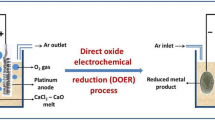
Abstract
The reduction of a metal oxide by calcium in a CaCl2 melt produces metal and calcium oxide (CaO) dissolved in the molten chloride. To minimize waste salts, the reacted salts have to be regenerated. This can be done either by converting CaO to CaCl2 by chlorination with Cl2 and adding calcium metal or by electrochemical deoxidation of CaO. In the first method, the addition of calcium metal increases the amount of reacted salts, and consequently the waste salts, while in the second method the quantity of reacted salts remains constant. The electrochemical deoxidation of CaO occurs in one step and the produced oxygen is evacuated. This article describes a study of the electrochemical deoxidation of CaO using a solid oxide membrane.
Similar content being viewed by others
References
P.D. Ferro, B. Mishra, and W.A. Averill, “Electroreduction of Calcium Oxide,” Light Metals 1991, ed. Elwin L. Rooy (Warrendale, PA: The Minerals, Metals & Materials Society, 1991), pp. 1197–1204.
A. Matthiessen, “On the Preparation of the Metals of the Alkalis and Alkaline Earths by Electrolysis,” J. Chem. Soc. of London, 8 (1856), pp. 27–30.
P.D. Ferro et al., “Recovery of Calcium from the Effluent of Direct Oxide Reduction Process,” Residues and Effluents, Processing and Environmental Considerations, ed. R.G. Reddy, W.P. Imrie, and P.B. Queneau (Warrendale, PA: The Minerals, Metals & Materials Society, 1991), pp. 539–550.
J.H. Goodwin, “The Electrolytic Production of Calcium,” J. Am. Chem. Soc., 25 (1903), pp. 873–876.
J.H. Goodwin, “Electrolytic Calcium,” J. Am. Chem. Soc., 27 (1905), pp. 1403–1415.
S.A. Tucker and J.B. Whitney, “Some Observations on the Production of Metallic Calcium by Electrolysis,” J. Am. Chem. Soc., 28 (1906), pp. 84–87.
U.B. Pal, D.E. Woolley, and G.B. Kenney, “Emerging SOM Technology for the Green Synthesis of Metals from Oxides,” JOM, 53 (10) (2001), pp. 32–35.
J.A. Sanchez and R. Monnier, “Etude du Diagramme de Phases du Système CaCl2-Ca par ATD,” Rev. int. Hautes Tempér. Réfract., 16 (1979), pp. 5–11.
K.M. Axler and G.L. DePoorter, “Solubility Studies of the Ca-CaO-CaCl2 System,” Materials Science Forum, Trans Tech Publications, 73–75 (1991), pp. 19–24.
P.D. Ferro et al., “Application of Ceramic Membrane in Molten Salt Electrolysis of CaO-CaCl2,” Waste Management, 17 (7) (1997), pp. 451–461.
Von G. Wehner, “Zur kenntnis des Calcium-Subchlorides,” Z. Anorg. Allg. Chem., 276 (1954), pp. 72–76.
A.V. Virkar et al., “Internal Precipitation of Molecular Oxygen and Electrochemical Failure of Zirconia Solid Electrolytes,” J. Am. Ceram. Soc., 73 (11) (1990), pp. 3382–3390.
C. Deportes et al., editors, Electrochimie des Solides (Grenoble, France: Presses Universitaires de Grenoble, 1994).
L. Dessemond, “Spectroscopie d’impédance des Fissures dans la Zircone Cubique,” Grenoble I.N.P. Ph.D. thesis (1992).
Author information
Authors and Affiliations
Additional information
A. Martin is with LEPMI and CEA/Valduc; D. Lambertin, G. Bourges, and L. Pescayre are with CEA/Valduc; J.-C. Poignet and J. Fouletier are with LEPMI; and M. Allibert is with LTPCM.
Rights and permissions
About this article
Cite this article
Martin, A., Lambertin, D., Poignet, J.C. et al. The electrochemical deoxidation of metal oxides by calcium using a solid oxide membrane. JOM 55, 52–54 (2003). https://doi.org/10.1007/s11837-003-0177-9
Issue Date:
DOI: https://doi.org/10.1007/s11837-003-0177-9