Abstract
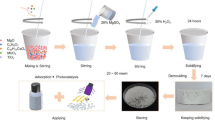
For the circulation of sodium carbonate in treating tungsten concentrate by the autoclave soda leaching, preparation of double ammonium-sodium paratungstate (DASP) from Na2WO4-Na2CO3 solution and its transformation to ammonium paratungstate (APT) were studied experimentally. The result shows that DASP can be obtained by the reaction of CO2-(NH4)2CO3 with Na2WO4-Na2CO3 solution, and almost all of Na remain in the spent liquid, which can be returned to the leaching process of tungsten concentrate. Moreover, the transformation of DASP to APT can be achieved by the reaction of DASP with (NH4)2CO3 or (NH4)2SO4. In (NH4)2CO3 solution, APT can be prepared by hydrothermal reaction at 120°C, but the tungsten conversion ratio is only 63.94% for 6 h. In the (NH4)2SO4 system, irregularly stacked APT and more than 90% of the tungsten conversion ratio can be achieved by hydrothermal transformation, whereas DASP can completely transform to quadrate APT by in situ reaction at 120°C for 6 h. The results presented are conducive to improve the soda leaching method and achieve cleaner, economical, and efficient preparation of APT.




Similar content being viewed by others
REFERENCES
R.P.S. Gaur, JOM 58, 45 (2006).
Y.L. Wang, S.H. Yang, and H. Li, Int. J. Refract. Met. Hard Mater. 54, 284 (2016).
E. Lassner and W.-D. Schubert, Tungsten: Properties, Chemistry, Technology of the Element, Alloys and Chemical Compounds (New York: Kluwer Academic, 1999), pp. 133–250.
J.P. Martins, Hydrometallurgy 42, 221 (1996).
Z. Zhao, J.T. Li, S.B. Wang, H.G. Li, M.S. Liu, P.M. Sun, and Y.J. Li, Hydrometallurgy 108, 152 (2011).
Z.W. Zhao, L.P. Xiao, C.H. Guo, and X.Y. Chen, Trans. Nonferrous Met. Soc. China 20, 2379 (2010).
E. Lassner, Int. J. Refract. Met. Hard Mater. 13, 35 (1995).
H.G. Li, Trans. Nonferrous Met. Soc. China 14, 366 (2004).
H. Singh and O.P. Pandey, Ceram. Int. 39, 6703 (2013).
G.Q. Zhang, W.J. Guan, L.S. Xiao, and Q.X. Zhang, Hydrometallurgy 165, 233 (2016).
Z.W. Zhao, L.P. Xiao, C.H. Guo, and X.Y. Chen, Trans. Nonferrous Met. Soc. China 20, 2379 (2010).
K. Vadasdi, Int. J. Refract. Met. Hard Mater. 13, 45 (1995).
M. Shi, Z.Y. Tang, and X.Y. Chen, Rare Met. Cem. Carbides 2, 1 (2015).
H.Q. Liang and S.H. Yu, Shanghai Jinshu 7, 1 (1986).
E.V. Peresypkina, A.V. Virovets, S.A. Adonin, P.A. Abramov, A.V. Rogachev, P.L. Sinkevich, V.S. Korenev, and M.N. Sokolov, J. Struct. Chem. 55, 295 (2014).
H.G. Li, J.G. Yang, and K. Li, Tungsten Metallurgy (Changsha: Central South University Press, 2010) (in Chinese).
Q. Shi, Master of science thesis (Changsha: Central South University, 2013). (in Chinese)
X.B. Li, Q.S. Zhou, and Q. Sh, CN Patent NO:102963933A, 2012-12-01. (in Chinese)
GB/T 4324.17-2012, Method of Chemical Analysis of Tungsten [S] (Beijing: China Standards Press, 2013). (in Chinese)
S.S. Al-Jaroudi, A. Ul-Hamid, A.I. Mohammed, and S. Saner, Powder Technol. 175, 115 (2007).
J.W. Van Put, Int. J. Refract. Met. Hard Mater. 13, 61 (1995).
Y.J. Li, P.M. Sun, H.G. Li, P.T. Su, Z.W. Zhao, and M.S. Li, J. Cent. South Univ. 4, 32 (1997).
Acknowledgements
This work is financially supported by the National Natural Science Foundation of China (No. 51274243).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, X., Lu, Z., Yun, W. et al. Preparation of Double Ammonium-Sodium Paratungstate and Its Transformation to Ammonium Paratungstate. JOM 70, 2523–2528 (2018). https://doi.org/10.1007/s11837-018-3037-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-018-3037-3