Abstract
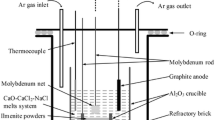
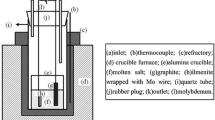
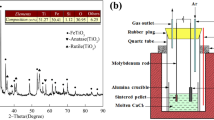
Electrolysis-assisted calciothermic reduction method is proposed and successfully used to prepare ferrotitanium alloy from ilmenite by using equal-molar CaCl2-NaCl molten salt as electrolyte, molybdenum rod as cathode, and graphite as anode at 973 K with cell voltages of 3.2–4.4 V under inert atmosphere. Thermodynamics analysis of the process is presented, and the products obtained are examined with x-ray diffraction, scanning electron microscopy, and energy-dispersive spectroscopy. It is demonstrated that the calciothermic reduction of ilmenite is a stepwise process since intermediate CaTiO3 is observed in the products partially reduced. In the calciothermic reduction process, the reduction of FeTiO3 first gives rise to the formation of Fe and CaTiO3, which as intermediates will further react with calcium metal to form ferrotitanium alloys. This is in good agreement with the prediction of thermodynamics. Experimental results also show that increasing cell voltage can accelerate the formation of calcium metal through electrolysis of CaO and CaCl2 and, hence, promote the calciothermic reduction of ilmenite. As the electrolytic zone and reduction zone are combined in the same bath, the theoretical energy requirement for the production of FeTi in the calciothermic process is lower than that in the aluminothermic process.






Similar content being viewed by others
References
M. Panigrahi, P.K. Paramguru, R.C. Gupta, E. Shibata, and T. Nakamura, High Temp. Mat. Process. -Isr. 29, 495 (2010).
B. Sakintuna, F. Lamari-Darkrim, and M. Hirscher, Int. J. Hydrog. Energy 32, 1121 (2007).
M. Panigrahi, E. Shibata, A. Iizuka, and T. Nakamura, Electrochim. Acta 93, 143 (2013).
M. Panigrahi, A. Iizuka, E. Shibata, and T. Nakamura, J. Alloys Compd. 550, 545 (2013).
A.A. Francis and A.A. EI-Midany, J. Mater. Process. Technol. 199, 279 (2008).
N.J. Welham, Miner. Eng. 9, 1189 (1996).
V.M. Sokolov, V.D. Babyuk, Y.A. Zhydkov, and Y.Y. Skok, Miner. Eng. 21, 143 (2008).
G.Z. Chen, Miner. Process. Extr. Metall. (Trans. Inst. Min. Metall. C) 124, 106 (2014).
M. Hu, C. Bai, X. Liu, X.I. Lv, and J. Du, J. Min. Metall. B 47, 193 (2011).
X. Liu, M. Hu, C. Bai, and X. Lv, High Temp. Mater. Process. -Isr. 33, 377 (2014).
G.Z. Chen, D.J. Fray, and T.W. Farthing, Nature 407, 361 (2000).
R.O. Suzuki, J. Phys. Chem. Solids 66, 461 (2005).
K. Ono and R.O. Suzuki, JOM 54, 59 (2002).
T.H. Okabe, R.O. Suzuki, T. Oishi, and K. Ono, Mater. Trans. JIM 32, 485 (1991).
R.O. Suzuki and S. Fukui, Mater. Trans. 45, 1665 (2004).
A.M. Abdelkader, K.T. Kilby, A. Cox, and D.J. Fray, Chem. Rev. 113, 2863 (2013).
R.O. Suzuki and S. Inous, Metall. Mater. Trans. B 34, 277 (2003).
R.O. Suzuki, K. One, and K. Teranuma, Metall. Mater. Trans. B 34, 287 (2003).
R.O. Suzuki, JOM 59, 68 (2007).
R.O. Suzuki, M. Aizawa, and K. Ono, J. Alloys Compd. 288, 173 (1999).
M. Baba, Y. Ono, and R.O. Suzuki, J. Phys. Chem. Solids 66, 466 (2005).
T. Kikuchi, M. Yoshida, S. Matsuura, S. Natsui, E. Tsuji, H. Habazaki, and R.O. Suzuki, J. Phys. Chem. Solids 75, 1041 (2014).
J. Jia, B. Xu, B. Yang, D. Wang, and D. Liu, JOM 65, 630 (2013).
M. Peretti, JOM 61, 44 (2009).
A. Martin, D. Lambertin, J.C. Poignet, M. Allibert, G. Bourges, L. Pescayre, and J. Fouletier, JOM 55, 52 (2003).
D.A. Wenz, I. Johnson, and R.D. Wolson, J. Chem. Eng. Data 14, 250 (1969).
K.M. Axler and G.L. DePoorter, Mater. Sci. Forum 73, 19 (1991).
Acknowledgement
The authors acknowledge the financial support of the National Natural Science Foundation of China (Project Nos. 51274108 and 21263007).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhou, Z., Hua, Y., Xu, C. et al. Preparation of Ferrotitanium from Ilmenite by Electrolysis-Assisted Calciothermic Reduction in CaCl2-NaCl Molten Salt. JOM 68, 532–539 (2016). https://doi.org/10.1007/s11837-015-1723-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-015-1723-y