Abstract
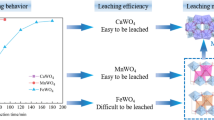
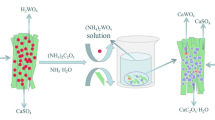
Complete wolframite conversion in sulfuric acid is significant for expanding the applicability of the sulfuric acid method for producing ammonium paratungstate. In this paper, the conversion of wolframite in treating a mixed wolframite–scheelite concentrate by sulfuric acid was studied systematically. The results show that the conversion of wolframite in sulfuric acid is more difficult than that of scheelite, requiring rigorous reaction conditions. A solid H2WO4 layer forms on the surfaces of the wolframite particles and becomes denser with increasing H2SO4 concentration, thus hindering the conversion. Furthermore, the difficulty in wolframite conversion can be mainly attributed to the accumulation of Fe2+ (and/or Mn2+) in the H2SO4 solution, which can be solved by reducing Fe2+ (and/or Mn2+) concentration through oxidization and/or a two-stage process. Additionally, the solid converted product of the mixed wolframite–scheelite concentrate has an excellent leachability of tungsten in an aqueous ammonium carbonate solution at ambient temperature, with approximately 99% WO3 recovery. This work presents a route for manufacturing ammonium paratungstate by treating the mixed concentrate in sulfuric acid followed by leaching in ammonium carbonate solution.




Similar content being viewed by others
References
D.H. Yang, R.R. Srivastava, M.S. Kim, D.D. Nam, J.C. Lee, and T.H. Hai, Met. Mater. Int. 22, 897 (2016).
E. Lassner, W. Schubert, E. Lüderitz, and H.U. Wolf, Tungsten, Tungsten Alloys, and Tungsten Compounds. Ullmann’s Encyclopedia of Industrial Chemistry (Weinheim: Wiley-VCH Verlag GmbH & Co. KGaA, 2012). https://doi.org/10.1002/14356007.a27_229.
R.P.S. Gaur, JOM 58, 45 (2006).
E. Lassner, Int. J. Refract. Met. Hard Mater. 13, 35 (1995).
J.I. Martins, Min. Proc. Ext. Met. Rev. 35, 23 (2014).
K. Vadasdi, Int. J. Refract. Met. Hard Mater. 13, 45 (1995).
Y.M. Potashnikov, A.M. Gamol’skii, and M.V. Mokhosoev, F.M. Zh. Neorg. Khim. 15, 502 (1970).
A.O. Kalpakli, S. Ilhan, C. Kahruman, and I. Yusufoglu, Hydrometallurgy 121–124, 7 (2012).
G.H. Xuin, D.Y. Yu, and Y.F. Su, Hydrometallurgy 16, 27 (1986).
S. Gurmen, S. Timur, C. Arslan, and I. Duman, Hydrometallurgy 51, 227 (1999).
J.T. Li and Z.W. Zhao, Hydrometallurgy 163, 55 (2016).
J.I. Martins, A. Moreira, and S.C. Costa, Hydrometallurgy 70, 131 (2003).
J.I. Martins, Ind. Eng. Chem. Res. 42, 5031 (2003).
F.A. Forward, and A.I. Vizsolyi, United States Patent, No. 3193347 (1965).
X.B. Li, L.T. Shen, Q.S. Zhou, Z.H. Peng, G.H. Liu, and T.G. Qi, Hydrometallurgy 171, 106 (2017).
A.N. Zelikman, A.S. Medvedev, and Z.O. Kadyrova, Izv. V.U.Z. Tsvetn. Metall. 3, 69 (1986).
H. Xie, The novel technology of extracting wolframite with sulfuric acid, (Changsha, Master of Science Thesis, Central South University, China 2011) (in Chinese).
S.S. Al-Jaroudi, A. Ul-Hamid, A.R.I. Mohammed, and S. Saner, Powder Technol. 175, 115 (2007).
X.B. Li, X.M. Xu, Q.S. Zhou, Z.H. Peng, G.H. Liu, and T.G. Qi, et al., Int. J. Refract. Met. Hard Mater. 52, 151 (2015).
S.C. Srivastava, S.R. Bhaisare, D.N. Wagh, and C.P.S. Iyer, Bull. Mater. Sci. 19, 331 (1996).
A. Roine, HSC Chemistry, vers. 9.0, Outotec Research Oy, Pori (Finland), Mar. 2016. http://www.outotec.com/products/digital-solutions/hsc-chemistry/.
C. Horner, Chem. Geol. 27, 85 (1979).
J.G. Speight, Lange’s Handbook of Chemistry, 16th ed. (New York: McGraw-Hill Book Co., 2005) (Sec. one.).
Acknowledgements
This work is financially supported by the National Natural Science Foundation of China (No. 51274243).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shen, L., Li, X., Zhou, Q. et al. Wolframite Conversion in Treating a Mixed Wolframite–Scheelite Concentrate by Sulfuric Acid. JOM 70, 161–167 (2018). https://doi.org/10.1007/s11837-017-2691-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-017-2691-1