Abstract
Creep-rupture behavior and microstructural response in alumina-forming austenitic (AFA) alloys with two different precipitation strengthening mechanisms, “Laves-phase + M23C6 carbide” and “coherent L12 γ′-Ni3(Al,Ti),” were explored as “model” cases of multi-phase, multi-scale heat-resistant AFA alloys for 650–750°C use. These alloys will be used to guide and verify computational alloy design and life-prediction modeling under an on-going eXtremeMAT project through the Office of Fossil Energy and Carbon Management, US Department of Energy. Computational thermodynamics were used to design and predict the amounts of strengthening and deteriorating secondary phases at 750°C. Creep-rupture lives of the alloys tested at 750°C and 100 MPa were in a range of 4000–9000 h, and the microstructure at the gage/grip after creep-rupture testing was compared with isothermally aged alloys for 1500 h, as well as the calculated phases. Detailed microstructure characterization includes phase identification, volume fraction measurement, and compositional analysis, which were correlated with the creep-rupture properties. High-temperature oxidation resistance was also screened and compared with commercial, chromia-forming heat-resistant steels. These model alloys also provide the basis for further design and optimization of next generation AFA alloys with improved creep resistance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alloy design strategies for high-temperature structural materials strongly depend on the combined requirements from target applications (e.g., heat-exchangers, steam generators, tubes/pipes, gas turbines, production furnaces, chemical/petrochemical plant components, etc.), in which the materials are to be exposed to various harsh environments including either static or dynamic stress conditions, different levels of oxidizing/corrosive environments, and elevated temperatures.1,2,3 The balance among mechanical properties, surface protection, manufacturability, and cost affordability is key for material selection, and these features must always be considered and satisfied in any new alloy designs. Commercially available heat-resistant steels and alloys may not always satisfy all these demands, resulting in compromising aggressiveness of the service condition fitting with the material limitation, such as lowering upper-limit service temperatures or applied pressures during operation. As one of the practical solutions, an alloy strategy combining “protective external Al2O3 (alumina)-scale formation” and “austenitic stainless steel alloys” was proposed by some research groups in the 1970s,4,5 and a family of creep-resistant, alumina-forming austenitic (AFA) stainless steel alloys was successfully developed at Oak Ridge National Laboratory in the early 2000s.6,7 The key innovation was the design of chemistry and microstructure to enable a high-strength austenitic alloy that selectively oxidizes to form a protective external alumina-scale instead of the typical Cr2O3 (chromia)-based scales that form on most stainless steels. Alumina scales are more stable and protective than chromia-based scales, particularly at elevated temperatures and in environments containing corrosive water vapor, carbon, and sulfur-based species.8 The inexpensive Fe-base alloy design preserves the cost effectiveness and advantage compared to the performance-competitive, expensive Ni-base alloys.
However, the addition of Al in Fe-base alloys for high-temperature use was challenging from various aspects. Because of the strong BCC-Fe stabilizing effect of Al on Fe-base alloys, AFA alloys always require considerable amounts of FCC-Fe stabilizing elements, such as Ni, Mn, Cu, and C, to maintain an austenite matrix for high-temperature strength.9,10 Among them, Ni is the most favorable element for controlling the balance of high-temperature strength and oxidation resistance, and a larger Ni addition typically results in the better performance. The Ni content primarily controls the raw material cost because of its expensive price compared to the other major elements,7 and therefore, an optimum Ni addition that balances both alloy properties and cost needs to be considered during the alloy development and the down selection processes.
The AFA alloys can cover a wide range of service temperatures and environments by selecting a proper combination of the key elements. In other words, the concept of the AFA alloy development is not targeting a single alloy composition but proposing a new class of heat-resistant steel alloys applicable in a wide temperature range from 650 to 1100°C.11,12,13 The AFA alloys evaluated to date are based on Fe, 2.5 to 4.0 wt.% Al, 12 to 25 wt.% Cr, 12 to 35 wt.% Ni, 0.6 to 3.3 wt% Nb, 0.03 to 0.5 wt% C, and further additions selected among of B, Cu, Hf, Mn, Mo, Si, Ta, Ti, V, W, Y, and Zr, in either wrought or cast forms, with various target temperatures and applications.6,7,8,9,10,11,12,13,14,15,16 Based on an experimentally accumulated database regarding microstructure, oxidation resistance, and creep-rupture property of the AFA alloys evaluated to date, relatively straightforward compositional criteria were developed to achieve the targeted AFA performance characteristic. The list below summarizes the guidelines regarding how to select the alloy compositions.
-
(1)
Identify the allowable Ni content range based on the targeted material cost, service temperature, strengthening mechanism to be applied, comparison with competitive materials, etc.
-
(2)
Maintain an austenite single-phase matrix (minimize/eliminate ferrite phase) at the target temperature, by balancing major and minor alloying elements such as Cr, Al, Nb, C, Mn, Si, etc.
-
(3)
Allow a relatively high Cr content, which reduces the required Al content to form a protective alumina-scale (excess Al content destabilizes austenite, which negatively impacts high-temperature strength)
-
(4)
Avoid or minimize the use of Ti and V (and N), and add Nb (or Ta), to control the strengthening secondary phase precipitation without interfering with the formation of the external alumina-scale
-
(5)
Maximize the supersaturation of secondary phases in the austenite matrix for precipitation strengthening at elevated temperatures
Since AFA alloys require combined additions of several key alloying elements at the same time, the formation of multiple and various types of secondary phases (i.e., intermetallic compounds, carbides, borides, etc.) is always accompanied with the AFA alloys. Therefore, computational thermodynamics play a significantly important role in the alloy development to narrow down the composition range with controlled strengthening secondary phase formation (or minimized deteriorating secondary phase formation) for the optimization of high-temperature mechanical performance. For example, the required Nb addition for a stable alumina-scale formation ranges from ~0.6 to 3 wt% (depending on the target temperature and/or combination with other alloying elements 14,15), and the required C addition for stabilizing the austenite matrix is in the range of ~0.05–0.2 wt%8, which results in the formation of NbC and Fe2Nb-Laves for almost all AFA alloys developed to date. Therefore, the alloy design needs to incorporate control of microstructure evolution with these phases suitable for high-temperature mechanical properties (i.e., maximizing supersaturation of them as the secondary phases for precipitation strengthening).10 Increasing Cr and/or C contents also promotes secondary M23C6 (M: mainly Cr) formation, instead of NbC, for precipitation strengthening.8 The Laves-Fe2Nb precipitation does not exhibit any significant negative impact on the creep deformation resistance16 but rather prevents or retards the grain boundary sliding as these phases form on grain boundaries at elevated temperatures.17,18 In the case that the cost of relatively high Ni content (> 30 wt%) is acceptable, the alloy designs with relatively high Cr content (~20 −25 wt%) targeting very high-temperature oxidation resistance (up to ~1100°C)12 or strengthening with coherent L12-Ni3Al precipitation15,19,20 are applicable. It is also important to eliminate or minimize some phases which deteriorate the creep performance, such as M7C3 (M: mainly Cr), Sigma, and G phase.12,21
The mechanical properties at elevated temperatures also rely on microstructural factors of the strengthening or deteriorating secondary phase precipitates including type, size and its distribution, volume fraction, morphology, number density, and chemistry,22 and therefore, they play another important role in controlling or predicting the material responses (e.g., deformation resistance, rupture life, etc.) under a stressed condition. However, these factors are mostly time-dependent, and it is not easy to predict the precipitation kinetics, especially for the alloys with multiple secondary phase precipitations. As AFA alloys contain multiple secondary phases that both positively and negatively impact the mechanical properties, two major computational techniques are key for successful material design and development: (1) a reliable prediction of thermodynamically equilibrated phases including precipitation kinetics, and (2) material life assessment of newly designed, precipitate strengthened heat-resistant alloys through physics-based modeling and simulation.
The present work is part of a larger on-going multi-national laboratory program, “eXtremeMAT,” under the Office of Fossil Energy and Carbon Management, US Department of Energy.23 The goals of this program include establishment of new computational lifetime assessment (particularly creep) and alloy design methodologies for high-temperature structural materials under extreme environments using physics-based, mechanistically derived material lifetime and performance predictors along with data analytics. A key goal of eXtremeMAT is to predict the influence of microstructure and to some extent material chemistry on microstructure evolution and performance of both laboratory-assessed material and commercial scale components. The models are being guided and verified by experimental creep and microstructural studies of established commercial alloys such as 347H stainless steel. As part of this experimental component of eXtremeMAT, “model” developmental AFA alloys with two different multi-phase/multi-scale strengthening precipitates structures based on “Laves-phase and carbides” and “coherent L12 γ′-Ni3(Al,Ti)” are also being explored as a test bed to guide and verify the modeling efforts.
The targeted service temperature for the AFA alloys in the present work is in the 650-750°C range. The selection of the model AFA alloy compositions follow the composition guidelines described above, together with computational thermodynamic calculations to achieve the target strengthening secondary phase formation at 750°C. The proposed alloys have a variation of Ni content (nominally 20, 25, and 35 wt%), which are to be compared with commercially available heat-resistant steels and alloys containing similar Ni contents, based on a consideration of cost-effectiveness of the materials. Microstructure characterization including phase identification, volume fraction measurement, and compositional analysis is conducted, and the results are to be compared with the thermodynamic calculation. The main objective of the present work is to provide detailed microstructural findings and creep behavior of model AFA alloys for future use in the eXtremeMAT creep modeling efforts. Although the model AFA alloys are primarily designed to explore aspects of microstructure and creep, compositional design considerations to also achieve oxidation resistance via protective alumina scale formation are also made, with screening level oxidation data reported. As such, the model AFA alloys can also serve as a basis for further design and optimization to achieve next generation AFA alloys with improved creep and oxidation resistance.
Experimental Procedure
Alloy Selection
Nominal and analyzed compositions of the model AFA alloys in this study are summarized in Table I. X201 and X251, containing nominally 20 and 25 wt% Ni, respectively, were designed for “Laves + carbide” strengthening and X351 and X354 with nominally 35 wt% Ni for “L12 strengthening. For X351 and X354, the higher Ni content allows the alloy design with coherent L12 formation for high-temperature strengthening. The X201 and 251 varied only in Ni content, whereas X354 was a variation of X351 with lower Ti (1 vs. 2 wt%) and higher Nb (3 vs. 2.5 wt%) levels, which can significantly impact the balance between creep and oxidation resistance in L12 strengthened AFA. Target alloy compositions were selected with the design strategy described in the previous section. Major elements such as Cr, Ni, Al, Nb, Ti, and C were balanced through the iteration of thermodynamic calculation within a narrow composition range of each element to control the formation of strengthening secondary phases at 750°C, which should be dissolved in the austenite matrix by a solution annealing. The other minor elements were added to expect multiple reasons, such as the austenite stabilization (Mn, Cu), typical impurities in industrial grade products (Mn, Si, V, Ti), additional strengthening effect (Cu, Mo, W), improved oxidation resistance (Zr, B), and so on.8,9,10,13,14,15 Figure 1 illustrates the calculated phase equilibria of the alloys in the present study which were predicted using a commercial thermodynamic software, Thermo-Calc, with the TCFE9 (for X201 and X251, Fig. 1a and b, respectively) and TCNI8 databases (for X351 and X354, Fig. 1c and d, respectively). In X201 and X251, Laves-(Fe,Cr)2(W,Mo,Nb) and M23C6 carbide were selected as the strengthening secondary phases at 750°C, in which these phases would be dissolved into the austenite matrix above ~900°C and 1150°C, respectively. The predicted volume fractions of the phases were ~2 vol% at 750°C for both alloys. The formation of B2-NiAl phase was also predicted below ~770°C. NbC would form during solidification, and the amount would be mostly unchanged between 750 and 1200°C, suggesting that no secondary NbC precipitation would be expected in these alloys. The calculation also predicted the formation of Sigma phase and BCC-Cr, which were known to deteriorate the high-temperature creep performance, although the formation was predicted below ~650°C. In X351 and X354, the formation of L12-Ni3(Al,Ti), NbC, Laves-Fe2Nb, and Sigma phases was predicted at 750°C. No B2-NiAl phase formation was predicted in these alloys, at least above 500°C. The melting point was predicted to be ~1170°C, and therefore, solution annealing should be conducted below this temperature. The calculated volume fractions of the L12 phase in X351 and X354 at 750°C were ~30 and 12 vol%, respectively. Significant reduction of the predicted L12 phase in X354 was due to a low Ti addition compared to X351.
Alloy Preparation
Button-shape ingots (~700 g) with the target AFA alloy compositions were prepared by arc melting with pure element feedstock. The ingots were re-melted and drop cast into a water-cooled Cu mold to obtain a laboratory-scale ingot with a size of ~ 25 × 25 × 127 mm. After sectioning into small pieces with a size of ~25 × 25 × 50 mm, the ingots were homogenized at 1200°C (for X201 and X251), 1150°C (for X351), or 1100°C (X354) in Ar cover gas for 1 h. They were subsequently forged into a pancake-shape from 50 mm to 13 mm thick, and then further forged (X201, X251, and X351) or rolled (X354) into plate-form samples with 7 mm thickness (total 88% reduction through the thermo-mechanical treatments). The plate samples were solution-annealed at the same temperatures of the homogenization process in Ar cover gas for 1 h, followed by water quenching. The microstructure after final annealing consisted of fully recrystallized austenite grain structure with undissolved, coarse NbC particles (size: 1-3 μm) dispersed uniformly. The averaged austenite grain sizes for X201, X251, X351, and X354, measured by mean-intercept method, were 50, 58, 83, and 47 μm, respectively. The specimens for property evaluation were machined from the plate samples, and all tests were conducted in the solution-annealed condition.
Oxidation Test
An accelerated, aggressive oxidation test condition of 100-h cycles at 850°C in air + 10% water vapor (850 cc/min, velocity 1.95 cm/s) was chosen to provide rapid feedback on the alumina-forming capability of the model AFA alloys following testing protocol described in Ref.24 Coupon specimens with a size of ~1 × 10 × 20 mm were sectioned from the plate samples by an electrical discharge machining (EDM), and then the specimen surface was ground using #600 SiC paper prior to the oxidation test. The mass of each specimen was measured before and after each 100-h cyclic exposure to evaluate the mass gain as a function of accumulated exposure time. In addition, oxidation exposures were conducted in 500 h cycles at 750°C in laboratory air for both oxidation and microstructural evolution assessment, along with comparison of microstructures formed in 750°C creep testing described in the following sections. In particular, oxidation coupon specimens after three cycles (= 1500 h) were characterized for the surface oxide-scale structure and the underlying isothermally aged alloy microstructure at 750°C for 1500 h.
Creep-Rupture Test
Uni-axial tensile creep-rupture test at 750°C in laboratory air and 100 MPa was conducted with a constant loading condition. The tensile creep specimens with a button head at the grip and a round-shape gage section (3.2 mm in diameter and 31.8 mm in length) were machined from the plate samples. An extensometer connecting to a couple of linear variable differential transformers was attached at the specimen grips to monitor the displacement (creep deformation) during testing.
Microstructure Characterization
Microstructure characterization of the AFA alloys before and after property evaluation was performed by using scanning electron microscopy (SEM), transmission electron microscopy (TEM), and atom probe tomography (APT). For SEM observation, cross-sectioned specimens were mounted into an epoxy resin, and then a conventional metallographic sample preparation was applied with final polishing using a colloidal silica solution. Phase identification was made with x-ray energy-dispersive spectroscopy (EDS) at 15 kV, and the image processing and volume fraction measurements were conducted using ImageJ.25 For TEM observation, disk specimens with 3 mm diameter were sectioned from the aged or creep-rupture tested specimens by EDM, ground by SiC paper up to #600, and then twin-jet electro-polished with a solution of methanol + nitric acid at −15°C and 13 V. Phase identification was performed by the selected area diffraction patterns (SADPs) combined with the EDS spot/map analysis. APT specimens were fabricated with an FEI Nova 200 dual-beam scanning electron microscope (SEM)/focused ion beam (FIB) instrument using the method described by Thompson et al.26 The APT experiments were run using a CAMECA LEAP 4000XHR in laser mode with a 30-K base temperature, 60-70 pJ laser power, 0.2% detection rate, and 200-kHz pulse repetition rate. The APT results were analyzed using CAMECA’s IVAS 3.8 software.
Results and Discussion
Oxidation Resistance
Figure 2 presents mass change data (2a) and SEM-backscattered electron (BSE) images (2b through 2d) showing representative surface cross-sections of X251, X351, and X354 exposed at 750°C in air for a total of 1500 h. The surface of X251 generally consisted of an outer transient oxide (gray) overlying a thin, micron to submicron layer consistent with continuous, protective alumina (occasional oxide nodules in the 5–15 μm range were also observed). A similar scale was formed on X201 (not shown). These duplex oxide scale configurations are typical for a protective alumina-scale formation on AFA alloys, which need to balance oxidation resistance with mechanical properties.6,13,27 On the other hand, the surface of X351 exhibited a complex external Cr-rich scale overlying an internal oxidation zone consistent with internal alumina and aluminum nitride. The observed results indicated that the alloy composition of X351 was not capable of establishing a protective alumina scale at 750°C in air. Although high Ni (+ high Nb) additions tend to increase the capability to form external, continuous protective alumina in the AFA composition range,13,27 the high Ti level in X351 resulted in internal oxidation and nitridation of Al, similar to previously reported alloy composition-oxidation trends.15,28 The X354 with lower Ti and higher Nb exhibited moderately improved oxidation resistance compared to X351, with cross-section features consistent with a composition at the borderline for protective alumina formation.
The oxidation behavior of the model AFA alloys was also screened under an accelerated, aggressive condition of 100 h cycles at 850°C in air + 10 % H2O (Fig. 3). Water vapor is widely encountered in high-temperature energy conversion and combustion system environments as well as chemical/petrochemical processing and can drive rapid component degradation.7,8,15,29,30,31,32 It is well established that alumina-forming alloys exhibit up to order(s) of magnitude level superior oxidation resistance to chromia-forming alloys in the presence of water vapor above 600–650°C.8,24,29,30,31 This is due to Cr oxy-hydroxide volatilization and subsequent increased alloy Cr depletion in water vapor environments triggering a loss of protective chromia formation, whereas alumina is far more stable in water vapor as water vapor enhances the tendency for internal oxidation.24,29,30,31,32 A transition to accelerated oxidation results from less protective spinel and related oxide phase formation in costly Ni-Cr based alloys, and breakaway oxidation is attributed to fast-growing Fe-rich oxides in lower cost Fe/Ni-Cr based alloys.24,31,32 These transitions are dependent on exposure conditions, primarily H2O level, flow rate, and temperature, as well as alloy Cr and Ni contents, alloy grain size, and component thickness.24,29,30,31,32 In the present work, the 850°C air + 10% H2O oxidation exposures were used as a highly corrosive rapid feedback condition to help assess the extent to which the model AFA alloys show potential for protective alumina scale formation. These alloys were selected primarily for microstructure-creep exploration to feed into eXtremeMAT creep modeling efforts, and the promising oxidation results allow for future alloy optimization.
Mass change of AFA alloys exposed at 850°C in air + 10% water vapor in 100 h cycles, plotted as a function of total exposure time; (a) alloys strengthened by Laves + carbides and (b) those by L12. For comparison purpose, the mass changes of commercially available, chromia- forming heat-resistant steels tested at 800 or 700°C in air + 10% water vapor 8, 15 are also plotted.
The Laves + carbide strengthened X201 and X251 exhibited relatively slow oxidation kinetics with total mass gains of only ~0.5 and 0.3 mg/cm2, respectively, after 4000 h of exposure at 850°C in air + 10% H2O (Fig. 3a). The data for the X251 with 25Ni are consistent with protective alumina scale formation, with the moderately higher oxidation rate for X201 with 20Ni likely falling very near the borderline for alumina formation in the 850°C air + 10% H2O environment. This finding is consistent with past work showing that higher Ni levels in AFA alloys can improve oxidation resistance.7,8,27 Both compositions show good potential for long-term use in the targeted 650–750°C temperature range. In comparison, 347 (Fe-18Cr-10Ni wt% based) and Super304H (Fe-18Cr-9Ni + Cu, N wt% based) exhibited oxidation kinetics consistent with a transition to rapid, nonprotective Fe-rich oxide formation after a few hundred hours of exposure at only 700°C in air + 10 % H2O due to volatilization and Cr depletion effects (Fig. 3a).8 At 800°C in air + 10% H2O, the higher Cr and Ni 310HCbN (HR3C, Fe–25Cr–20Ni wt% based) showed a relatively rapid increase in mass followed by a transition to mass loss (downward bend in the mass change curve) consistent with Cr oxy-hydroxide volatilization, which will result in Cr depletion and eventually a transition to rapid Fe-rich oxide growth with prolonged exposure times.8,31,32
The L12 strengthened X351 showed faster oxidation than the model Laves + carbide alloys, reaching a specific mass gain of 1 mg/cm2 after 2500 h at 850°C in air + 10% H2O (Fig. 3b). Although the kinetics were qualitatively parabolic, this rate of oxidation indicates that a protective alumina scale was not formed. The Ti addition in X351 is known to negatively impact oxidation resistance in AFA alloys by promoting internal oxidation of Al, although it is important to promote strengthening L12 precipitate formation.15,28 It is considered that the Ti content in X351 is past the threshold to achieve a protective alumina scale at 850°C air + 10% H2O for the Al, Cr, Nb, and Ni levels used in this alloy. In contrast, X354 containing a lower Ti level (1 vs. 2 wt%) and higher Nb (3 vs. 2.5) and Cr (15 vs. 14Cr wt%) levels than X351 (Table I) exhibited improved oxidation resistance at 850°C air + 10% H2O (Fig. 3b). The oxidation mass change data fell between that of X351 and X251, with a small decrease in mass at 2500-3000 h of exposure suggestive of minor scale spallation or volatility. Preliminary cross-sectional characterization (not shown) indicated borderline alumina scale formation for X354, similar to that observed at 750°C in air (Fig. 2), with patches of inner continuous alumina along with regions of internal attack, but with partially continuous alumina at the alloy-internal oxide interface. These findings highlight the competing impacts of Ti, Nb, and Cr levels on oxidation resistance in AFA alloys, but also suggest that the X354-base alloy would show a promise for further alloy development and use in the targeted 650–750°C temperature range. It is also hypothesized that an increased Al content in X354 may result in fully protective alumina formation, which is part of a future study. The promise of X354 type AFA in oxidation resistance is also proved by comparing with the commercial L12 strengthened A286 (Fe-25Ni-14.5Cr-0.4Al-2Ti wt% based), which showed a rapid transition to mass loss and Fe-rich oxide formation after only a few hundred hours at 700 and 800°C in air + 10% H2O [15], as shown in Fig. 3b.
Aged Microstructure
Figure 4 shows the cross-sectional SEM-back-scatter electron (BSE) images of the model AFA alloys after oxidation testing with a total 1500 h exposure at 750°C. The images were taken from near the center of the specimen thickness and quite far away from the surface oxides, and they are considered to represent as an isothermally aged microstructure. The images of X201 (Fig. 4a) and X251 (Fig. 4b) were similar to each other, consisting of multiple sub-micron size secondary phase precipitates including Laves-Fe2Nb (bright contrast), B2-NiAl (dark contrast), M23C6 (similar contrast to the matrix), as well as primary coarse NbC (bright with blurred outline) particles with ~1–3 μm size, which were consistent with the types of the phases predicted by the calculation in Fig. 1. It should be emphasized that most of the grain boundaries were decorated by Laves, B2, and M23C6 alternatively. The image of X351 (Fig. 4c) indicated a different secondary phase configuration from X201/X251, with relatively coarse dispersion of Laves, B2, Sigma, and NbC precipitates with an ~2-5 μm size in the austenite matrix. The grain boundaries were also decorated mostly with Laves and B2 phase. The austenite matrix also showed dark and spherical precipitate dispersions with a size < 100 nm, which correspond to coherent L12-Ni3(Al,Ti) precipitates. The image of X354 (Fig. 4d) also showed a similar configuration of the secondary phases including NbC, Laves, B2, and Sigma, as well as a dispersion of spherical L12 precipitates in the austenite matrix. Although the image may not clearly depict the morphology of the L12 precipitates, the number density seems quite lower than that in X351, which is consistent with the prediction from the thermodynamic calculation in Fig. 1. It should be emphasized that the B2 phase was predicted with very little (X201/X251) or no (X351/X354) formation in all alloys at 750°C, which was one of the major discrepancies between the experimental and thermodynamic calculation.
In addition to the SEM observation, a detailed TEM microstructure characterization inside the austenite matrix was also conducted. Figure 5 illustrates representative microstructures of X251 (5a) and X351 (5b) after isothermal exposure at 750°C for 1500 h. M23C6 and B2 precipitates, with sizes of ~100 nm and ~500 nm, respectively, were observed inside the X251 matrix, in which the phase identification was made through both EDS and SADPs. The M23C6 precipitation was sparse and not uniformly observed in the matrix, so that no quantitative measurements of the size and number density were performed. The dark-field image with [011] L12 super-lattice reflection in X351 showed a dense, spherical L12 precipitate dispersion. The average precipitate size was identified as ~100 nm, although the number density was not successfully measured from these observations.
The SEM-EDS map analysis with a phase identification function was utilized to measure the volume fraction of the secondary phases in the model AFA alloys after aging at 750°C for 1500 h. Figure 6 compares the SEM-BSE images (Fig. 6a and b) and the EDS phase identification maps (Fig. 6c and d) for X201 and X351. The phase identification detected six different phases in X201 (Fig. 6c) including FCC-Fe, NbC, two different Laves (W, Mo-rich and Nb-rich), B2, and M23C6. X351 (Fig. 6d) showed almost the same phases except for Sigma instead of M23C6. By comparing the EDS with the SEM-BSE images, the phase identification map of X201 (and X251 as well) did not clearly capture the size and the morphology of the secondary phases, since the sub-micron size precipitation was too small compared to the anticipated spatial resolution (> 1 μm) of the SEM-EDS measurement. In the case of X351, the L12 precipitates could not be identified because of the size limitation similar to X201. The other secondary phases in the phase identification map mostly appeared at the corresponding locations in the SEM-BSE image, although the interphase boundaries were still unclear. To improve the spatial resolution for the secondary phase volume fraction measurement, an image processing was applied to the SEM-BSE images in Fig. 6a and b, which converted the different contrast regions (bright, right gray, dark gray, etc.) into the different colors but consistent with the phase identification map, as shown in Fig. 6e and f, respectively. The color-processed image demonstrated further improvement of the size and the morphology identification of the phases quantitatively.
Table II summarizes the volume fraction of the phases in the model AFA alloys at 750°C obtained from the thermodynamic calculations with TCFE9 (for X201/X251, Table 2a and b, respectively) or TCNI9 (for X351/X354, Table IIc and d) and the measured volume fractions using SEM-EDS phase identification maps and processed BSE images. The measured volume fractions from the phase identification map were averaged from three different images acquired from different locations in each aged specimen, and the volume fractions from the processed images were obtained from one image of each aged specimen. The calculated phase volume fractions in X201 and X251 were very similar to each other, in which Laves and M23C6 were the dominant secondary phases (1.54–1.83%), and B2 followed (0.19-0.42%). NbC also showed a similar volume fraction to the others (1.11–1.15%), although it formed not at 750°C as a secondary phase but existed as a primary phase from solidification or solution annealing at 1200°C. The measured volume fractions from the phase identification maps were higher than the calculated amounts, especially for Laves and B2. The phase identification map did not precisely capture the sizes and the morphology of the secondary phase precipitates because of low special resolution, so that the volume fractions could be overestimated. However, the volume fractions from the processed images revealed more Laves and B2 phases than those from the phase identification maps, suggesting considerable gaps between the calculation and the actual phase fractions. The measured volume fraction of NbC, on the other hand, remained close to the calculated volume fraction. For X351, the B2 formation was not predicted at 750°C in the calculation, but the aged specimen exhibited a non-negligible amount of the B2 precipitates. This is another noticeable gap between the thermodynamic calculation and the experimentally obtained volume fractions in the AFA alloys, showing less predicted amount of B2-NiAl at a given temperature. In fact, a similar trend was also reported by the authors previously.9 Other than B2, X351 also showed more Laves and less Sigma phase in the phase identification map. The same trend was also captured from the processed images, although a non-negligible gap still exists between the phase identification map and the processed image. Based on these results, the phase identification map analysis was not conducted for X354. The measured volume fraction from the processed image of X354 suggested more Laves and B2 and less Sigma, similar to X351.
It is briefly concluded that the gaps of the secondary phase volume fractions between the calculation and the measurements from the processed SEM-BSE images are obvious, and the obtained results need to be archived and provided for thermodynamic database correction. In the meantime, it should also be emphasized that the formation kinetics of Sigma phase in austenitic stainless steels is typically slow,33 so that a total 1500 h aging at 750°C may not be sufficient to reach the thermodynamically equilibrated condition, and additional experimental data are required.
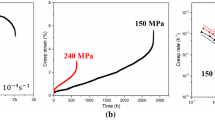
Creep-Rupture Performance
Creep-rupture tests of the model AFA alloys were conducted at 750°C and 100 MPa. Figure 7 illustrates the creep-rupture curves (Fig. 7a) and the Larson-Miller parameter plots of the model AFA alloys (Fig. 7b). The latter also plotted commercially available heat-resistant steels Sanicro 25 (Fe-23Cr-25Ni-W-Co-Nb-C-N),34 Super304H (Fe-18Cr-9Ni-3Cu-Nb-C-N), and 347HFG (Fe-18Cr-11Ni-Nb-C)35 for comparison purpose. The rupture lives of X201 and X251 strengthened by Laves and carbide (M23C6) were 4018 and 5422 h, respectively. At the same test condition, the creep rupture lives of 347HFG and Super304H were 335 h and 1533 h, respectively,35 and that of Sanicro25 could be estimated to be around ~5000–5500 h,34 suggesting that the creep-rupture performances of X201/X251 were significantly better than those of 347HFG and Super304H, and X251 achieved comparable performance to Sanicro25 in the studied test condition. The creep-rupture lives of X351 and X354 strengthened by coherent L12 precipitates were 9012 and 7347 h, respectively, which were far better than that of X201/X251. The results represented the advantage of the coherent L12 strengthening mechanism compared to Laves + carbide strengthening, which could be attributed to a significant difference in the predicted volume fraction of the strengthening precipitates in the austenite matrix, in which X201/X251 relied on ~2 vol% of M23C6 precipitates whereas X351/354 were strengthened from ~12–30 vol% of L12 precipitates. For a detailed consideration of the precipitation strengthening, the measurement of precipitate size and number density will be studied in the future. The longer rupture-life in X351 than that in X354 could also be partly attributed to the larger volume fraction of coherent L12 phases in X351 compared to X354.
The minimum creep rates (MCRs) of X201 and X251 were 1.11 × 10−9 and 9.25 × 10−10 s−1, respectively, which were quite similar to each other, indicating that these alloys exhibited a similar deformation resistance attributed to similar microstructure to each other. X351 exhibited lower MCR (3.39 × 10−10 s−1) than that of X201/X251, whereas the MCR of X354 (1.94 × 10−9 s−1) was even higher than that of X201/X251, suggesting that the creep-deformation resistance through the coherent L12 precipitation in the matrix is strongly influenced by the number of the L12 precipitates. The tertiary creep stages for X201 and X251 appeared earlier than those of X351 and X354, which probably represented less thermal stability for strengthening secondary phase containing microstructure in X201/X251 compared to those of X351/X354. It should also be noted that the creep rupture elongation of X351 was only 4% whereas that of X354 was 15%, and X201/X251 exceeded 20%, representing that the longer rupture lives could be a tradeoff for the lower creep ductility.
Although only one test condition was conducted in this study, the present AFA alloys demonstrated a creep strength similar to that of the state-of-the-art austenitic stainless steel Sanicro25, +20~40% of Super 304H, and +50~70% of 347HFG. It should be emphasized that these commercial heat-resistant steels are chromia-formers, so that the present AFA alloys have an additional advantage of the surface protectiveness in various industrial environments due to protective alumina-scale formation. This leads to an expectation of long-term component lives in the present AFA alloys from two different perspectives: environmental compatibility and creep-deformation resistance.
Microstructure of Creep-Rupture Tested Specimens
Figure 8 summarizes the SEM-BSE images acquired from the model AFA alloys after creep-rupture testing at the gage and grip sections. The microstructure evolution at the gage section is considered to have a significant influence of the creep deformation, and the grip section has very little effect of the stress in the microstructure evolution and is therefore considered an isothermally aged specimen.
For X201 (Fig. 8a and b) and X251 (Fig. 8c and d), the creep deformation has two major impacts on the microstructure evolution: (1) all secondary phases including Laves, B2, and M23C6 coarsened faster, especially on the grain boundaries, than those in the grip section, and (2) the B2 precipitates in the austenite matrix were spheroidized and relatively smaller than the disk-shaped B2 precipitates in the grip section. The former suggests that the deformation promoted the elemental transportation, such as a pipe diffusion through the deformation dislocation, which accelerated the coarsening kinetics of the observed secondary phase precipitates. Although the precipitation sizes at the grain boundaries were almost doubled in the gage section compared to the grip section, the configurations of the alternative secondary phase precipitation remained. It suggests that the grain boundary sliding was effectively prevented by multiple secondary phase precipitates on the grain boundary and then increased the macroscopic deformation resistance under the stress condition. It should also be noted that the precipitate size and distribution in the grip section, considered as isothermally exposed at 750°C for 4018 h (X201) or 5422 h (X251), showed no significant difference from the specimens aged for 1500 h, which reveals a high thermal stability of the microstructure during isothermal aging in the present condition as well as a significant impact of the creep deformation on microstructure evolution.
For X351 (Fig. 8e and f), the SEM-BSE images showed no noticeable difference in microstructure between the gage and grip sections, suggesting no significant impact of the creep deformation on the microstructure evolution in the present test condition. The grain boundaries were fully decorated by multiple secondary phase precipitates including Laves, B2, and Sigma phase precipitates. Dispersion of coarse precipitates in the austenite matrix (not shown) were observed in both sections with no significant difference in the macroscopic configuration to each other. A similarly dense dispersion of spherical L12 precipitates was also observed in the austenite matrix in both the gage and grip sections. A large difference from the specimen aged for 1500 h (Fig. 4c) was that the dominant secondary phase precipitates on the grain boundaries changed from Laves to Sigma. Microstructure characterization of X354 specimens is currently in progress, and the results will be summarized in the future.
Effect of Isothermal Exposure and Creep-Deformation on the Secondary Phase Precipitates
Figure 9 compares the calculated and the measured volume fractions of the secondary phase precipitates (other than M23C6 and L12) in the X201, X251, and X351 after isothermal aging for 1500 h and creep-rupture testing at 750°C. The volume fractions of the secondary phase precipitates were measured using the contrast-converted (processed) SEM-BSE images; the approach is shown in Fig. 6e and f. For all case, the amount of NbC did not significantly change with respect to exposure time. However, the volume fraction of Laves precipitates decreased as the exposure time increased. The volume fraction of B2 in X201 was slightly high after 1500 h exposure, but that for the other alloys was mostly unchanged after prolonged exposure. For X351, the volume fraction of Sigma increased from 0.2% to 4.7% as the exposure time increased from 1500 h to 9012 h. By comparing with the exposure time dependence, the effect of creep deformation on the volume fractions seemed not so significant.
Since the aging or the creep-rupture testing was initiated in the solution-annealed condition, all observed secondary phase precipitates (except for primary NbC) formed during thermal exposure at 750°C. Therefore, it is considered that Laves precipitates nucleate in the early stage of aging and grow rapidly to exceed the thermodynamically equilibrated volume fraction. The B2 precipitation is likely accompanied by Laves formation since very limited Al dissolution in Laves phase could promote Al-rich B2 precipitation adjacent to Laves precipitates.14,21 As the thermal exposure proceeds, the volume fraction of Laves phase reduces toward the thermodynamically equilibrated value. Although a prolonged exposure test has not been conducted, the exposure time at the grip section of the creep-rupture specimens (~4000 to 9000 h at 750°C) is sufficiently long to be considered in equilibrium.33 For X351, Sigma precipitates form and grow slowly, and therefore, longer exposure would be required to assess the actual phase equilibrium for comparison to the calculated phase fraction.
Individual phase compositions were analyzed by APT for the series of the model AFA alloys in the aged and the creep-rupture conditions and then compared with the thermodynamically calculated results as a part of gap analysis between the calculation and the actual materials. Figure 10 summarizes a part of the analyzed results to date, focusing on X351. When comparing with the calculation results, there are several noticeable gaps and characteristics in the analyzed compositions as listed below: (1) FCC-Fe (austenite matrix) contains slightly less Al and Ni (possibly due to the formation of the non-predicted B2-NiAl) and more Cr, (2) L12 contains slightly more Al and Fe, (3) MC (NbC) contains less C and more Ti, (4) Laves contains more Mo, Ni, and Si, and also a small amount of Al dissolution, (5) Sigma contains more Fe, Al, and Si and less Cr, Mo and Ni, and (6) no apparent effects of exposure time or creep deformation on the analyzed compositions appeared. The analyzed compositions are considered to closely correlate with the volume fractions of the secondary phase precipitates in both equilibrium and kinetics viewpoints, which also have a strong impact on the strengthening mechanisms. Therefore, the obtained results combined with the volume fractions will be archived with the detailed material pedigree and the test conditions, and then a part of the microstructural database will be built to be used for alloy design optimization as well as life-prediction modeling activities in future.
Comparison of the compositions of the matrix and the secondary phase precipitates in X351 calculated by Thermo-Calc with TCNI8 and measured using Atom probe tomography (APT); (a) the calculated equilibrated composition at 750°C, (b) aged for total 1500 h at 750°C, and (c) the grip section and (d) the gage section of the creep-rupture specimen tested at 750°C/100 MPa/9012 h.
The results presented are still preliminary and require further updates such as the microstructural factors of L12 precipitates, missing APT characterization, and similar compositional analysis of X201, X251, and X354 as a function of isothermal exposure and creep deformation, which are currently in progress. The microstructural database with different strengthening mechanisms and compositional variation should help with extending the alloy design capability of multiple secondary phase precipitation strengthened materials.
Conclusion
In this study, two “model” cases of multiple secondary phase precipitation strengthening AFA alloys have been explored targeting 650–750°C use, with the alloy designs of the formation of “Laves-phase and carbides (for the alloys X201 and X251)” and “coherent L12 γ′-Ni3(Al,Ti) (for the alloys X351 and X354)” as strengthening secondary phase precipitates. These alloys are to be used to guide and verify computational alloy design and life-prediction modeling under an on-going multi-national laboratory program “eXtremeMAT” through the Office of Fossil Energy and Carbon Management, US Department of Energy. Computational thermodynamics was used to select the alloy compositions with maximized supersaturation of the strengthening secondary phases at 750°C after solution annealing at 1100–1200°C. The presented AFA alloys exhibited a creep-rupture performance at 750°C and 100 MPa superior to that of the commercially available, chromia-forming Super304H, and comparable to that of Sanicro25, together with good oxidation resistance in aggressive oxidation screening at 850°C in air + 10% water vapor. The oxidation behavior of X201 and X251 at 750–850°C was consistent with protective, external alumina-scale formation, X351 did not have a good scale for oxidation protection, while X354 exhibited borderline protective alumina scale formation with a discontinuous alumina scale. The X201, X251, and X354 offer attractive alloy bases for further co-optimization of creep and oxidation resistance. Detailed microstructure characterization after isothermal exposure and creep-rupture testing at 750°C suggested that (1) the types of secondary phase precipitates were mostly consistent with thermodynamic calculations, (2) there were significant gaps in the volume fractions between the experimental results and the calculations, and (3) higher volume fractions of the precipitates, especially for B2-NiAl and Laves-Fe2Nb, were observed in the alloys studied, which is opposed to the prediction through computational thermodynamics. Also, (4) the creep-deformation promoted the precipitate coarsening and spheroidization in X201/X251, but no significant effect of the creep-deformation was observed in X351. In addition, (5) it was found that Laves precipitation in X351 was promoted in the early stage of isothermal exposure, but after long-term exposure, the volume fraction of the Laves phase was reduced, while that of the Sigma phase was increased, whereas (6) the chemical composition of each phase remained consistent through all exposure times and after creep deformation. The obtained microstructural responses are to be archived and utilized for improvement of a thermodynamic database to predict microstructure evolution, as well as the material life assessment through physic-based modeling and simulation, which is connected to computation-based alloy development of multiple secondary phase precipitation strengthening alloys like AFA alloys.
References
R. Viswanathan and W. Bakker, J. Mater. Eng. Perform. 10, 81. (2001)
R. Viswanathan and W. Bakker, J. Mater. Eng. Perform. 10, 96. (2001)
A. Di Gianfrancesco, Materials for Ultra-Supercritical and Advanced Ultra-Supercritical Power Plants (Woodhead Publishing, Duxford, UK, 2017).
T. Fujioka, M. Kinugasa, S. Iizumi S, Teshima, and I Shimizu, U.S. Patent 3,989,514 (1976).
J.A. McGurty, U.S. Patent 4,086,085 (1978).
Y. Yamamoto, M.P. Brady, Z.P. Lu, P.J. Maziasz, C.T. Liu, B.A. Pint, K.L. More, H.M. Meyer, and E.A. Payzant, Science 316, 433. (2007).
M.P. Brady, Y. Yamamoto, M.L. Santella, P.J. Maziasz, B.A. Pint, C.T. Liu, Z.P. Lu, and H. Bei, JOM 60, 12. (2008).
M.P. Brady, J. Magee, Y. Yamamoto, D. Helmick, and L. Wang, Mater. Sci. Eng. A 590, 101. (2014).
Y. Yamamoto, M.L. Santella, C.T. Liu, N.D. Evans, P.J. Maziasz, and M.P. Brady, Mater. Sci. Eng. A 524, 176. (2009).
Y. Yamamoto, M.L. Santella, M.P. Brady, H. Bei, and P.J. Maziasz, Metall. Mater. Trans. A 40A, 1868. (2009).
Y. Yamamoto, M.P. Brady, G. Muralidharan, P.A. Pint, P.J. Maziasz, D. Shin, B. Shassere, S.S. Babu, C.H. Kuo, Proceedings of the ASME 2018 Symposium on Elevetad Temperature Application of Materials for Fossil, Nuclear, and Petrochemical Industries, (ASME, Seattle, WA, 2018), ETAM2018-6727.
G. Muralidharan, Y. Yamamoto, M.P. Brady, D. Leonard, E. Cakmak, T. Ros, S. Fauske, G. Hadley, R. Pankiw, J. Myers . Proceedings of 2nd International Symposium on The Recent Developments in Plate Steels, AISI (Memphis, TN, USA, 2018). pp. 513-520.
M.P. Brady, K.A. Unocic, M.J. Lance, M.L. Santella, Y. Yamamoto, and L.R. Walker, Oxid. Met. 75, 337. (2011).
Y. Yamamoto, M.P. Brady, M.L. Santella, H. Bei, P.J. Maziasz, and B.A. Pint, Metall. Mater. Trans. A 42A, 922. (2011).
Y. Yamamoto, G. Muralidharan, and M.P. Brady, Scr. Mater. 69, 816. (2013).
Y. Yamamoto, M. Takeyama, Z.P. Lu, C.T. Liu, N.D. Evans, P.J. Maziasz, and M.P. Brady, Intermetallics 16, 453. (2008).
M. Takeyama, Mater. Sci. Forum 539–543, 3012. (2007).
B. Kuhn, M. Talik, T. Fischer, X. Fan, and Y. Yamamoto, J. Lopez Barrilao. Metals 10, 463. (2020).
B. Hu, G. Trotter, Z. Wang, S. Chen, Z. Cai, and I. Baker, Intermetallics 90, 36. (2017).
A. Peterson and I. Baker, Mater. Sci. Eng. A 806, 140602. (2021).
Y. Yamamoto, M.P. Brady, Z.P. Lu, C.T. Liu, M. Takeyama, P.J. Maziasz, and B.A. Pint, Metall. Mater. Trans. A 38A, 2737. (2007).
H. Han, J. Shen, J Xie, Scientific Reports, 8, 15411 (2018)
National Energy Technology Laboratory (NETL), “Accelerating the Development of Extreme Environment Materials”, https://edx.netl.doe.gov/extrememat/
R. Pillai, S. Dryepondt, M. Lance and B. Pint, High Temperature Oxidation Behavior of Fe- and Ni-Based Alloy Foils in Water Vapor at 850°C Marie Romedenne; Paper presented at the CORROSION 2021 (Paper Number: NACE-2021-16594), Virtual, 19 April 2021
J. Rasband and W.S. ImageJ, U. S. National Institutes of Health, Bethesda, Maryland, USA, https://imagej.nih.gov/ij/, 1997-2018.
K. Thompson, D. Lawrence, D.J. Larson, J.D. Olson, T.F. Kelly, and B. Gorman, Ultramicroscopy 107(2–3), 131. (2007).
M.P. Brady, Y. Yamamoto, M.L. Santella, and L.R. Walker, Oxid. Met. 72, 311. (2009).
M.P. Brady, Y. Yamamoto, M.L. Santella, and B.A. Pint, Scr. Mater. 57, 1117. (2007).
E. Opila, Mater. Sci. Forum 461–464, 765. (2004).
E. Opila, N.S. Jacobson, D.L. Myers, and E.H. Copland, JOM 58(1), 22. (2006).
S.R.J. Saunders, M. Monteiro, and F. Rizzo, Prog. Mater. Sci. 53, 775. (2008).
R. Peraldi, and B.A. Pint, Oxid. Met. 61, 463. (2004).
T. Sourmail, Mater. Sci. Technol. 17(1), 1. (2001).
Sandvik datasheet, SANICRO® 25, TUBE AND PIPE, SEAMLESS, https://www.materials.sandvik/en/materials-center/material-datasheets/tube-and-pipe-seamless/sanicro-25/
National Institute of Materials and Sciences (NIMS) creep data sheet, https://smds.nims.go.jp/creep/en/
Acknowledgements
The authors thank Dongwon Shin, Dean Pierce, and Edgar Lara-Curzio for helpful comments on this manuscript. This work was performed in support of the US DOE Office of Fossil Energy and Carbon Management through the eXtremeMAT(XMAT) program under Crosscutting Technology High Performance Materials Research Program. Atom probe tomography (APT) was performed at ORNL’s CNMS, which is a US DOE office of science user facility. The authors would like to thank James Burns for assistance in performing APT sample preparation and running the APT experiments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher′s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This manuscript has been authored by UT-Battelle, LLC, under contract DE-AC05-00OR22725 with the US Department of Energy (DOE). The US government retains and the publisher, by accepting the article for publication, acknowledges that the US government retains a nonexclusive, paid-up, irrevocable, worldwide license to publish or reproduce the published form of this manuscript, or allow others to do so, for US government purposes. DOE will provide public access to these results of federally sponsored research in accordance with the DOE Public Access Plan (http://energy.gov/downloads/doe-public-access-plan).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yamamoto, Y., Brady, M.P., Ren, QQ. et al. Creep Behavior and Phase Equilibria in Model Precipitate Strengthened Alumina-Forming Austenitic Alloys. JOM 74, 1453–1468 (2022). https://doi.org/10.1007/s11837-022-05203-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-022-05203-5