Abstract
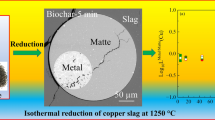
Copper and cobalt are two of the most valuable metals that can be recovered from copper converter slag. In the reduction–vulcanization process, copper is reduced before cobalt, while FeS vulcanizes Cu2O into Cu2S and forms the matte phase. The matte phase can dissolve the reduced metals as solvent. In this study, the distribution coefficient of cobalt between metallic cobalt in matte and CoO in slag, namely L Co, was calculated to be 5000–8500 at the reaction temperature of 1600–1700 K, while the distribution coefficient between CoS and CoO, namely \(L_{\text{Co}}^{{^{\prime } }}\), was calculated to be between 6 and 8. The distribution coefficient of copper between metallic copper in matte and Cu2O in slag, namely L Cu, was calculated to be in the range of 7500–8500, while the coefficient between Cu2S and Cu2O, namely \(L_{\text{Cu}}^{{^{\prime } }}\), was calculated to be in the range of 60,000–75,000.




Similar content being viewed by others
References
C.Y. Fu, Principle of Nonferrous Metallurgy, 2nd ed. (Beijing: Metallurgical Industry Press, 2007), pp. 64–72.
J.Y. Zhang, Physical Chemistry of Metallurgy (Beijing: Metallurgical Industry Press, 2004), pp. 312–315.
R. Sridhar, J.M. Toguri, and S. Simeonov, Metall. Mater. Trans. B 28, 191 (1997).
Y.J. Liu, Introduction to Elemental Geochemistry (Beijing: Geological Publishing House, 1987), pp. 220–222.
X.J. Zhai, Heavy Metal Metallurgy (Beijing: Metallurgical Industry Press, 2011), pp. 320–330.
P.F. Tan and C.F. Zhang, J. Cent. S. Univ. Technol. 4, 36 (1997).
E.J. Grimsey and X. Liu, Metall. Mater. Trans. B 26, 229 (1995).
S.K. Wei, Thermodynamics of Metallurgical Processes (Shanghai: Shanghai Science and Technology Press, 1980), p. 67.
R. Shimpo, S. Goto, and O. Ogawa, Can. Metall. Q. 25, 113 (1986).
R. Shimpo, Y. Watanabe, S. Goto, and H.Y. Sohn, Advances in Sulfide Smelting (Beijing: Metallurgical Industry Press, 1990), p. 283.
S.N. Sinha and M. Nagamori, J. Electron Microsc. 20, 461 (1991).
K.C. Teague, D.R. Swinbourne, and S.A. Jahanshahi, Mater. Trans. B 32, 47 (2001).
N. Jacinto, S.N. Sinha, M. Nagamori, and H.Y. Sohn, Mater. Trans. B 14, 136 (1983).
R. Shimpo, O. Ogawa, and S. Goto, Mater. Trans. A 24, 1882 (1993).
Acknowledgements
The authors gratefully acknowledge financial support from the National Science Foundation of P. R. China for Research Project 51234008 and the Beijing Technical Development Project (00012132).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Du, K., Li, H. & Zhang, M. Calculation of Distribution Coefficients of Cobalt and Copper in Matte and Slag Phases in Reduction–Vulcanization Process of Copper Converter Slag. JOM 69, 2379–2382 (2017). https://doi.org/10.1007/s11837-017-2542-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-017-2542-0