Abstract
Dissolution and solubility of palladium in iron silicate melts saturated with alumina–iron spinel at 1300°C has been measured using an equilibration-drop quenching technique combined with electron probe microanalysis and laser ablation–inductive coupled plasma–mass spectrometry analysis from polished sections. Composition of the resulting Fe-Pd alloy allowed estimation of the activity of palladium at different oxygen partial pressures, and, thus, the solubilities of palladium in the studied slags in conditions typical of copper and nickel smelting as well as slag cleaning at pO2=10-5 to 10-10 atm. The mechanism of palladium dissolution in the studied iron silicate slags was oxidation by formation of the monovalent oxide species PdO0.5 over the entire oxygen activity range of this study. Testing the applicability of the various palladium isotopes for quantitative analyses of Pd in these types of matrices resulted in a good fit of measured concentrations of 104Pd and 105Pd with interference-corrected 106Pd and 108Pd.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Palladium is one of the most important precious metals used in electrical and electronic equipment, as well as playing a critical role in catalytic converters. Palladium and platinum have similar properties, and thus, depending on their prices in metal exchanges, their use in the major application fields may change from platinum to palladium and back to platinum in a cyclical way. In general, palladium is a much less well-studied platinum group metal (PGM) in geochemical and metallurgical processes than platinum. Sullivan et al.1 recently compiled the available solubility data for palladium in silicate melts as a function of oxygen partial pressure. They also summarized most of the earlier studies in geochemistry as a function of temperature and pressure.
Nishijima and Yamaguchi2 determined PGM distributions between ternary Al2O3-CaO-SiO2 slags at copper saturation and 1450°C, measuring the oxygen activities with an EMF cell. Their distribution results were essentially independent of the (CaO + MgO)/(CaO + MgO + SiO2) ratio (w/w). Shuva et al.3, Avarmaa et al.4,5 and Sukhomlinov et al.6 determined the distribution coefficients of palladium between selected secondary copper smelting slags and a copper alloy. Yamaguchi7,8 measured palladium distributions between copper and CaO-FeOx slags at 1300°C and MgO saturation. Henao et al.9 carried out measurements on PGMs as trace elements in a copper matte–silicate slag system at MgO saturation at 1300°C. Avarmaa et al.10 and Piskunen et al.11 studied palladium distributions in copper and nickel matte smelting conditions at silica saturation, and conducted direct phase composition measurements with laser ablation–inductive coupled plasma–mass spectrometry (LA-ICP-MS).
Tomiska12 summarized the previous experimental data on the Fe-Pd system and carried out Knudsen cell measurements on ternary Au-Fe-Pd alloys. Ghosh et al.13 carried out a critical thermodynamic assessment of the Fe-Pd alloy system using the available thermodynamic and phase equilibrium information.
This study experimentally investigated palladium behavior in copper smelting and converting conditions (1300°C, pO2 = 10-10–10-5 atm) without the presence of copper. The simultaneous presence of copper and palladium in LA-ICP-MS measurements is a demanding task, as copper argide (65Cu40Ar+), potentially formed by the Ar ICP14 in copper-bearing systems, has almost the same molar mass as the 105Pd isotope and they are therefore impossible to separate by a mass spectrometer running at low mass resolution. This problematic mass interference was a driver for the current measurements on Cu-free experiments, providing higher accuracy Pd distribution coefficient determinations with a pure palladium equilibrium phase instead of a dilute copper alloy4,5,6 or copper-bearing matte10,11. Moreover, as PGMs are also recovered via metallic iron-slag systems,15 this study also provides valuable data and information for those technologies.
Experimental
The experimental technique involved sample equilibration at a controlled temperature, 1300 ± 3°C, and in a flowing gas atmosphere, followed by rapid quenching and direct elemental analyses of the equilibrium phases with EPMA (electron probe microanalysis) and LA-ICP-MS from polished sections. The materials used in the experiments have been collected in Table S1 of the online Electronic Supplementary Material file. They were mixed and homogenized by grinding in an agate mortar with a metal alloy-to-slag ratio of 1:9 and a Fe/SiO2 ratio of 1.3 (w/w) in slag. The CO-CO2 gas mixtures used to generate the different oxygen partial pressures at 1300°C were controlled using thermal mass flow controllers (DFC 26; Aalborg, USA) of different flow ranges, with an accuracy of ± 1% of full range. The gases were supplied by AGA-Linde (Finland) with purities of 99.97 vol.% CO and 99.9992 vol.% CO2.
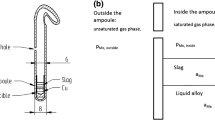
The details of the experimental furnace and the gas train have been described earlier.16,17 This study continues the PGM solubility studies in a similar Fe alloy–slag system with the same experimental arrangement and procedure as previously published for platinum.16 The sample and an alumina crucible as the sample holder in the hot zone of the experimental furnace are shown in Fig. 1(a). All the other experimental details, including the furnace, sample mass, equilibration time series, and analytical equipment, are available in the previous publication regarding platinum solubility in alumina–iron silicate slag.16 All the presented experiments were equilibrated for 48 h. A typical microstructure of the equilibrated-quenched sample is shown in Fig. 1(b).
Sample Characterization
The elemental compositions of the quenched metal and slag were analyzed with EPMA, of which the analytical details have been presented earlier.16 The only change was that pure Pd was used as a standard for analyzing Pd Lα peaks in the Pd-Fe alloy, as opposed to pure Pt for Pt Lα. The averages of eight EPMA points have been reported as well as their standard deviations. The slag composition results are presented in more detail elsewhere.18
The slag and spinel phases were also analyzed by LA-ICP-MS, since the concentrations of palladium were below the detection limit of EPMA. The details of this equipment have also been presented earlier.16 For slag analysis, NIST 612 SRM19 was employed as the external standard and 29Si as the internal standard. NIST 610, BHVO-20, and BCR-2G19,20 were analyzed as unknowns for monitoring the analysis accuracy. For spinels, GSD-1G20 was chosen as the external standard and 57Fe as the internal. The Pd concentration in GSD-1G was determined by analyzing this standard material as an unknown, using NIST 612 as an external standard and 57Fe as internal. The value obtained for Pd concentration was 33 ppm. The Glitter software package21 was applied for the raw data processing, i.e., the baseline reduction and quantifications.
The palladium concentrations were measured for all the existing isotopes 104Pd, 105Pd, 106Pd, 108Pd, and 110Pd by the LA-ICP-MS technique. In this study, due to the absence of copper in the experimental charges, the isotopes 104Pd and 105Pd provide interference-free measurements and could be used, as such, to present the final results. The interference caused by the formation of 89Y16O+ for 105Pd was evaluated negligible ( < 2%) as the concentration of Y in NIST612 standard is only 38.3 ppmw (Y/Pd ratio = 36.5), and its influence was evaluated as 20% of the counts measured on 105Pd when using high Y/Pd ratio (381.8) standard NIST610.22 The 106Pd, 108Pd, and 110Pd isotopes have isobaric interferences from Cd-isotopes when using NIST 612 SRM as the primary standard material and additionally 94Zr16O+ for 110Pd.23 Because Cd exists in the standards, but not in the experimental samples, the measured palladium results based on the 106Pd and 108Pd isotopes were lower than the true concentrations. Nevertheless, results for isotopes of 106Pd and 108Pd can be interference-corrected by using formulae presented in the literature.4,24 Consequently, selected samples were re-analyzed for a range of Pd and Cd isotopes to allow correction of 106Pd and 108Pd and to verify the consistency of calculated Pd concentrations based on four different Pd isotopes (Table I).
As can be seen in Table I, palladium concentrations in slags based on different isotope measurements are internally consistent. This provides a good indication that the use of corrected 106Pd and 108Pd isotopes are applicable for systems when there are interference problems with the 104Pd and 105Pd isotopes, such as copper-containing slags (65Cu40Ar+) with the 105Pd isotope.4 In this study, we employed the 105Pd isotope results, as this isotope has a higher natural abundance, of 22.33%,25 than 104Pd (11%). The detection limits for the elements studied with EPMA and LA-ICP-MS obtained in this study have been summarized in Table S2 of the Electronic Supplementary Material file.
Only minor heterogeneities in some time-resolved MS spectra were observed in the samples, as indicated by the experimental uncertainties collected in Tables I and II. The time-resolved MS-signal profiles were generally smooth as a function of time, as indicated in Fig. 2; only occasional spikes were present. This indicates that only a few nanonuggets of palladium26 were possibly present in the iron silicate slags prior to or after the quench.
Results and Discussion
The primary concentration data for palladium in the slag based on 105Pd measurements are shown in Table II and Fig. 3 as a function of oxygen partial pressure. The vertical error bars show the experimental analytical standard deviations (± 1σ) calculated from the independent LA-ICP-MS analysis points of each sample, and the horizontal error bars for log10pO2 as a fixed value of ± 0.2 based on the full range accuracy of mass flow controllers and gas flow rates presented earlier.16 The re-analyzed sample results for 105Pd (see Table I) fit well with the primary results measured and shown in Table II.
Due to the dissolution of iron during equilibration, the composition of the palladium alloy was strongly affected by the prevailing oxygen partial pressure of the system. The EPMA data of the solid alloy are shown in Table II and Fig. 4. Using these data, the solubility of palladium (i.e., in equilibrium with pure metal and excluding the effects of dissolved iron) in the slag can be evaluated using the relationship:
where a[Pd] is the activity of palladium in the metallic Fe-Pd alloy,13 referred to pure solid palladium, and c(Pd) is the measured concentration of palladium in the slag at that activity. This corrected chemical solubility of palladium [*c(Pd)] in alumina spinel saturated iron silicate melt at 1300°C as a function of the oxygen partial pressure is presented in Fig. 5.
Figure 5 shows that the solubility of palladium in silicate melts at alumina–iron spinel saturation is strongly increasing as a function of the prevailing oxygen activity. Thus, over the entire oxygen partial pressure range studied, it was dissolved as an oxide species in the molten iron silicate melt.
Selected experimental points were measured from the solid alumina–iron spinel phase and its palladium concentration in equilibrium with the molten slag. The spinel was essentially a binary solution of FeAl2O4 and Fe3O4, as its silicon concentration at the studied oxygen partial pressure range was very low, between 0.035 and 0.055 wt.% (Si), equivalent to 0.075 and 0.12 wt.% SiO2. The obtained distribution coefficient of palladium between liquid slag (s) and solid spinel (sp), Ls/spPd = (wt.% Pd)slag/(wt.%Pd)spinel, shows a minor enrichment in the spinel at pO2 = 10-6–10-5 atm with Ls/sp(Pd) = 0.45 ± 0.2.
The apparent distribution coefficient of palladium between the metal alloy and molten silicate has been defined conventionally27 as:
where [%Pd]alloy and (%Pd)slag are the concentrations of palladium in the corresponding phases. The functional relationship between L and the oxygen partial pressure can be obtained using the oxidic dissolution reaction of palladium in the slag, which can be written as:
where x refers to the oxidation degree of the palladium oxide present in the molten slag. The dissolving palladium in Eq. (3) was written as an alloy, due to dissolution of iron from the slag. Using the equilibrium constant equation of Eq. (3) combined with the apparent distribution coefficient La/sPd in Eq. (2), the reorganized form for the thermodynamic distribution coefficient ‘La/sPd is:
In this case, the composition of the palladium alloy, the activity coefficient of palladium f[Pd], as well as the number of moles in the alloy, nT[alloy], vary so significantly that they must be taken into account when calculating the oxygen activity slope of the metal–slag distribution coefficient. In Eq. (4), the symbols nT(slag) and nT[alloy] refer to the number of moles in 100 g slag and alloy, respectively, K is the equilibrium constant of Eq. (3), and f[Pd] and f(PdOx) are the Raoultian activity coefficients of palladium in the solid alloy and palladium oxide in the silicate melt, respectively. The obtained concentrations and activity data of palladium13 allowed us to estimate the dissolving oxide form of palladium in the slag according to the thermodynamic distribution coefficient derived in Eq. (4) and presented in Fig. 6. Additionally, the apparent distribution coefficient and reference studies are included in Fig. 6.
The thermodynamic distribution coefficient of palladium between pure metallic Pd and molten iron silicate slag saturated with solid alumina–iron spinel at 1300°C. The apparent distribution coefficient values obtained in this study, as well as results of several earlier studies, have been plotted for comparison.
According to Eq. (4), the oxidation mechanism of palladium or the oxidation degree in the slag can be obtained from the slope of log ‘La/sPd versus log pO2 plot. The slope of the line was constant at 0.25 over the entire oxygen partial pressure range studied, as shown in Fig. 6. This suggests that the dissolving oxide species of palladium in the slag was PdO0.5 or that palladium was present in the molten iron silicate as a monovalent cation Pd+. This agrees with our previous studies5,6 in the copper–slag system, where the palladium form in slags was evaluated to be PdO0.5. Moreover, the observed oxidation mechanism of this study is in good agreement with the measurements of Borisov and Palme28 and Laurenz et al.29 Thus, no further discussion about the presence of Pd° at present oxygen partial pressures as delivered by Sullivan et al.1 is necessary for iron-rich silicate slags. The relatively high palladium concentration (~ 100 ppm) found in the iron silicate slag at high oxygen partial pressures suggests reducing the slag cleaning conditions for successful palladium recovery. This may be related to the high stability of palladium oxide (standard Gibbs free energy of formation) at elevated temperatures.5 All the reference studies in Fig. 6 present distribution coefficients of palladium between copper and different slags, except for the geological study by Borisov and Palme.28
Yamaguchi8 and Nishijama and Yamaguchi2 reported copper–slag distribution coefficients for Pd which were approximately 1.5 orders of magnitude lower in logarithmic scale than the values obtained in the present study. The data by Shuva et al.3 at 1300°C suggested much higher palladium solubility in the slag and a strong influence of MgO concentration as well as of the slag basicity (CaO + MgO)/SiO2. The technique used in those studies was a bulk analysis by ICP-atomic emission spectroscopy of the slag where metal entrainment in sampling may cause major errors. Thus, in the cases of a high metal-to-slag distribution coefficient (> 100) the trace element concentrations in the slag can be seriously distorted. In the above cases,2,3,8 the distribution coefficients reported were 1.5–2 orders of magnitude in log scale smaller than obtained in this study, where true chemically dissolved concentrations were measured by direct phase analyses of the glassy silicate phase without the need for mechanical phase separation.
The distribution coefficients of Pd between copper and iron silicate slag at 1300°C by Avarmaa et al.5 and Sukhomlinov et al.6 are slightly higher than the ones obtained in this study. The values obtained by Avarmaa et al. between copper and high-alumina iron silicate slag4 correspond to the present values in reducing conditions but are also slightly on the higher side in oxidizing atmospheres. A comparison of the present experimental solubilities of palladium with geochemical data from low-iron basaltic silicates at 1300–1350°C and 1 atm total pressure by Sullivan et al.1 and Borisov and Palme28 are in good agreement with the values obtained in this study at 1300°C. The distribution coefficients of Pd between pure Pd (a = 1) and basalts were slightly on the higher side in oxidizing conditions but similar to our results in reducing conditions.1,28
The influence of copper on the distribution coefficient of palladium has been small when compared to our previous studies4,5,6 in copper-saturated systems, as the distribution coefficients obtained previously were of the same order of magnitude despite the presence of metallic copper, especially in the reducing conditions. Additionally, this study clarifies the behavior of palladium at reducing, low-oxygen partial pressure conditions, in which the concentrations in slags at copper saturation were previously below the detection limits of LA-ICP-MS.4,6 Here, we have also provided strong evidence that with FeOx-SiO2-Al2O3 slags containing copper, palladium results can be presented according to concentration results achieved with isotope 104Pd or with corrected results by isotopes 106Pd and 108Pd. Nevertheless, when new systems containing additional elements are characterized with LA-ICP-MS, all possible isobaric interferences need to be considered and carefully examined.
Conclusion
Palladium is a critical element of the EU, the availability of which in the future needs to be secured. This study provided new insights to its behavior, recyclability, and recoverability via ferrous and non-ferrous smelting conditions. In order to confirm the solubility of palladium in alumina-rich iron silicate slags, an experimental series was completed with direct contact between palladium and the slag. The experimental conditions were typical copper and nickel smelting/converting as well as slag cleaning conditions at pO2 = 10−5 to 10−10 atm and 1300°C. The obtained results formed a coherent dataset with a constant thermodynamic distribution coefficient versus the pO2 slope of 0.25, indicating a solution of Pd as the PdO0.5 (Pd+) form in slag, for the metal–slag distribution coefficient between 104 and 105.5.
References
N. Sullivan, Z. Zajecz, and J. Brenan, Geochim. Cosmochim. Acta 231, 15. (2018).
W. Nishijima, and K. Yamaguchi, J. Jpn. Inst. Metals Mater. 78, 267. (2014).
M. Shuva, M. Rhamdhani, G. Brooks, S. Masood, and M. Reuter, Metall. Mater. Trans. B 48B, 317. (2017).
K. Avarmaa, H. O’Brien, L. Klemettinen, and P. Taskinen, J. Mater. Cycles Waste Manag. 22, 642. (2020).
K. Avarmaa, L. Klemettinen, H. O’Brien, and P. Taskinen, Miner. Eng. 133, 95. (2019).
D. Sukhomlinov, L. Klemettinen, K. Avarmaa, H. O’Brien, and P. Taskinen, Metall. Mater. Trans. B 50B, 1752. (2019).
K. Yamaguchi, in Proc. Extraction 2018, ed. by. B. Davis, M. Moats & S. Wang (Ottawa, Canada, August 26–29, 2018) (Springer, TMS Warrendale (PA), 2018), p. 797
K. Yamaguchi, in Proceedings of the 6th European Metallurgical Conference (EMC2011), (Düsseldorf, Germany, June 26–29, 2011) (GDMB, Clausthal-Zellerfeld, 201), p. 171
H. Henao, K. Yamaguchi, and S. Ueda, in Proc. Sohn Int. Symp. ed. by F. Kongoli & R.G. Reddy (San Diego, US, August 27–31, 2006) (TMS, Warrendale (PA), 2006) p. 723
K. Avarmaa, H. O’Brien, H. Johto, and P. Taskinen, J. Sustain. Metall. 1, 216. (2015).
P. Piskunen, K. Avarmaa, H. O’Brien, L. Klemettinen, H. Johto, and P. Taskinen, Metall. Mater. Trans. B 49B, 98. (2017).
J. Tomiska, Thermochim. Acta 535, 42. (2012).
G. Ghosh, C. Kantner, and G. Olson, J. Phase Equilib. 20, 295. (1999).
M. Guillong, L. Danyushevsky, M. Walle, and M. Raveggi, J. Anal. At. Spectrom. 26, 1401. (2011).
M. Benson, C. Bennett, J. Harry, M. Patel, and M. Cross, Resour. Conserv. Recycl. 31, 1. (2000).
L. Klemettinen, K. Avarmaa, H. O’Brien, A. Jokilaakso, and P. Taskinen, JOM 72, 2770. (2019).
L. Klemettinen, K. Avarmaa, and P. Taskinen, J. Sust. Metall. 3, 772. (2017).
L. Klemettinen, K. Avarmaa, A. Jokilaakso, and P. Taskinen, J. Alloys Compd. https://doi.org/10.1016/j.jallcom.2020.157539 (2020).
K. Jochum, U. Weis, B. Stoll, D. Kuzmin, Q. Yang, I. Raczek, D. Jacob, A. Stracke, K. Birbaum, D. Frick, D. Günther, and J. Enzweiler, Geostand. Geoanal. Res. 35, 397. (2011).
K. Jochum, M. Willbold, I. Raczek, B. Stoll, and K. Herwig, Geostand. Geoanal. Res. 29, 285. (2005).
E. van Achterberg, C. Ryan, S. Jackson, and W. Griffin, Laser Ablation ICP-MS in the Earth Science: Principles and Applications, Short Course Series #29 (Mineralogical Association of Canada, St John, Newfoundland, 2001), p 239.
K. Jochum, U. Weis, B. Stoll, D. Kuzmin, Q. Yang, I. Raczek, and D. Günther, Geostand. Geoanal. Res. 35, 397. (2011).
N. Sugiyama, and Y. Shikamori, J. Anal. Atom. Spectrom. 30, 2481. (2015).
P. Sylvester, Laser-Ablation in the Earth Sciences: Principles and Application (Mineralogical Association of Canada, 2001), p 203.
IUPAC Periodic Table of Elements and Isotopes, https://www.isotopesmatter.com/applets/IPTEI/IPTEI.html Accessed on 4 Dec 2020
V. Malavergne, E. Charon, J. Jones, P. Cordier, K. Righter, D. Deldicque, and L. Hennet, Earth Planet. Sci. Lett. 434, 197. (2016).
A. Yazawa, Erzmetall 33, 377. (1980).
A. Borisov, and H. Palme, Mineral. Petrol. 56, 297. (1996).
V. Laurenz, R. Fonseca, C. Ballhaus, and P. Sylvester, Geochim. Cosmochim. Acta 74, 2989. (2010).
Acknowledgements
Financial support by CMEco (Grant # 7405/31/2016) and SYMMET (Grant # 3891/31/2018) programs of Business Finland are gratefully acknowledged. Additional funding from the Alfred Kordelin Foundation, Finnish Cultural Foundation as well as the Emil Aaltonen Foundation (KA), and the Finnish Steel and Metals Producers’ Fund (LK) is also appreciated. The EPMA measurements were carried out by Mr. Lassi Pakkanen of the Geological Survey of Finland. This study utilized the Academy of Finland’s RawMatTERS Finland Infrastructure (RAMI) housed at Aalto University, GTK and VTT.
Funding
Open access funding provided by Aalto University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Avarmaa, K., Klemettinen, L., O’Brien, H. et al. Solubility of Palladium in Alumina-Iron Silicate Melts. JOM 73, 1871–1877 (2021). https://doi.org/10.1007/s11837-021-04672-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-021-04672-4