(Incremental) time (s)
- C :
-
Concentration of oxygen (\(\mathrm{mol}\,\mathrm{m}^{-3}\))
- \(C_{0}\) :
-
Concentration of oxygen in water (\(\mathrm{mol}\,\mathrm{m}^{-3}\))
- a :
-
Areal coefficient (\(\mathrm{m}^{2}\))
- k :
-
Reaction (corrosion) constant (\(\mathrm{s}^{-1}\))
- \(N_{\mathrm{O}}\) :
-
Moles of filled oxygen (\([\text { }]\))
- s :
-
Thickness of oxide scale (m)
- \(s_{\mathrm{c}}\) :
-
Critical thickness of oxide scale for failure (m)
- \(A_{\mathrm{OX}}\) :
-
Area of oxide (\(\mathrm{m}^{2}\))
- M :
-
Molar mass (\(\mathrm{gr}\,\mathrm{mol}^{-1}\))
- \(\sigma _{\mathrm{uc}}\) :
-
Ultimate compressive strength (Pa)
- \(k_{\mathrm{B}}\) :
-
Boltzmann constant (\(\mathrm{J}\,\mathrm{K}^{-1}\))
- \(\delta V_{\mathrm{PL}}\) :
-
Infinitesimal oxide volume (\(\mathrm{m}^{3}\))
- \(R_{\mathrm{PB}}\) :
-
Pilling-bedworth ratio (\([\text { }]\))
- dr :
-
Infinitesimal radial variation (m)
- \({\rm d}\theta \) :
-
Infinitesimal azimuthal variation (\([\text { }]\))
References
A. Neil North and D. MacLeod. Corrosion of metals, in Conservation of Marine Archaeological Objects (Elsevier, 1987), pp. 68–98
H.H. Strehblow and P. Marcus. Fundamentals of corrosion, Corrosion Mechanisms in Theory and Practice, pp. 1–104 (2012)
D.R. Knittel and A. Bronson, Corrosion 40(1), 9 (1984).
P. Bossis, D. Pecheur, K. Hanifi, J. Thomazet, and M. Blat, Comparison of the high burn-up corrosion on m5 and low tin zircaloy-4, in Zirconium in the Nuclear Industry: 14th International Symposium. ASTM STP 1467, 494 (2006).
A.S. Zaimovskii, Soviet Atom. Energy 45(6), 1165 (1978).
R.A. Causey, D.F. Cowgill, and R.H. Nilson, Review of the Oxidation Rate of Zirconium Alloys Report (Sandia National Laboratories, 2005)
E. Hillner, Zirconium Nucl. Ind. 633, 211 (1977)
A.T. Motta, A. Yilmazbayhan, J. Marcelo, G. da Silva, R.J. Comstock, G.S. Was, J.T. Busby, E. Gartner, Q. Peng, Y.H. Jeong, and J.Y. Park, J. Nucl. Mater. 371(1), 61 (2007).
A.A. Kharkov, A.A. Alkhimenko, N.O. Shaposhnikov, and E.L. Alekseeva, Mater. Phys. Mech. 47(3), (2021)
A. Contreras, M. Salazar, A. Albiter, R. Galván, and O. Vega, Assessment of stress corrosion cracking on pipeline steels weldments used in the petroleum industry by slow strain rate tests (Arc Welding, IntechOpen Zagreb, Croatia, 2011), p. 144
A.H. Akhi and A.S. Dhar, Eng. Fract. Mech. 250, 107778 (2021)
Y. Qin, V. Litvinov, W. Chassé, J. Sun, and Y. Men, Polymer 252, 124938 (2022)
I.V. Ryakhovskikh and R.I. Bogdanov, Eng. Fail. Anal. 121, 105134 (2021)
Z. Wang, P. Wang, D. Zeng, T. Shi, and W. Deng, Materials 15(3), 801 (2022)
C. Lin and H. Ruan, Electrochimica Acta 395, 139196 (2021)
C. Cui, R. Ma, and E. Martínez-Pañeda, J. Mech. Phys. Solids 147, 104254 (2021)
B. Cox, J. Nucl. Mater. 336(2), 331 (2005).
B. Cox, J. Nucl. Mater. 170(1), 1 (1990).
A.T. Motta, A. Couet, and R.J. Comstock, Annu. Rev. Mater. Res. 45, 311 (2015).
D.J. Young, High Temperature Oxidation and Corrosion of Metals, vol. 1 (Elsevier, Berlin, 2008)
T. El Maaddawy and K. Soudki, Cem. Concrete Compos. 29(3), 168 (2007).
T.R. Allen, R.J.M. Konings, and A.T. Motta, Compreh. Nucl. Mater. 5, 49 (2012).
P. Jacques, F. Lefebvre, and C. Lemaignan, J. Nuclear Mater. 264(3), 239 (1999).
S. Kass, J. Nucl. Mater. 29(3), 315 (1969).
C.M. Eucken, P.T. Finden, S. Trapp-Pritsching, and H.G. Weidinger, Influence of chemical composition on uniform corrosion of zirconium-base alloys in autoclave tests. in Zirconium in the Nuclear Industry Eighth International Symposium, (ASTM International, 1989) 3
G.S. Frankel, Fundamentals of Corrosion Kinetics (Springer, 2016), pp.17–32.
P. Jacques, F. Lefebvre, and C. Lemaignan, J. Nucl. Mater. 264(3), 249 (1999).
K. Forsberg, M. Limback, and A.R. Massih, Nucl. Eng. Des. 154(2), 157 (1995).
B. Lustman and F. Kerze, The Metallurgy of Zirconium, vol. 4 (McGraw-Hill Book Company, 1955)
E. Alat, J. Hu, D. Wolfe, and A. Motta. Corrosion and ion irradiation behavior of ceramic-coated nuclear fuel cladding, in Zirconium in the Nuclear Industry: 19th International Symposium (ASTM International, 2021), pp. 149–171.
N. Lin, Q. Liu, J. Zou, D. Li, S. Yuan, Z. Wang, and B. Tang, RSC Adv. 7(22), 13517 (2017).
G.P. Sabol and G.D. Moan, in Zirconium in the Nuclear Industry: Twelfth International Symposium (ASTM International, 2000), vol. 1354
M. Reyes, A. Aryanfar, S.W. Baek, and J. Marian, J. Nucl. Mater. 509, 550 (2018).
A.T. Motta, L. Capolungo, L.-Q. Chen, M.N. Cinbiz, M.R. Daymond, and D.A. Koss, J. Nucl. Mater. 518, 440 (2019).
M. Preuss, A review of early findings within the collaborative research programme muzic: Mechanistic understanding of zirconium corrosion, in Zirconium in the Nuclear Industry: 19th International Symposium (ASTM International, 2021)
J. Li, M. Elboujdaini, B. Fang, R.W. Revie, and M.W. Phaneuf, Corrosion 62(4), 316 (2006).
M. Mermoux and C. Duriez, J. Raman Spectrosc. 52(12), 2131 (2021).
A.R. Khasanova, J. Phys. Conf. Ser. 2176, 012051 (2022)
K. Wilczynska, M. Bono, D. Le Boulch, M. Fregonese, V. Chabretou, N. Mozzani, and L. Barbie, Development and validation of a new experimental device for studies of iodine stress corrosion cracking of zirconium alloys, in 19th International Conference on Environmental Degradation of Materials in Nuclear Power Systems-Water Reactors (2019)
M. Kimura, N. Totsuka, T. Kurisu, K. Amano, J. Matsuyama, and Y. Nakai, Corrosion 45(4), 340 (1989).
B. Gwinner, F. Balbaud-Célérier, P. Fauvet, N. Gruet, P. Laghoutaris, F. Miserque, and R. Robin, Corros. Sci. 201, 110284 (2022).
Y. Li, C. Ge, Y. Liu, G. Li, X. Dong, G. Zongxing, and Y. Zhang, Int. J. Min. Metall. Mater. 29(4), 586 (2022).
X.-F. Ma, Y.-W. Wu, J. Tan, C.-Y. Meng, L. Yang, W.-A. Dang, and X.-J. He, Surf. Coat. Technol. 358, 521 (2019).
Q.S. Chen, C.H. Liu, J.P. Long, J. Wang, R.Q. Zhang, H.Y. Yang, W. Zhang, F.Y. Yao, S. Zhao, and Q. Zhang, Mater. Res. Exp. 6(8), 086511 (2019).
X. Sun, F. Gong, M. Hao, W. Lei, C. Yin, Z. Sun, and R. Xiao, Appl. Surf. Sci. 582, 152484 (2022).
B.-H. Choi, A. Chudnovsky, and K. Sehanobish, Int. J. Fract. 145(1), 81 (2007).
S. Mohanty, S. Majumdar,and K. Natesan, A Review of Stress Corrosion Cracking/Fatigue Modeling for Light Water Reactor Cooling System Components (Nuclear Engineering Division Argonne National Laboratory, Argonne, IL, 2012)
X. Guo, L. Junqiang, P. Lai, Z. Shen, W. Zhuang, Z. Han, L. Zhang, and S. Lozano-Perez, Corros. Sci. 202, 110300 (2022).
C. Tang, M. Große, S. Ulrich, M. Klimenkov, and U.H. Jürgen, Surf. Coat. Technol. 419, 127263 (2021).
H. Mehrer, Diffusion in solids under pressure, in Defect and Diffusion Forum (Trans Tech Publ, 2011), vol. 309, pp. 91–112.
F.-Z. Xuan, S.-S. Shao, Z. Wang, and S.-T. Tu, J. Phys. D Appl. Phys. 42(1), 015401 (2008).
R.L. De Orio, H. Ceric, and S. Selberherr, Microelectron. Reliab. 50(6), 775 (2010).
M. Ahammed and R.E. Melchers, Int. J. Press. Vessels Pip. 69(3), 267 (1996).
S.-X. Li, S.-R. Yu, H.-L. Zeng, J.-H. Li, and R. Liang, J. Pet. Sci. Eng. 65(3–4), 162 (2009).
T.A. Bubenik, R.J. Olson, D.R. Stephens, and R.B. Francini. Analyzing the pressure strength of corroded line pipe, in Proceedings of the International Conference on Offshore Mechanics and Arctic Engineering (American Society of Mechanical Engineers, 1992), pp. 225–225.
F.A.V. Bazána and A.T. Beck, Corros. Sci. 74, 50 (2013).
P.R. Bergethon, Flow in a chemical potential field: diffusion, in The Physical Basis of Biochemistry (Springer, 1998), pp. 445–454.
I.A. Blech and C. Herring, Appl. Phys. Lett. 29(3), 131 (1976).
M. Ganser, F.E. Hildebrand, M. Klinsmann, M. Hanauer, M. Kamlah, and R.M. McMeeking, J. Electrochem. Soc. 166(4), H167 (2019).
C.O. De González and E.A. García, Appl. Surf. Sci. 44(3), 211 (1990).
A.J. Bard and L.R. Faulkner, Electrochemical Methods: Fundamentals and Applications, vol. 2 (Wiley, New York, 1980)
R.H. Nielsen, J.H. Schlewitz, H. Nielsen, and Updated by Staff. Zirconium and zirconium compounds. Kirk-Othmer Encyclopedia of Chemical Technology (2000)
D.R. Lide, CRC Handbook of Chemistry and Physics, vol. 85 (CRC Press, 2004)
S.D. Cramer, B.S. Covino Jr., C. Moosbrugger, B.R. Sanders, G.J. Anton, N. Hrivnak, J. Kinson, C. Polakowski, K. Muldoon, S.D. Henry et al., ASM Handbook, vol. 13 (ASM international Materials Park, Ohio, 2003)
J.J. Duderstadt and L.J. Hamilton, Nuclear Reactor Analysis (Wiley, 1976)
X.Y. Zhang, M.H. Shi, C. Li, N.F. Liu, and Y.M. Wei, Mater. Sci. Eng. A 448(1–2), 259 (2007).
F.A. Gabr, J. Ferrandis, D. Baron, and P. Chantoin, Pressure and composition of gas mixtures in fuel rods for pressurised water reactors by an ultrasonic sensor, in International Conference on WWER Fuel Performance, Modelling and Experimental Support, Varna, Bulgaria (2003)
R. Kirchheim, Acta Metallurgica et Materialia 40(2), 309 (1992).
E. Hillner, D.G. Franklin, and J.D. Smee, J. Nuclear Mater. 278(2–3), 334 (2000).
P.F. Weck, E. Kim, V. Tikare, and J.A. Mitchell, Dalton Trans. 44(43), 18769 (2015).
J. Chakrabarty and W.J. Drugan, J. Appl. Mech. 55, 253 (1988).
S. Silvano, Mathematical model of the lame’problem for simplified elastic theory applied to controlled-clearance pressure balances. Preprint arXiv:1007.0813 (2010)
A. Aryanfar, I.I.I.W. Goddard, and J. Marian, Corros. Sci. 158, 108058 (2019).
X. Chunhua and W. Gao, Mater. Res. Innov. 3(4), 231 (2000).
D.G. Peters, H.R. Franklin, and R.B. Adamson, in Sixth International Symposium on Zirconium in the Nuclear Industry (ASTM STP 824, American Society for Testing and Materials, 1984) p. 507
S.B. Dalgaard, Extended abstracts of the electrochem- ical society, washington, dc, usa. pp. 82. ASTM STP 824 (1976)
F. Garzarolli, W. Jung, H. Schoenfeld, A.M. Garde, G.W. Parry, and P.G. Smerd, Waterside corrosion of zircaloy fuel rods. final report. Technical report, Kraftwerk Union AG (1982)
H. Stehle, W. Kaden, and R. Manzel, Nucl. Eng. Des. 33(2), 155 (1975).
H.C. Berg, Diffusion: microscopic theory, in Random Walks in Biology (Princeton University Press, 2018), pp. 5–16.
Acknowledgements
The authors would like to thank the support from Masri Institute at American University of Beirut, Grant Award No. 103919 for the student Abdel Rahman El Tallis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing financial interests to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Here, we present the detailed steps for establishing the relationships shown in the article.
1. Elastic Stress \(\sigma _{\mathrm{EL}}\) (Eq. 7)
Imposing the internal and external pressures \(P_{\mathrm{I}}\) and \(P_{\mathrm{O}}\), the radial \(\sigma _{r}\) and hoop \(\sigma _{\theta }\) elastic stresses develop, as shown in Fig. 1b which are theonly function of radius r due to polar symmetry. Cutting out the infinitesimal element shown in Fig. 2b, the balance relationships in the horizontal direction would be:
therefore:
where by simplification and ignoring the higher-order terms, we arrive at Lame’s relationship as:71,72
Regarding the strain \(\epsilon \), if u is the infinitesimal displacement in the radial direction, from the Hook’s generalized law, one gets:71
Solving for the radial \(\sigma _{r}\) and hoop \(\sigma _{\theta }\) stresses and replacing in terms of the infinitesimal displacement, u, one gets:
replacing the Eq. 39 into the compatibility Eq. 5 and simplifying we get:
where integrating leads to:
From Fig. 1b, assuming the \(\{R_{\mathrm{I}},R_{\mathrm{O}}\}\) are inner and outer radii of the pipe, respectively, and assigning the boundary conditions for the compressive stresses as \(\sigma _{r}\left( R_{\mathrm{I}}\right) =-P_{\mathrm{I}}\) and \(\sigma _{r}\left( R_{\mathrm{O}}\right) =-P_{\mathrm{O}}\) , the hoop stress \(\sigma _{P}\) is finally obtained as:
where A and B are constants obtained by the following relationships:
Since the compressive stress causing the failure is the hoop direction, therefore \(\sigma _{P}=\sigma _{\theta }\) , and hence Eq. 7 is obtained as:
which is the compressive stress generated by the boundedness in the azimuthal (i.e., hoop) direction.
2. Corrosion Stress (Eq. 17)
3. Corrosion Stress (Eq. 21)
4. Equation 23
Translating Eq. 22 into a 1D radial direction, we get:
The \(\sigma _{r}\) is obtained from Eqs. 40 and 41, therefore: \({\displaystyle \frac{\partial \sigma _{r}}{\partial r}=-\frac{2B}{r^{3}}}\), replacing gives:
and the coefficients \(\alpha \) and \(\beta \) are obtained, respectively, as:
5. Equation 29
In order to have a stable solution, we need to have \(Q_{1}>0\), hence:
replacing the \(\alpha \) and \(\beta \) values gives:
which means that:
Rights and permissions
About this article
Cite this article
Aryanfar, A., El Tallis, A.R. & Marian, J. Coupling the Corrosion-and Pressure-Assisted Stress Buildup Within the Zirconium in PWR Pipes. JOM 75, 120–131 (2023). https://doi.org/10.1007/s11837-022-05503-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-022-05503-w