Abstract
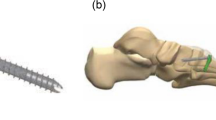
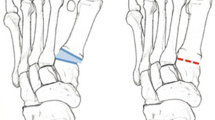
Hallux valgus can be addressed through realignment and arthrodesis of the first tarsometatarsal joint. This study aimed to characterize the biomechanical performance of two implant constructs in a matched-pair cadaveric model. Simulated first tarsometatarsal arthrodesis utilized either a lag screw plus locking plate or nitinol staple construct placed in 90-degree configuration. In situ digital image correlation was performed on fresh-frozen cadaveric specimens to characterize 1st tarsometatarsal gapping during cyclic loading. Interfacial characteristics were analyzed with gap displacement allowed by the implant following cyclical loading. The locking plate and nitinol staple constructs gapped 1.367 ± 0.917 mm and 2.116 ± 0.8934 mm, respectively, under 50 N. Removing load, the locking plate and nitinol staple constructs measured 0.3898 ± 0.3787 mm and 0.673 ± 0.5467 mm of residual gapping, respectively. In our cadaver model, both constructs maintained compression and residual stability of the first tarsometatarsal joint gap under 3 mm. Contrary to our synthetic bone study, the locking plate constructs provided statistically superior performance during loading compared to nitinol staples.











Similar content being viewed by others
References
M.J. Coughlin, C.P. Jones, R. Viladot, P. Glanó, B.R. Grebing, M.J. Kennedy, and F. Alvarez, Foot Ankle Int. 25, 537. https://doi.org/10.1177/107110070402500805 (2004).
J.K. Ellington, M.S. Myerson, J.C. Coetzee, and R.M. Stone, Foot Ankle Int. 32, 674. https://doi.org/10.3113/FAI.2011.0674 (2011).
J.S. Kim, H.K. Cho, K.W. Young, J. Kim, and K.T. Lee, Clin. Orthop. Surg. 9, 514. https://doi.org/10.4055/cios.2017.9.4.514 (2017).
E. So, B. Van Dyke, M.R. McGann, R. Brandao, D. Larson, and C.F. Hyer, Foot Ankle Surg. 58, 62. https://doi.org/10.1053/j.jfas.2018.08.010 (2019).
S. Nix, M. Smith, and B. Vicenzino, Foot Ankle Res. 3, 1. https://doi.org/10.1186/1757-1146-3-21 (2010).
P.E. Scranton, J.C. Coetzee, and D. Carreira, Foot Ankle Int. 30, 341. https://doi.org/10.3113/fai.2009.0341 (2009).
P. Sztefek, M. Vanleene, R. Olsson, R. Collinson, A.A. Pitsillides, and S. Shefelbine, J. Biomech. 43, 599. https://doi.org/10.1016/j.jbiomech.2009.10.042 (2010).
M.A. Sutton, J.J. Orteu, and H.W. Schreier, Image Correlation for Shape Motion and Deformation Measurements (Springer, Boston, 2009), pp 1–37.
R. Ghorbani, F. Matta, and M. Sutton, Exp Mech. 55, 227. https://doi.org/10.1007/s11340-014-9906-y (2014).
M.A. Sutton, J. Yan, X. Deng, C.S. Cheng, and P.D. Zavattieri, Opt. Eng. 46, 1. https://doi.org/10.1117/1.2741279 (2007).
J.D. Helm, S.R. McNeil, and M.A. Sutton, Opt. Eng. 35, 1911. https://doi.org/10.1117/1.600624 (1996).
M.N. Rossol, J.H. Shaw, H. Bale, R.O. Ritchie, D.B. Marshall, and F.W. Zok, J. Am. Ceram. Soc. 96, 2362. https://doi.org/10.1111/jace.12468 (2013).
V.C. Shen, C.H. Bumgardner, L. Actis, J. Ritz, J. Park, and X. Li, Clin. Biomech. 71, 29. https://doi.org/10.1016/j.clinbiomech.2019.10.011 (2020).
J.M. Cottom, and J.S. Baker, Foot Ankle Spec. 10, 227. https://doi.org/10.1177/1938640016676341 (2017).
E.A. Friis, Mechanical Testing of Orthopaedic Implants (Woodhead Publishing, Cambridge, 2016), pp 231–253.
K. Klos, B. Gueorguiev, T. Mückley, R. Frober, G.O. Hofmann, K. Schwieger, and M. Windolf, Foot Ankle Int. 31, 158. https://doi.org/10.3113/fai.2010.0158 (2010).
B. Campbell, P. Schimoler, S. Belagaje, M.C. Miller, and S.F. Conti, J. Orthop Surg. Res. 12, 1. https://doi.org/10.1186/s13018-017-0525-z (2017).
A.T. Scott, J.K. DeOrio, H.E. Montijo, and R.R. Glisson, Clin. Biomech. 25, 271. https://doi.org/10.1016/j.clinbiomech.2009.12.006 (2010).
A. Aiyer, N.A. Russell, M.H. Pelletier, M. Myerson, and W.R. Walsh, Foot Ankle Spec. 9, 232. https://doi.org/10.1177/1938640015620655 (2015).
D. Drummond, T. Motley, V. Kosmopoulos, and J. Ernst, Foot Ankle Surg. 57, 466. https://doi.org/10.1053/j.jfas.2017.10.025 (2018).
O.N. Schipper, S.E. Ford, P.W. Moody, B. Van Doren, and J.K. Ellington, Foot Ankle Int. 39, 172. https://doi.org/10.1177/1071100717737740 (2018).
C.C. Dock, K.L. Freeman, J.C. Coetzee, R.S. Mcgaver, and M.R. Giveans, Foot Ankle Orthop. 5, 1. https://doi.org/10.1177/2473011420944904 (2020).
E.J. Luger, M. Nissan, A. Karpf, E.L. Steinberg, and S. Dekel, J. Bone Jt. Surg. 81, 199. https://doi.org/10.1302/0301-620x.81b2.9353 (1999).
Q.J. Hoon, M.H. Pelletier, C. Christou, K.A. Johnson, and W.R. Walsh, J. Exp. Orthop. 3, 19. https://doi.org/10.1186/s40634-016-0055-3 (2016).
T.L. Bredbenner, and R.H. Haug, Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 90, 574. https://doi.org/10.1067/moe.2000.111025 (2000).
F. O’Neill, F. Condon, T. McGloughlin, B. Lenehan, C. Coffey, and M. Walsh, Bone Jt. Res. 1, 50. https://doi.org/10.1302/2046-3758.14.2000044 (2012).
J. Kraus, M.J. Ziegele, M. Wang, and B. Law, Foot Ankle Orthop. 6, 1. https://doi.org/10.1177/24730114211026934 (2021).
Acknowledgements
We would like to thank BioMedical Enterprises for providing the implant hardware. We would also like to acknowledge MedCure as well as Dr. David Moyer and Kerrie May-Nikstaitis of Surgical Skills Training Center and Gross Anatomy Lab at the University of Virginia for their assistance.
Funding
Funding was provide by DePuy Synthes Spine.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Park is a paid speaker/consultant for DePuy Synthes and performed the surgical implantation of all arthrodesis constructs. He was not involved in the testing or characterization of the constructs.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shen, V.C., Bumgardner, C., Actis, L. et al. In Situ Deformation of First Tarsometatarsal Arthrodesis Implants with Digital Image Correlation: A Cadaveric Study. JOM 74, 3357–3366 (2022). https://doi.org/10.1007/s11837-022-05391-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-022-05391-0