Abstract
Gassing, outbursts of high-rate gas flows through the taphole, is a phenomenon that sometimes occurs during tapping of silicon furnaces. In addition to being an HSE challenge, this might also affect the tapping, while the warm gas might harm the equipment in the tapping area. The pressure above the metal bath, influencing the gassing, is affected by permeability, and the gas flow through the charge materials in the furnace. Pressure drop and permeability have been measured for different gas velocities through particle beds of different charge mixtures, with the raw materials and particle sizes used in industrial silicon production. The experimental results have been upscaled to conditions in industrial furnaces through modeling flow in a packed bed. The findings are compared with results from measurements of pressure drop in industrial furnaces.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metallurgical silicon (Si) and ferrosilicon (FeSi) are produced in submerged arc furnaces, in which the main raw materials are quartz plus carbon reductants, such as coal, charcoal, and woodchips. For FeSi production, iron sources are also added. Electrical energy, around 10–13 MWh/ton alloy for Si production, is another important input factor, which is supplied through the electrodes. The reduction of silica to Si takes place by the overall reaction (1). In industrial furnaces, the reactions take place in several steps, as described by Schei et al.1
Both a high silicon yield and a stable furnace operation are needed to achieve good results and low energy consumption. In industrial Si and FeSi production, this will be a result of interaction between several chemical reactions taking place in the same reactor, as well as mass and energy transfer. Even flow of evolved gases is crucial to obtain a stable furnace operation that contributes to good economic results and to ensure stable tapping without gassing. The effect of raw material sizing on gas flow has been investigated.
The Silicon Processes
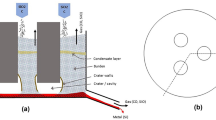
Furnace excavations during recent years2 have established a schematic of the inner structure of a Si furnace, given, with the overall reactions, in Fig. 1.
The raw materials are fed at the top of the charge mixture, where the temperature is around 700–1300°C.3 Liquid Si, at approximately 1800°C,4 is tapped at the bottom of the furnace. Off-gas, composed mainly of CO and SiO, leaves the charge at the charge top, where it burns to CO2 and SiO2. To obtain a high silicon yield, the off-gas must have a low SiO content. To obtain a good furnace performance, there must be an even gas flow and a good charge permeability in the furnace.
Raw materials fed at the top of the charge descend into the furnace, where they are heated and react with ascending gases, according to the reactions given below. Their reaction mechanism is not part of the current study and not further discussed. In the lower, high-temperature part of the furnace, quartz is reduced to SiO by reactions (5) and (6). Some of the produced SiO will react with SiC in the high-temperature zone, producing Si according to reaction (7). SiO and CO- gas, produced by reactions (5–7) in the high-temperature zone, ascend through the partly reacted charge mixture. SiO will, at lower temperatures, around 1600°C, in the upper part of the furnace, react with carbon, producing SiC according to reaction (4). SiO gas that is not consumed by reactions (4) or (7) may be captured in the charge by reactions (2) and (3), producing a sticky impermeable Si-SiC-SiO2 mixture, commonly referred to as “SiO condensate”. The remaining SiO will escape at the charge top and lead to decreased Si yield.
All enthalpy values are calculated by HSC.5
An effective production of Si requires that the reactions with SiO2, reactions (5) and (6), in the upper part of the furnace are slow and governed by kinetics, while the reaction between SiO-gas and carbon, reaction (4), is fast and close to equilibrium. The final production of Si in the lower part of the furnace, reaction (7), has not been much investigated, but a fast reaction rate will be an advantage. The reaction sequence involves both highly endothermic and highly exothermic reactions. Their extent depends on, and interacts with, the temperature profile in the furnace. This varies with furnace design and produced alloy quality, as well as furnace operation and distance from the electrodes. An average heating rate of 5.5°C/min and a material flow of around 0.3 m/h has earlier been estimated6 as normal for Si furnaces. Modeled temperature profiles for selected Si and FeSi furnaces7 indicate that the temperature does not increase linearly with increasing furnace depth. The raw materials are heated quickly up to around 1600°C in the upper part of the furnace. In most of the furnace volume above the crater zone, the temperature is between 1600°C and 1800°C. The heating is here much slower, at around 0.5°C/min.
the extent of the various reactions determines the amount of gases produced or consumed in the different zones of the furnace. Together with temperature, this will determine the volume of gas and the gas flow rate in different parts of the furnace. Pressure and pressure drop are in addition affected by the permeability of the solid bed, which is a mixture of the raw materials and partly reacted charge.
Gas Flow and Pressure Drop in Si Furnaces
In an ideal Si furnace, in which all the added quartz reacts with Si according to reaction (1), and the gas is ideal, 2 mol of CO gas is evolved for each mol of Si produced. Thus, production of 1 kg of Si generates around 1,75 m3 of CO at 25°C. At higher temperatures, as in the high-temperature zone of the furnace, this will be higher: at 1800°C, it will be around 12 m3. A 40-MW furnace, using 10 MWh/ton, will thus produce nearly 2 m3/s of CO gas at 25°C. Thus, each second, around 1 L of condensed materials will be converted to more than 2 m.3 of gas,6 as illustrated in Fig. 2. It has been estimated that, in an industrial Si-furnace, the available space between the solid particles is similar to about 10 s of gas production.6
Inner structure of an Si-furnace and the reactions that take place in different parts of the furnace. Adapted from Ref. 2.
Schematic of gas evolution in an Si furnace.6
Industrial measurements of the pressure drop under two of the three electrodes in a 40-MW furnace3 showed a variation between 30 mbar and 140 mbar, as seen in Fig. 3. Most of the time, the pressure drop is around 20–50 mbar, with some peaks up to 140 mbar. The peaks are assumed to be the result of clogging due to condensation by reactions (2) and (3), while the lower pressure drop is assumed to be a consequence of the permeability in the charge mixture.
Industrial measurements of pressure under two of the three electrodes in a 40-MW Si furnace.10
Pressure in the crater zone has been modeled by Kadkhodabeigi,7 with a focus on tapping. The pressure that is built up here can be released in two main ways, either upwards through channels in the charge bed, or outwards through the tap hole. Both cases give gas jets with SiO-CO with a high temperature. They pose a safety risk, as well as a negative impact on the environment. This is a major HSE concern in the tapping area. To reduce gassing from the tap hole is thus an important objective for many Si producers.
To reduce or avoid gassing through the tap hole, a good upwards flow of the large gas volumes is required. The charge mixture and partly reacted charge must then have a high permeability. This is often related to a low amount of fines, e.g., the amount of small particles, and, overall, to all size distribution. Fines can be both a part of the raw materials fed to the furnace and formed by decrepitation of raw material during heating. Usually, carbon materials in the size range of 1–30 mm and 10–150 mm quartz are the main part of the raw material mixture. Larger pieces of wood chips, up to above 100 mm wide and often less than 10 mm thick, are also often included. Quartz will decrepitate to fines during heating, both by heating in the charge mixture and by thermal shock when it meets the charge surface that has a temperature of 700–1300°C.8 The amount of fines that is generated varies strongly with the quartz source.9 The “condensate” created by reactions (2) and (3) will in many cases glue particles together, and form a layer or crust with very low permeability, that may have a higher effect on the gas flow than fines in the raw materials. In most silicon-producing furnaces, this is broken up with stoking to improve both the upwards flow of gases and the downwards flow of solid materials.
The main question that is raised here is what makes up the fines: which sizes and size distributions are critical in different conditions and so should be avoided. A quantification of critical size distribution is the main basis for raw material specifications, as well as the criteria for strength and decrepitation tests. The aim of the current work is thus to contribute both to quantify critical size distributions and to a better understanding of the effect on furnace performance of different sizes and size distributions of the raw material mix.
The presented experimental and modeling work have been mainly carried out as a summer job, project, and MSc by Edfeldt10 and Nordhus.11 This paper summarizes the results and discusses their impact on industrial Si production.
Methodology
The effect of raw material sizing on the pressure drop in charge mixtures for Si production has been investigated for charge mixtures made from industrial coal, quartz, and woodchips, In the experiments with woodchips, the charge was a mixture of 53% quartz, 28% coal (around 5 mm), and 19% woodchips. When woodchips were not included, the charge was a mixture of 55% quartz and 45% coal. The ratios were calculated based on the fixed carbon needed for reaction (1) In addition, a charge of only quartz was studied. The materials were screened to designated their size distributions. Industrial woodchips were too large for the test rig and manually were cut to designated sizes. Properties of the raw materials other than sizing were not regarded as relevant for the pressure drop investigations and are not included.
A custom-built test rig [12], shown in Fig. 4, was used for the measurement of the pressure drop under different conditions.
Test rig for measuring pressure drop in charge mixtures. In some of the experiments, the charge was filled to 10 cm instead of 40 cm above the highest pressure sensor.10
The test rig was filled with cold raw materials of the designated type and mixture with the chosen size distribution. It could be filled in homogenously mixed or in layers. Particles in the size range 0.25–10 mm were used. Air was blown in at the bottom of the cylinder through a porous disk. Gas flow was controlled, and the gas velocity was increased continuously during the experiment, from 0 m/s and up to 1.8 m/s, lower in some experiments. Pressure was measured continuously at three different levels, at the bottom, in the middle, and 30 cm above bottom, and, in the upper part, 60 cm above the bottom of the rig. Gas blown into raw materials not earlier exposed to gas flow will move the materials and create channels. To see the effect of earlier exposure to high gas velocities, gas, with the same increase in velocities from 0 m/s to 1.8 m/s, was blown a second time through the charge mixture, run 2, with continuous pressure measurements, as in run 1.
The charge in Si furnaces has been regarded as a packed bed, and the gas flow is assumed to be laminar. In the initial experiments, the pressure drop was modeled by Darcy’s law.11 For upscaling to conditions in industrial Si furnaces, and for later experiments, Ergun's law (8) was used.11 The pressure drop over a packed bed was then calculated as a function of the volumetric flux:
where \(\Delta P\) is the pressure drop, \(H\) is the height of the packed bed, \(V\) is the volumetric flux corresponding to the fluid velocity in reference to an empty bed, \(\varepsilon \) is the void fraction of the packed bed, \(\rho \) is the density of the fluid, \(\mu \) is the viscosity of the fluid, \({d}_{p}\) is the equivalent spherical diameter of the particles, and \(\varphi \) is the particle sphericity? A more simplified model for predicting the pressure drop, the Darcy–Forecheimer equation, has also been used:
where \({k}_{1}\) and \({k}_{2}\) can be determined by fitting of the experimental data and describe the effect of the unknown material parameters, respectively.
Measured Pressure Drops in Charge Mixtures at Laboratory Scale
A charge mixture of quartz and coal mixed with woodchips represents the upper zone in the furnace, the unreacted charge. Lower down in the furnace at higher temperatures, the woodchips have been calcined and reacted. The charge mixture is here assumed to be a mixture of only coal and quartz. The effect of “condensates” layers with low permeability has not been investigated. The pressure drop in these comes in addition to the pressure drop in the packed bed of raw materials investigated here.
Channels, that were created the first time gas was blown through the materials not earlier exposed to gas flow, changed the distribution of materials. As a result of the new conditions, the pressure drop measured the second time gas is blown through the charge mixture (run 2) is different from the pressure drop measured during the first gas flow (run 1). The materials can be moved upwards and redistributed to the channels, or parts of the charge mixture can be lifted, as shown by the examples from the experimental test rig in Fig. 5. In most of the cases in the laboratory-scale experiments, the pressure drop was slightly lower the second time. The materials may have been disturbed and moved even with the first relatively low gas flows when the first-time gas was blown through the materials. The measured difference in pressure drop is thus often smaller than expected from the observed disturbance of the materials. The difference is larger when woodchips are included in the charge mixture, as seen in Fig. 6.
Examples of disturbed materials after gas flow: (a) channel formation in 0–0.25 mm quartz mixed with coal > 5 mm, (b) coal, 2.0–2.35 mm mixed with 4.75–10 mm quartz transported to the top of the charge as a separate layer, (c) charge of 5 mm coal and 0.35–2 mm quartz has been lifted. Adapted from Ref. 11.
Measured pressure drop in experiments with packed bed with 2.0–4.75 mm quartz and 5 mm coal with and without woodchips. Run 1 (continuous lines) Run 2 (dotted lines). Adapted from from Ref. 11.
A charge that has already been exposed to gas flow is regarded as best representing the situation in an industrial furnace. Run 2 is thus used as a basis for most future discussion. Since not all parts of a furnace have been exposed to such gas flows, this must be used with care. The observed movement of the particles is a result of fluidization. The charge mixture is then no longer a packed bed, so other flow regimes must be used for simulation and upscaling. For the smallest particle sizes, fluidization starts at the lowest gas velocities, but all the investigated coal–quartz charge mixtures, with up to 5 mm coal and 10 mm quartz, were fluidized at gas velocities above 1.2 m/s.
Woodchips are normally added to the charge mixture to obtain a more porous charge mix, partly due to the gases formed during drying and partly due to the shape. Woodchips of a type used as raw material in industrial Si production have been used without further characterization in the current experimental investigations. The effect of variations in size and shape of the woodchips have not been investigated here When woodchips are included in the charge mixture, the pressure drop for the higher gas velocities is lower than for a charge mixture without woodchips, as seen in Fig. 6. There is also a larger difference in pressure drop between the first- and second-time gas blown through the charge, with a considerably lower pressure drop the second time.
The pressure drop in the charge mixture depends on the particle size distribution of the involved materials, here quartz and coal, and whether they are homogenously mixed or segregated into layers. The effect of quartz in a separate layer versus a mixed quartz–coal charge has been investigated for different quartz sizes mixed with 5 mm coal. Comparing the two cases in Figs. 7 and 8 shows that the particle size of the quartz has a higher impact on pressure drop than the layer of quartz. However, it has been observed11 that layers of quartz are more easily moved and disturbed than quartz mixed with coal. For both cases, particles smaller than 2 mm give a much higher pressure drop than particles larger than 2 mm.
Charge with 5 mm coal and different quartz sizes. Mixes.11
Charge with 5 mm coal and different quartz sizes, with layers of quartz.11
The effect of particle size on pressure drop at different gas velocities was investigated through a series of experiments, in which coal in the size fractions 0–2 mm, 2–3.35 mm, 3.35 mm, and < 5 mm were mixed with each of the following size fractions of quartz: 0–0.35 mm, 0.25–2 mm, 2–4.75 mm and 4.75–10 mm. Coal only acted as packed bed when mixed with 2–4.75 mm quartz. Nor did quartz in the size 0–0.25 mm act as a packed bed. When 0.25–2 mm quartz was mixed with coal in different sizes, the variations in pressure drop between parallel experiments were too large to see any clear effects. It is assumed that this is due to disturbance of the distribution of such small particles, and that similar phenomena will also be experienced in industrial furnaces.
For packed beds, smaller particles gave the highest pressure drop. A major difference was observed between particles larger than and particles smaller than 2 mm. Gas flows with the same velocity gave a considerably higher pressure drop in a charge with particles smaller than 2 mm than in charge mixtures with larger particles. There was larger spread in the measured data for the smallest particles sizes. The observed trends were similar for all the investigated particle size combinations.
For charge mixtures with the smallest particles, 0–2 mm, and gas velocities around 0.3 m/s, the pressure drop was around 50–80 mbar, higher for small quartz particles than for small coal particles. For charge mixtures with particles larger than 2 mm, pressure drop at such low gas velocities was only around 10 mbar. It did not reach 50 mbar until the gas velocity was as high as around 1 m/s. The difference between particles smaller than 2 mm and larger particles is significant for both small quartz and small coal particles, as seen in Figs. 9 and 10. A similar picture was seen for other charge mixtures.
Pressure drops with increasing gas velocity and various sizes of coal for flow through a charge of 2–4.75 mm quartz mixed with coal. Average pressure values are marked with dots, estimated average drop is given as a solid line, while the 95% confidence intervals are marked with dotted lines.11
Pressure drops with increasing gas velocity and various sizes of quartz for flow through a charge of 2–3.35 mm coal mixed with quartz. Average pressure values are marked with dots, estimated average drop is given as a solid line, while the 95% confidence intervals are marked with dotted lines.11
Simulated Pressure Drops in Charge Mixtures in Industrial Furnaces
Pressure drop in industrial furnaces was, as a first approach, simulated based on measured pressure drops for charge mixtures with 5 mm coal and 4.76–10 mm quartz and woodchips, based on the parameters in Table 1. Pressure drop in the packed bed will also depend on the sphericity of the particles. Quartz particles are nearly spherical, with a sphericity close to 1, while woodchips will have a much lower sphericity.
Simulated pressure drop in a 40-MW Si furnace for different sphericities and particle sizes are shown in Fig. 11. From Fig. 3, it can be seen that the basis level, outside peaks, of pressure drop in an industrial furnace is around 30 mbar,11 as shown in Fig. 6. For particles larger than 4.76 mm and with a sphericity in the actual range, around 0.75–1, the pressure drop is lower than 30 mbar, in line with what is seen in stable periods in Si furnaces.
Simulated pressure drop from gas flow through a packed bed in a 40-MW Si furnace for different sizes of quartz and varying sphericity of the particles. Basis pressure drop in an industrial furnace is also indicated.11
Discussion
High pressure in the crater has been measured in industrial Si furnaces as sudden peaks above a low level of 30–50 mbar.10 Modeling7 has indicated that build-up of high pressure will affect tapping, and might be a main cause for gassing during tapping. In addition, high-pressure build-up in the crater zone will be released by blows of gas through the charge mixture, leading to losses of SiO, giving a lower Si yield. Measurements at the laboratory scale show that gas flow through a packed bed with quartz and coal particles larger than 2 mm will not give a build-up of pressure above the normal level of around 40 mbar.11 Gas velocities up to above 1 m/s, which is relevant, was used in the experimental set-up. Simulation to upscale this indicates that particles above 4.76 mm will not contribute to the build-up of high pressure.10 Industrial Si furnaces contain layers or zones of “SiO condensates” with low permeability.2 It is assumed that these zones will give a build-up of high pressure, and that this is released when the pressure is high enough to break through this “crust”. Formation of SiO, and its back reaction to solid and liquid phases at different levels in the furnace, is thus assumed to affect gassing during tapping.
Raw materials such as quartz and coal with a size above 4.76 mm, and most likely also above 2 mm, will under normal conditions not have a major impact on the build-up of peaks with high pressure. However, particles smaller than 2 mm behave differently, and gas flow through a packed bed of these generates a much higher pressure drop at the experimental scale. Particles smaller than 2 mm might also contribute to build-up of pressure in industrial furnaces. A large amount of such particles could thus possibly affect gassing during tapping. The pressure drops increase with increasing gas velocities. With higher furnace load, more material reacts, and more gas is generated. A charge with a given particle size distribution is thus expected to give a higher pressure drop in a furnace operated with high power than a furnace operated with lower power. Gas volume increases with increasing temperature, and gas velocities as well as pressure drop through the charge, will thus depend on the maximum temperature and the temperature profile in the furnace.
Simulations as well as the discussion above assumes gas flow through a packed bed. The experimental investigations showed that the charge was fluidized for all particle sizes when the gas velocities was above 1.2 m/s.11 Some of the finest particles were fluidized for all the investigated gas velocities. The smaller particles were in addition transported upwards with the gas flow and redistributed to other positions, creating open voids and channels. The charge will thus segregate and cannot be regarded as a homogenous mixed fixed bed. Segregation is assumed to also take place in industrial furnaces, although this has not been investigated and verified.
Conclusion
Experimental investigations of the pressure drop for different gas velocities in charge mixtures for Si furnaces showed major differences in the pressure drop between particles smaller than and larger than 2 mm. Particles smaller than 2 mm give a higher pressure drop. High gas velocities and small particle sizes will give fluidization of the bed and segregation in the charge.
Peaks of high pressure in Si furnaces, and gassing through the tap hole due to this, are most likely not a result of fines in the charge mixture, but caused by “SiO condensates” clogging the charge.
References
A. Schei, J.K. Tuset, and H.Tveit, Production of High Silicon Alloys, (Tapir forlag, Trondheim, Norway, 1998), ISBN: 82-519-1317-9
M. Ksiazek, M. Tangstad, and E. Ringdalen,Five Furnaces Five Different Stories, (Paper presented at Silicon for the Chemical and Solar Industry XIII, Kristiansand, Norway, 2016)
S. T. Johansen, H. Tveit, S. Grådahl, A. Valderhaug, and J. A. Byberg, Environmental Aspects of Ferro-Silicon Furnace Operations-An Investigation of Waste Gas Dynamics, (Paper presented at INFACON 8, Beijing, China 1998)
M. Ksiazek, H.M. Hustad, A. Nymoen, J. Holtan, S. Grådahl, S. Kaukonen, and M. Tangstad,Measurement of Metal Temperature During Tapping of an Industrial FeSi Furnace, (Furnace tapping conference, Kruger National Park South Africa, 2018), pp 14–20, ISBN 978-1-928410-11-9
E. Ringdalen, S. Bao, H. Tveit, and E. Nordnes, Melting Properties of Quartz and Their Effect on Industrial Si and FeSi Production. (Paper presented at International Congress & Exhibition "Non-ferrous Metals and Minerals + ISCOBA conference; 2019–09–16), ISSN 2518-332X
H Tveit, E. Ringdalen, and H. Edfeldt, Important Parameters that contrOl the Gas Pressure in a Silicon Furnace, (Silicon for the chemical Industry XV, Trondheim, 2020), ISBN 978-82-997357-9-7
M. Kadkhodabeigi, Modelling of Tapping Processes in Submerged Arc Furnaces, PhD Thesis, Norwegian University of Science and Technology, Department of Materials Science and Engineering, Trondheim May 2011 ISBN 978-82-471-2841-1 (5)
E. Ringdalen and M. Tangstad, In: Proceedings of International Smelting Technology Symposium (Incorporating the 6th advances in sulphide smelting) held during TMS 2012, ed. by J.P. Downey, T.P. Battle, J.F. White, pp. 195–204. ISBN 978-1-11829-116-0
K.F. Jusnes, M. Tangstad, and E. Ringdalen, Cracks, Disintegration and Phases Transformations in Quartz, Silicon for the Chemical and Solar Industry 14 (Svolvær, Norway, 2018).
H. Edfeldt, Pressure drop in charge mixture in silicon production, Master Thesis, (Norwegian University of Science and Technology, Department of Materials Science and Engineering, Trondheim January 2020)
N. Astrid, Gas Flow in Furnaces for Si-Production, Project Work in TMT4500 (NTNU Trondheim, Norway, 2021).
Acknowledgements
This work has been funded by the Norwegian Ferroalloy Research Association (FFF) and the Research Council of Norway through the RCN projects 267621 Controlled Tapping and 326581 Recursive. They are both acknowledged.
Funding
Open access funding provided by SINTEF.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there are no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ringdalen, E., Einarsrud, K.E. & Nordhus, A. Gas Flow and Pressure Drop in Charge Material in Silicon Production. JOM 74, 3971–3979 (2022). https://doi.org/10.1007/s11837-022-05431-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-022-05431-9