Abstract
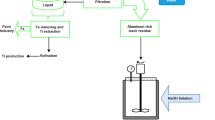
Aluminothermic reduction-electrolysis using an inert anode process is proposed to extract oxygen and metals from Minnesota Lunar Simulant-1 (MLS-1). Effective aluminothermic reduction between dissolved MLS-1 and dissolved metal aluminum was achieved in cryolite salt media. The product phases obtained by aluminothermic reduction at 980°C for 4 h were Al, Si, and Al5FeSi, while the chemical components were 79.71 mass% aluminum, 12.03 mass% silicon, 5.91 mass% iron, and 2.35 mass% titanium. The cryolite salt containing Al2O3 was subsequently electrolyzed with Fe0.58-Ni0.42 inert anode at 960°C for 4 h. Oxygen was evolved at the anode with an anodic current efficiency of 78.28%. The results demonstrate that this two-step process is remarkably feasible for the extraterrestrial extraction of oxygen and metals. This process will help expand the existing in situ resource utilization methods.



Similar content being viewed by others
References
I.A. Crawford, Prog. Phys. Geog. 39, 137 (2015).
G.J. Taylor and L.M.V. Marte, Adv. Space Res. 31, 2403 (2003).
W.H. Siegfried, Acta Astronaut. 44, 755 (1999).
M.B. Duke, B.R. Blair, and J. Diaz, Adv. Space Res. 31, 2413 (2003).
G.B. Sanders and W.E. Larson, J. Aerosp. Eng. 26, 5 (2013).
W.D. Carrier, J. Geotech. Geoenviron. Eng. 129, 956 (2003).
Y. Zhao and F. Shadman, AIChE J. 36, 1433 (1990).
Y. Kobayashi, H. Sonezaki, R. Endo, and M. Susa, ISIJ Int. 50, 35 (2010).
C.C. Allen, R.V. Morris, and D.S. McKay, J. Geophys. Res.: Planets 101, 26085 (1996).
A.H.C. Sirk, D.R. Sadoway, and L. Sibille, ECS Trans. 28, 367 (2010).
A.M. Liu, Z.N. Shi, J.L. Xu, X.W. Hu, B.L. Gao, and Z.W. Wang, JOM 68, 1518 (2016).
A.M. Liu, J.Z. Guan, K.Y. Xie, and Z.N. Shi, J. Northeast. Univ. Nat. Sci. 37, 522 (2016).
W. Steurer, Space Resources: Materials, ed. M.F. Mckay, D.S. Mckay, and M.B. Duke (Houston: Johnson Space Center, 1992), pp. 210–213.
M.J. Eick, P.R. Grossl, D.C. Golden, D.L. Sparks, and D.W. Ming, Geoderma 74, 139 (1996).
G.A. Landis, Acta Astronaut. 60, 906 (2007).
G. Corrias, R. Licheri, R. Orrù, and G. Cao, Acta Astronaut. 70, 69 (2012).
C. Schwandt, J.A. Hamilton, D.J. Fray, and I.A. Crawford, Planet. Space Sci. 74, 49 (2012).
L.A. Taylor and W.D. Carrier III, AIAA J. 30, 2858 (1992).
H. Xiao, R. Hovland, S. Rolseth, and J. Thonstad, Metall. Mater. Trans. B 27, 185 (1996).
D.R. Sadoway, JOM 53 (5), 34 (2001).
Z.N. Shi, J.L. Xu, Z.X. Qiu, B.L. Gao, and Z.W. Wang, JOM 55 (11), 63 (2003).
P.W. Weiblen, M.J. Murawa, and K.J. Reid, Engineering, Construction, and Operations in Space II, ed. S.W. Johnson and J. Wetzel (New York: American Society of Civil Engineers, 1990), pp. 428–435.
E. Hill, M.J. Mellin, B. Deane, Y. Liu, and L.A. Taylor, J. Geophys. Res. 112, E02006 (2007).
J.S. Zhang and Z.X. Qiu, Light Met. 6, 10 (1988).
K. Grjotheim, C. Krohn, M. Malinovský, K. Matiašovský, and J. Thonstad, Aluminium Electrolysis: Fundamentals and the Hall-Héroult Process, 2nd ed. (Düsseldorf: Aluminium-Verlag, 1982), pp. 364–365.
E. Skybakmoen, A. Solheim, and A. Sterten, Metall. Mater. Trans. B 28, 81 (1997).
W.E. Haupin and W.C. McGrew, Aluminum 51, 273 (1975).
Z.X. Qiu, L.M. Fan, K. Grjotheim, and H. Kvande, J. Appl. Electrochem. 17, 707 (1987).
V. Chapman, B.J. Welch, and M. Skyllas-Kazacos, Electrochim. Acta 56, 1227 (2011).
Acknowledgements
The authors would like to acknowledge the financial support from the National Key Research and Development Program of China (No. 2017YFC0805101) and the National Natural Science Foundation of China (No. 51574071). Acknowledgment is also made to Dr. Rudolf Keller for enthusiastically providing the lunar soil simulant MLS-1.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xie, K., Shi, Z., Xu, J. et al. Aluminothermic Reduction-Molten Salt Electrolysis Using Inert Anode for Oxygen and Al-Base Alloy Extraction from Lunar Soil Simulant. JOM 69, 1963–1969 (2017). https://doi.org/10.1007/s11837-017-2478-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-017-2478-4