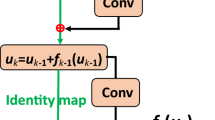
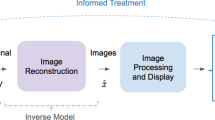
Abstract
Medical image reconstruction (MIR) is the elementary way of producing an internal 3D view of the patient. MIR is inherently ill-posed, and various approaches have been proposed to address to resolve the ill-posedness. Recent inverse problem aims to create a mathematically consistent framework for merging data-driven models, particularly based on machine learning and deep learning, with domain-specific information contained in physical–analytical models. This study aims to discuss some of the significant contributions of data-driven techniques to solve the inverse problems in MIR. This paper provides a detailed survey of MIR which includes the traditional reconstruction algorithm, machine learning and deep learning-based approaches such as GAN, autoencoder, RNN, U-net, etc., to solve inverse problems, evaluation metrics, and openly available codes used in the literature. This paper also summarises the contribution of the most recent state-of-the-art methods used in MIR. The potentially attractive strategic paths for future study and fundamental problems in MIR are also discussed.

















Similar content being viewed by others
References
Hsieh J et al (2013) Recent advances in CT image reconstruction. Curr Radiol Rep 1(1):39–51. https://doi.org/10.1007/s40134-012-0003-7
Kajla V et al (2018) Analysis of X-ray images with image processing techniques: a review. In: 2018 4th international conference on computing communication and automation (ICCCA). IEEE, pp 1–4. https://doi.org/10.1109/CCAA.2018.8777693
Crooks LE (1985) An introduction to magnetic resonance imaging. IEEE Eng Med Biol Mag 4(3):8–15. https://doi.org/10.1109/MEMB.1985.5006193
Jaszczak RJ et al (1980) SPECT: single photon emission computed tomography. IEEE Trans Nucl Sci 27(3):1137–1153. https://doi.org/10.1109/TNS.1980.4330986
Shukla AK, Kumar U (2006) Positron emission tomography: an overview. J Med Phys 31(1):13. https://doi.org/10.4103/0971-6203.25665
Andrew W, George CK (2003) Introduction to biomedical imaging. Med Phys 30(8):2267–2267. https://doi.org/10.1118/1.1589017
Kanitsar A et al (2001) Computed tomography angiography: a case study of peripheral vessel investigation. In: Proceedings visualization. VIS ’01. IEEE, pp 477–593. https://doi.org/10.1109/VISUAL.2001.964555
Cappabianco FA, Shida CS et al (2016) Introduction to research in magnetic resonance imaging. In: 2016 29th SIBGRAPI conference on graphics, patterns and images tutorials (SIBGRAPI-T). IEEE, pp 1–14. https://doi.org/10.1109/SIBGRAPI-T.2016.010
Hounsfield GN (1973) Computerized transverse axial scanning (Tomography): Part 1. Description of system. Br J Radiol 46(552):1016–1022. https://doi.org/10.1259/0007-1285-46-552-1016
Aarsvold JN, Miles NW (2004) Emission tomography: the fundamentals of pet and spect. Elsevier, Open WorldCat. http://www.123library.org/book_details/?id=42889
Geyer LL et al (2015) State of the art: iterative CT reconstruction techniques. Radiology 276(2):339–357. https://doi.org/10.1148/radiol.2015132766
Gordon R (1974) A tutorial on art (algebraic reconstruction techniques). IEEE Trans Nucl Sci 21(3):78–93. https://doi.org/10.1109/TNS.1974.6499238
Schofield R et al (2020) Image reconstruction: Part 1 – understanding filtered back projection, noise and image acquisition. J Cardiovasc Comput Tomogr 14(3):219–225. https://doi.org/10.1016/j.jcct.2019.04.008
Hara AK et al (2009) Iterative reconstruction technique for reducing body radiation dose at CT: feasibility study. Am J Roentgenol 193(3):764–771. https://doi.org/10.2214/AJR.09.2397
Wu Q et al (2017) The application of deep learning in computer vision. In: 2017 Chinese automation congress (CAC). IEEE, pp 6522–6527. https://doi.org/10.1109/CAC.2017.8243952
Jia Y et al (2014) Caffe: convolutional architecture for fast feature embedding. In: Proceedings of the 22nd ACM international conference on multimedia, association for computing machinery. ACM Digital Library, pp 675–78. https://doi.org/10.1145/2647868.2654889
Kukačka J et al (2017) Regularization for deep learning: a taxonomy. https://doi.org/10.48550/ARXIV.1710.10686
Ahishakiye E et al (2021) A survey on deep learning in medical image reconstruction. Intell Med 1(3):118–127. https://doi.org/10.1016/j.imed.2021.03.003
Zhou T et al (2022) Dense convolutional network and its application in medical image analysis. BioMed Res Int 2022:e2384830. https://doi.org/10.1155/2022/2384830
Krizhevsky A et al (2017) ImageNet classification with deep convolutional neural networks. Commun ACM 60(6):84–90. https://doi.org/10.1145/3065386
Tajbakhsh N et al (2016) Convolutional neural networks for medical image analysis: full training or fine tuning? IEEE Trans Med Imaging 35(5):1299–1312. https://doi.org/10.1109/TMI.2016.2535302
He K et al (2016) Deep residual learning for image recognition. In: 2016 IEEE conference on computer vision and pattern recognition (CVPR). IEEE, pp 770–78. https://doi.org/10.1109/CVPR.2016.90
Goodfellow IJ et al (2014) Generative adversarial networks. http://arxiv.org/abs/1406.2661
Yavuz M, Fessler JA (1998) statistical image reconstruction methods for randoms-precorrected PET scans. Med Image Anal 2(4):369–378. https://doi.org/10.1016/S1361-8415(98)80017-0
Cheng J, Hofmann B (2011) Regularization methods for ill-posed problems. In: Scherzer O (ed) Handbook of mathematical methods in imaging. Springer, New York, pp 87–109
Perelli A, Davies ME (2015) Compressive computed tomography image reconstruction with denoising message passing algorithms. In: 2015 23rd European Signal Processing Conference (EUSIPCO). IEEE, pp 2806–2010. https://doi.org/10.1109/EUSIPCO.2015.7362896
Liu H et al (2018) Image inpainting based on generative adversarial networks. In: 2018 14th International conference on natural computation, fuzzy systems and knowledge discovery (ICNC-FSKD). IEEE, pp 373–78. https://doi.org/10.1109/FSKD.2018.8686914
Pavlovic G, Tekalp AM (1992) Maximum likelihood parametric blur identification based on a continuous spatial domain model. IEEE Trans Image Process 1(4):496–504. https://doi.org/10.1109/83.199919
(2008) Algebraic and statistical reconstruction methods. In: Computed tomography. Springer, Berlin Heidelberg, pp 201–40. https://doi.org/10.1007/978-3-540-39408-2_6
Dobosz P (2012) An analytical approach to the image reconstruction problem using EM algorithm. In: Rutkowski L (ed) Artificial intelligence and soft computing, vol 7267. Springer, Berlin Heidelberg, pp 495–500
Gouia-Zarrad R (2014) Analytical reconstruction formula for n -dimensional conical radon transform. Comput Math Appl 68(9):1016–1023. https://doi.org/10.1016/j.camwa.2014.04.019
McCann MT, Unsen M (2019) Biomedical image reconstruction: from the foundations to deep neural networks. Found Trends Signal Process 13(3):283–357. https://doi.org/10.1561/2000000101
Fessler JA (2017) Medical image reconstruction: a brief overview of past milestones and future directions. http://arxiv.org/abs/1707.05927
Renker M et al (2011) Iterative image reconstruction techniques: applications for cardiac CT. J Cardiovasc Comput Tomogr 5(4):225–230. https://doi.org/10.1016/j.jcct.2011.05.002
Dong B et al (2015) Image restoration: a data-driven perspective. In Proceedings of the international congress of industrial and applied mathematics (ICIAM). Citeseer, pp 65–108
Rudin LI et al (1992) Nonlinear total variation based noise removal algorithms. Physica D 60(1):259–268. https://doi.org/10.1016/0167-2789(92)90242-F
Buades A et al (2011) Non-local means denoising. Image Process On Line 1:208–212. https://doi.org/10.5201/ipol.2011.bcm_nlm
Zhang K et al (2022) SOUP-GAN: super-resolution MRI using generative adversarial networks. Tomography 8(2):905–919. https://doi.org/10.3390/tomography8020073
Tensor Dictionary Learning with an Enhanced Sparsity Constraint for Sparse-View Spectral CT Reconstruction
Xie Q et al (2017) Robust low-dose CT sinogram preprocessing via exploiting noise-generating mechanism. IEEE Trans Med Imaging 36(12):2487–2498. https://doi.org/10.1109/TMI.2017.2767290
Zhang Y et al (2017) Low-dose lung ct image restoration using adaptive prior features from full-dose training database. IEEE Trans Med Imaging 36(12):2510–2523. https://doi.org/10.1109/TMI.2017.2757035
Andersen A (1984) Simultaneous algebraic reconstruction technique (SART): a superior implementation of the ART algorithm. Ultrason Imaging 6(1):81–94. https://doi.org/10.1016/0161-7346(84)90008-7
Willemink MJ et al (2013) Iterative reconstruction techniques for computed tomography part 1: technical principles. Eur Radiol 23(6):1623–1631. https://doi.org/10.1007/s00330-012-2765-y
Mango LJ (1994) Computer-assisted cervical cancer screening using neural networks. Cancer Lett 77(2–3):155–162. https://doi.org/10.1016/0304-3835(94)90098-1
Hamad YA et al (2018) Breast cancer detection and classification using artificial neural networks. In: 2018 1st annual international conference on information and sciences (AiCIS). IEEE, pp 51–57. https://doi.org/10.1109/AiCIS.2018.00022
Hassanien AE et al (2014) MRI breast cancer diagnosis hybrid approach using adaptive ant-based segmentation and multilayer perceptron neural networks classifier. Appl Soft Comput 14:62–71. https://doi.org/10.1016/j.asoc.2013.08.011
Knickerbocker JU et al (2018) Heterogeneous integration technology demonstrations for future healthcare, IoT, and AI computing solutions. In: 2018 IEEE 68th electronic components and technology conference (ECTC). IEEE Xplore, pp 1519–28. https://doi.org/10.1109/ECTC.2018.00231
Floyd CE (1991) An artificial neural network for SPECT image reconstruction. IEEE Trans Med Imaging 10(3):485–487. https://doi.org/10.1109/42.97600
Boublil D et al (2015) Spatially-adaptive reconstruction in computed tomography using neural networks. IEEE Trans Med Imaging 34(7):1474–1485. https://doi.org/10.1109/TMI.2015.2401131a
Wang G et al (2018) Image reconstruction is a new frontier of machine learning. IEEE Trans Med Imaging 37(6):1289–1296. https://doi.org/10.1109/TMI.2018.2833635
Grossi E, Buscema M (2007) Introduction to artificial neural networks. Eur J Gastroenterol Hepatol 19(12):1046–1054. https://doi.org/10.1097/MEG.0b013e3282f198a0
Singh R et al (2020) Artificial intelligence in image reconstruction: the change is here. Physica Med 79:113–125. https://doi.org/10.1016/j.ejmp.2020.11.012
McCann MT et al (2017) convolutional neural networks for inverse problems in imaging: a review. IEEE Signal Process Mag 34(6):85–95. https://doi.org/10.1109/MSP.2017.2739299
Shireesha M et al (2020) Image reconstruction using deep convolutional neural network. In: 2020 international conference on artificial intelligence and signal Processing (AISP). IEEE, pp 1–6. https://doi.org/10.1109/AISP48273.2020.9073016
Adler J, Oktem O (2018) Learned primal-dual reconstruction. IEEE Trans Med Imaging 37(6):1322–1332. https://doi.org/10.1109/TMI.2018.2799231
Zhang HM, Dong B (2019) A review on deep learning in medical image reconstruction. J Oper Res Soc China 8:311. https://doi.org/10.48550/ARXIV.1906.10643
Aharon M et al (2006) K-SVD: an algorithm for designing overcomplete dictionaries for sparse representation. IEEE Trans Signal Process 54(11):4311–4322. https://doi.org/10.1109/TSP.2006.881199
Tony CT, Wang L (2011) Orthogonal matching pursuit for sparse signal recovery with noise. IEEE Trans Inf Theory 57(7):4680–4688. https://doi.org/10.1109/TIT.2011.2146090
Caballero J et al (2014) Dictionary learning and time sparsity for dynamic MR data reconstruction. IEEE Trans Med Imaging 33(4):979–994. https://doi.org/10.1109/TMI.2014.2301271
X2CT-GAN: Reconstructing CT from Biplanar X-Rays with Generative Adversarial Networks
Kang E et al (2017) A deep convolutional neural network using directional wavelets for low-dose X-ray CT reconstruction. Med Phys 44(10):e360–e375. https://doi.org/10.1002/mp.12344
Putzky P, Welling M (2017) Recurrent inference machines for solving inverse problems. http://arxiv.org/abs/1706.04008
Paschalis P et al (2004) tomographic image reconstruction using artificial neural networks. Nucl Instrum Methods Phys Res Sect A 527(1–2):211–215. https://doi.org/10.1016/j.nima.2004.03.122
Jin KH, McCann MT et al (2017) Deep convolutional neural network for inverse problems in imaging. IEEE Trans Image Process 26(9):4509–4522. https://doi.org/10.1109/TIP.2017.2713099
Calatroni L et al (2015) Bilevel approaches for learning of variational imaging models. http://arxiv.org/abs/1505.02120
Chung C et al (2016) Learning optimal spatially-dependent regularization parameters in total variation image restoration. http://arxiv.org/abs/1603.09155
Rick Chang JH et al (2017) One network to solve them all—Solving linear inverse problems using deep projection models. http://arxiv.org/abs/1703.09912
Johnson J et al (2016) Perceptual losses for real-time style transfer and super-resolution. In: Bastian L (ed) Computer vision – ECCV 2016. Springer International Publishing, New York, pp 694–711
Rudzusika J et al (2021) Deep learning based dictionary learning and tomographic image reconstruction. http://arxiv.org/abs/2108.11730
Hammernik K et al (2018) Learning a variational network for reconstruction of accelerated MRI data: learning a variational network for reconstruction of accelerated MRI data. Magn Resonance Med 79(6):3055–3071. https://doi.org/10.1002/mrm.26977
Mardani M et al (2017) Deep generative adversarial networks for compressed sensing automates MRI. https://doi.org/10.48550/ARXIV.1706.00051
Wang G et al (2020) Deep learning for tomographic image reconstruction. Nat Mach Intell 2(12):737–748. https://doi.org/10.1038/s42256-020-00273-z
Bai J et al (2018) Limited-view CT reconstruction based on autoencoder-like generative adversarial networks with joint loss. In: 2018 40th Annual International conference of the IEEE engineering in medicine and biology society (EMBC). IEEE, pp 5570–74. https://doi.org/10.1109/EMBC.2018.8513659
Liang K et al (2018) Improve angular resolution for sparse-view CT with residual convolutional neural network. In: GH Chen (eds) Medical imaging 2018: physics of medical imaging. SPIE, p 55. https://doi.org/10.1117/12.2293319
Claus BE, Jin Y, Gjesteby LA, Wang G, De Man B (2017) Metal-artifact reduction using deep-learning based sinogram completion: initial results. In: Proceedings of 14th international meeting fully three-dimensional image reconstruction radiology nuclear medicine, pp 631–634
Ghani MU, Karl WC (2020) Fast enhanced CT metal artifact reduction using data domain deep learning. IEEE Trans Comput Imaging 6:181–193. https://doi.org/10.1109/TCI.2019.2937221
Chen Y et al (2012) Thoracic low-dose ct image processing using an artifact suppressed large-scale nonlocal means. Phys Med Biol 57(9):2667–2688. https://doi.org/10.1088/0031-9155/57/9/2667
Alzain AF et al (2021) Common computed tomography artifact: source and avoidance. Egypt J Radiol Nucl Med 52(1):151. https://doi.org/10.1186/s43055-021-00530-0
Mustafa W et al (2021) Sparse-view spectral CT reconstruction using deep learning. http://arxiv.org/abs/2011.14842
Lee D et al (2018) Deep residual learning for accelerated MRI using magnitude and phase networks. IEEE Trans Biomed Eng 65(9):1985–1995. https://doi.org/10.1109/TBME.2018.2821699
Gong K et al (2019) PET image reconstruction using deep image prior. IEEE Trans Med Imaging 38(7):1655–1665. https://doi.org/10.1109/TMI.2018.2888491
Qian H et al (2017) Deep learning models for PET scatter estimations. In: 2017 IEEE nuclear science symposium and medical imaging conference (NSS/MIC). IEEE, pp 1–5. https://doi.org/10.1109/NSSMIC.2017.8533103
Wolterink JM et al (2017) Generative adversarial networks for noise reduction in low-dose CT. IEEE Trans Med Imaging 36(12):2536–2545. https://doi.org/10.1109/TMI.2017.2708987
Yang G et al (2018) DAGAN: deep de-aliasing generative adversarial networks for fast compressed sensing MRI reconstruction. IEEE Trans Med Imaging 37(6):1310–1321. https://doi.org/10.1109/TMI.2017.2785879
Bubba TA et al (2019) Learning the invisible: a hybrid deep learning-shearlet framework for limited angle computed tomography. Inverse Probl 35(6):064002. https://doi.org/10.1088/1361-6420/ab10ca
Kutyniok G, Labate D (eds) (2012) Shearlets: multiscale analysis for multivariate data. Birkhäuser
Zhang Y, Yu H (2018) Convolutional neural network based metal artifact reduction in X-ray computed tomography. IEEE Trans Med Imaging 37(6):1370–1381. https://doi.org/10.1109/TMI.2018.2823083
Shan H et al (2019) Competitive performance of a modularized deep neural network compared to commercial algorithms for low-dose CT image reconstruction. Nat Mach Intell 1(6):269–276. https://doi.org/10.1038/s42256-019-0057-9
Auto encoder based dimensionality reduction and classification using convolutional neural networks for hyperspectral images
Yang Y et al (2017) ADMM-Net: a deep learning approach for compressive sensing MRI. http://arxiv.org/abs/1705.06869
Gupta H et al (2018) CNN-based projected gradient descent for consistent CT image reconstruction. IEEE Trans Med Imaging 37(6):1440–1453. https://doi.org/10.1109/TMI.2018.2832656
Wu D et al (2017) Iterative low-dose CT reconstruction with priors trained by artificial neural network. IEEE Trans Med Imaging 36(12):2479–2486. https://doi.org/10.1109/TMI.2017.2753138
Chen H et al (2018) LEARN: learned experts’ assessment-based reconstruction network for sparse-data CT. IEEE Trans Med Imaging 37(6):1333–1347. https://doi.org/10.1109/TMI.2018.2805692
Buzzard GT et al (2018) Plug-and-play unplugged: optimization-free reconstruction using consensus equilibrium. SIAM J Imaging Sci 11(3):2001–2020. https://doi.org/10.1137/17M1122451
Ulyanov D et al (2020) Deep image prior. http://arxiv.org/abs/1711.10925. https://doi.org/10.1007/s11263-020-01303-4
Thaler F et al (2018) Sparse-view CT reconstruction using wasserstein GANs. In: Knoll F (ed) Machine learning for medical image reconstruction. Springer, Cham, pp 75–82
Ben Yedder H et al (2018) Deep learning based image reconstruction for diffuse optical tomography. In: Knoll F (ed) Machine learning for medical image reconstruction. Springer, Cham, pp 112–119
Ben Yedder H et al (2019) Limited-angle diffuse optical tomography image reconstruction using deep learning. In: Shen D (ed) Medical image computing and computer assisted intervention – MICCAI, vol 11764. Springer, Cham, pp 66–74
Zhu B et al (2018) Image reconstruction by domain-transform manifold learning. Nature 555(7697):487–492. https://doi.org/10.1038/nature25988
Zhou B et al (2019) Limited angle tomography reconstruction: synthetic reconstruction via unsupervised sinogram adaptation. In: Chung ACS (ed) Information processing in medical imaging, vol 11492. Springer, Cham, pp 141–152
Oksuz I et al (2020) Deep learning-based detection and correction of cardiac MR motion artefacts during reconstruction for high-quality segmentation. IEEE Trans Med Imaging 39(12):4001–4010. https://doi.org/10.1109/TMI.2020.3008930
Sbalzarini IF (2016) Seeing is believing: quantifying is convincing: computational image analysis in biology. In: De Vos WH et al (eds) Focus on bio-image informatics, vol 219. Springer, Cham, pp 1–39
Paul G et al (2013) Coupling image restoration and segmentation: a generalized linear model/bregman perspective. Int J Comput Vis 104(1):69–93. https://doi.org/10.1007/s11263-013-0615-2
Sun L et al (2018) Joint CS-MRI reconstruction and segmentation with a unified deep network. http://arxiv.org/abs/1805.02165
Learning Sparsifying Transforms.
Huang Q et al (2019) FR-Net: joint reconstruction and segmentation in compressed sensing cardiac MRI. In: Coudière Y (ed) Functional imaging and modeling of the heart, vol 11504. Springer, New York, pp 352–360
Bhadra S et al (2020) Medical image reconstruction with image-adaptive priors learned by use of generative adversarial networks. http://arxiv.org/abs/2001.10830
Gu J et al (2019) Deep generative adversarial networks for thin-section infant MR image reconstruction. IEEE Access 7:68290–68304. https://doi.org/10.1109/ACCESS.2019.2918926
Kuanar S et al (2019) Low dose abdominal CT image reconstruction: an unsupervised learning based approach. In: 2019 IEEE international conference on image processing (ICIP). IEEE, pp 1351–55. https://doi.org/10.1109/ICIP.2019.8803037
Analysis and Evaluation of a Deep Learning Reconstruction Approach with Denoising for Orthopedic MRI
Quan TM et al (2018) Compressed sensing MRI reconstruction using a generative adversarial network with a cyclic loss. IEEE Trans Med Imaging 37(6):1488–1497. https://doi.org/10.1109/TMI.2018.2820120
Yang Y et al (2019) A stacked multi-granularity convolution denoising auto-encoder. IEEE Access 7:83888–83899. https://doi.org/10.1109/ACCESS.2019.2918409
Jiang M et al (2021) FA-GAN: fused attentive generative adversarial networks for MRI image super-resolution. Comput Med Imaging Graph 92:101969. https://doi.org/10.1016/j.compmedimag.2021.101969
Pain CD et al (2022) Deep learning-based image reconstruction and post-processing methods in positron emission tomography for low-dose imaging and resolution enhancement. Eur J Nucl Med Mol Imaging. https://doi.org/10.1007/s00259-022-05746-4
MirGAN: Medical Image Reconstruction using Generative Adversarial Networks.
Wang Y et al (2016) Auto-encoder based dimensionality reduction. Neurocomputing 184:232–242. https://doi.org/10.1016/j.neucom.2015.08.104
Chen M et al (2021) Deep feature learning for medical image analysis with convolutional autoencoder neural network. IEEE Trans Big Data 7(4):750–758. https://doi.org/10.1109/TBDATA.2017.2717439
Wei R, Mahmood A (2021) Recent advances in variational autoencoders with representation learning for biomedical informatics: a survey. IEEE Access 9:4939–4956. https://doi.org/10.1109/ACCESS.2020.3048309
Shin HC et al (2013) Stacked autoencoders for unsupervised feature learning and multiple organ detection in a pilot study using 4D patient data. IEEE Trans Pattern Anal Mach Intell 35(8):1930–1943. https://doi.org/10.1109/TPAMI.2012.277
Saravanan S, Sujitha J (2020) Deep medical image reconstruction with autoencoders using deep boltzmann machine training. EAI Endorsed Trans Pervas Health Technol 6(24):166360. https://doi.org/10.4108/eai.24-9-2020.166360
Chen H et al (2017) Low-dose CT with a residual encoder-decoder convolutional neural network. IEEE Trans Med Imaging 36(12):2524–2535. https://doi.org/10.1109/TMI.2017.2715284
Tezcan KC et al (2019) MR image reconstruction using deep density priors. IEEE Trans Med Imaging 38(7):1633–1642. https://doi.org/10.1109/TMI.2018.2887072
Koonjoo N et al (2021) Boosting the signal-to-noise of low-field MRI with deep learning image reconstruction. Sci Rep 11(1):8248. https://doi.org/10.1038/s41598-021-87482-7
Marhon SA et al (2013) Recurrent neural networks. In: Bianchini M (ed) Handbook on neural information processing, vol 49. Springer, Berlin Heidelberg, pp 29–65
Salehinejad H et al (2019) Synthesizing chest X-ray pathology for training deep convolutional neural networks. IEEE Trans Med Imaging 38(5):1197–1206. https://doi.org/10.1109/TMI.2018.2881415
Urolagin S et al (2012) Generalization capability of artificial neural network incorporated with pruning method. In: Thilagam PS (ed) Advanced computing, networking and security, vol 7135. Springer, Berlin Heidelberg, pp 171–178
Hochreiter S, Schmidhuber J (1997) Long short-term memory. Neural Comput 9(8):1735–1780. https://doi.org/10.1162/neco.1997.9.8.1735
Dey R, Salem FM (2017) Gate-variants of gated recurrent unit (GRU) neural networks. http://arxiv.org/abs/1701.05923
Chakravarty A, Sivaswamy J (2019) RACE-Net: a recurrent neural network for biomedical image segmentation. IEEE J Biomed Health Inf 23(3):1151–1162. https://doi.org/10.1109/JBHI.2018.2852635
Zhang J, Zuo H (2020) A deep RNN for CT image reconstruction. Med Imaging 11312:1136–1144. https://doi.org/10.1117/12.2549809
Qin C et al (2019) Convolutional recurrent neural networks for dynamic MR image reconstruction. IEEE Trans Med Imaging 38(1):280–290. https://doi.org/10.1109/TMI.2018.2863670
Kim TH et al (2019) LORAKI: autocalibrated recurrent neural networks for autoregressive MRI reconstruction in k-Space. http://arxiv.org/abs/1904.09390
Ikuta M (2021) A deep recurrent neural network with gated momentum unit for CT image reconstruction. https://doi.org/10.36227/techrxiv.15066138.v1
Oh C et al (2021) A K-space-to-image reconstruction network for MRI using recurrent neural network. Med Phys 48(1):193–203. https://doi.org/10.1002/mp.14566
Hosseini SA et al (2019) SRAKI-RNN: accelerated MRI with scan-specific recurrent neural networks using densely connected blocks. In: YM Lu (ed) Wavelets and sparsity XVIII. SPIE, p 46. https://doi.org/10.1117/12.2527949
Wang P et al (2020) Pyramid convolutional RNN for MRI reconstruction. http://arxiv.org/abs/1912.00543
Ma G et al (2019) Learning image from projection: a full-automatic reconstruction (FAR) net for sparse-views computed tomography. http://arxiv.org/abs/1901.03454
Chen L, Wu C (2019) A note on the expressive power of deep rectified linear unit networks in high-dimensional spaces. Math Methods Appl Sci 42(9):3400–3404. https://doi.org/10.1002/mma.5575
Low-dose CT image reconstruction using gain intervention-based dictionary learning
Lee M et al (2020) Sparse-view CT reconstruction based on multi-level wavelet convolution neural network. Physica Med 80:352–362. https://doi.org/10.1016/j.ejmp.2020.11.021
Huang Y et al (2020) Field of view extension in computed tomography using deep learning prior. In: Tolxdorff T (ed) Bildverarbeitung für die Medizin. Springer Fachmedien, New York, pp 186–191
Gröhl J et al (2018) Reconstruction of initial pressure from limited view photoacoustic images using deep learning. In: AA Oraevsky, LV Wang (eds) Photons plus ultrasound: imaging and sensing. SPIE, p 98. https://doi.org/10.1117/12.2288353
Hyun CM et al (2018) Deep learning for undersampled MRI reconstruction. Phys Med Biol 63(13):135007. https://doi.org/10.1088/1361-6560/aac71a
Shlezinger N et al (2021) Model-based deep learning. http://arxiv.org/abs/2012.08405
Aggarwal HK et al (2019) MoDL: model based deep learning architecture for inverse problems. IEEE Trans Med Imaging 38(2):394–405. https://doi.org/10.1109/TMI.2018.2865356
Liang K et al (2019) A model-based deep learning reconstruction for X-Ray CT. http://arxiv.org/abs/1910.06940
Biswas S et al (2019) Dynamic MRI using model-based deep learning and SToRM priors: MoDL-SToRM. Magn Reson Med 82(1):485–494. https://doi.org/10.1002/mrm.27706
Lyu Q et al (2021) Cine cardiac MRI motion artifact reduction using a recurrent neural network. IEEE Trans Med Imaging 40(8):2170–2181. https://doi.org/10.1109/TMI.2021.3073381
Gao Y et al (2019) A feasibility study of extracting tissue textures from a previous full-dose CT database as prior knowledge for bayesian reconstruction of current low-dose ct images. IEEE Trans Med Imaging 38(8):1981–1992. https://doi.org/10.1109/TMI.2018.2890788
Szegedy C et al (2015) Going deeper with convolutions. In: 2015 IEEE conference on computer vision and pattern recognition (CVPR). IEEE, pp 1–9. https://doi.org/10.1109/CVPR.2015.7298594
Guo S, Yang Z (2018) Multi-channel-ResNet: an integration framework towards skin lesion analysis. Inf Med Unlocked 12:67–74. https://doi.org/10.1016/j.imu.2018.06.006
Yang W et al (2017) Improving low-dose CT image using residual convolutional network. IEEE Access 5:24698–24705. https://doi.org/10.1109/ACCESS.2017.2766438
Mikolajczyk A, Grochowski M (2018) Data augmentation for improving deep learning in image classification problem. In: 2018 international interdisciplinary PhD workshop (IIPhDW). IEEE, pp 117–22. https://doi.org/10.1109/IIPHDW.2018.8388338
Syben C et al (2018) Deriving neural network architectures using precision learning: parallel-to-fan beam conversion. http://arxiv.org/abs/1807.03057
Deniz O et al (2020) Robustness to adversarial examples can be improved with overfitting. Int J Mach Learn Cybern 11(4):935–944. https://doi.org/10.1007/s13042-020-01097-4
Miller DJ et al (2020) Adversarial learning targeting deep neural network classification: a comprehensive review of defenses against attacks. Proc IEEE 108(3):402–433. https://doi.org/10.1109/JPROC.2020.2970615
Arulkumaran K et al (2017) Deep reinforcement learning: a brief survey. IEEE Signal Process Mag 34(6):26–38. https://doi.org/10.1109/MSP.2017.2743240
Kumar N et al (2021) Novel deep transfer learning model for COVID-19 patient detection using X-ray chest images. J Ambient Intell HumComput. 5:22. https://doi.org/10.1007/s12652-021-03306-6
Tiwari S (2017) A variational framework for low-dose sinogram restoration. Int J Biomed Eng Technol 24(4):356–367. https://doi.org/10.1504/IJBET.2017.085440
Yedder HB et al (2020) Deep learning for biomedical image reconstruction: a survey. https://doi.org/10.48550/ARXIV.2002.12351
Abadi M et al (2016) TensorFlow: large-scale machine learning on heterogeneous distributed systems. http://arxiv.org/abs/1603.04467
Bastien F et al (2012) Theano: new features and speed improvements. http://arxiv.org/abs/1211.5590
Rush AM (2020) Torch-Struct: deep structured prediction library. http://arxiv.org/abs/2002.00876
Paszke A et al (2019) PyTorch: an imperative style, high-performance deep learning library. http://arxiv.org/abs/1912.01703
Ketkar N (2017) Introduction to Keras. In: Ketkar N (ed) Deep learning with python: a hands-on introduction. Springer, Cham, pp 97–111
Vedaldi A, Lenc K (2015) MatConvNet: convolutional neural networks for MATLAB. In: Proceedings of the 23rd ACM international conference on multimedia. ACM, pp 689–92. https://doi.org/10.1145/2733373.2807412
Seide F, Agarwal A (2016) CNTK: microsoft’s open-source deep-learning toolkit. In: Proceedings of the 22nd ACM SIGKDD international conference on knowledge discovery and data mining. ACM, pp 2135–2135. https://doi.org/10.1145/2939672.2945397
Han Y, Ye JC (2018) Framing U-Net via deep convolutional framelets: application to sparse-view CT. IEEE Trans Med Imaging 37(6):1418–1429. https://doi.org/10.1109/TMI.2018.2823768
Ronneberger O et al (2015) U-Net: convolutional networks for biomedical image segmentation. http://arxiv.org/abs/1505.04597
Gibson E et al (2018) NiftyNet: a deep-learning platform for medical imaging. Comput Methods Prog Biomed 158:113–122. https://doi.org/10.1016/j.cmpb.2018.01.025
Pawlowski N et al (2017) DLTK: state of the art reference implementations for deep learning on medical images. http://arxiv.org/abs/1711.06853
Kamnitsas K et al (2017) Efficient multi-scale 3D CNN with fully connected CRF for accurate brain lesion segmentation. Med Image Anal 36:61–78. https://doi.org/10.1016/j.media.2016.10.004
Kamnitsas K et al (2016) DeepMedic for brain tumor segmentation. In: Crimi A (ed) Brainlesion: glioma, multiple sclerosis, stroke and traumatic brain injuries. Springer International Publishing, Cham, pp 138–149
Shen L et al (2019) Patient-specific reconstruction of volumetric computed tomography images from a single projection view via deep learning. Nat Biomed Eng 3(11):880–888. https://doi.org/10.1038/s41551-019-0466-4
Gadelha M et al (2019) Shape reconstruction using differentiable projections and deep priors. In: 2019 IEEE/CVF international conference on computer vision (ICCV). IEEE, pp 22–30. https://doi.org/10.1109/ICCV.2019.00011
Kulkarni K et al (2016) ReconNet: non-iterative reconstruction of images from compressively sensed measurements. In: 2016 IEEE conference on computer vision and pattern recognition (CVPR). IEEE, pp 449–58. https://doi.org/10.1109/CVPR.2016.55
Kang E et al (2017) Wavelet domain residual network (WavResNet) for low-dose X-ray CT reconstruction. http://arxiv.org/abs/1703.01383
Kang E et al (2018) Deep convolutional framelet denosing for low-dose CT via wavelet residual network. IEEE Trans Med Imaging 37(6):1358–1369. https://doi.org/10.1109/TMI.2018.2823756
Schlemper J et al (2017) A deep cascade of convolutional neural networks for MR image reconstruction. http://arxiv.org/abs/1703.00555
Guo Y et al (2016) Deep learning for visual understanding: a review. Neurocomputing 187:27–48. https://doi.org/10.1016/j.neucom.2015.09.116
Huang C et al (2016) Learning deep representation for imbalanced classification. In: Proceedings of the 2016 IEEE conference on computer vision and pattern recognition (CVPR). IEEE, pp 5375–84. https://doi.org/10.1109/CVPR.2016.580
Ioffe S, Szegedy C (2015) Batch normalization: accelerating deep network training by reducing internal covariate shift. http://arxiv.org/abs/1502.03167
Yu L et al (2017) automated melanoma recognition in dermoscopy images via very deep residual networks. IEEE Trans Med Imaging 36(4):994–1004. https://doi.org/10.1109/TMI.2016.2642839
Direct reconstruction of ultrasound elastography using an end-to-end deep neural network. Springerprofessional.De. https://www.springerprofessional.de/en/direct-reconstruction-of-ultrasound-elastography-using-an-end-to/16122350. Accessed 16 Sept 2021
Greffier J et al (2020) Image quality and dose reduction opportunity of deep learning image reconstruction algorithm for CT: a phantom study. Eur Radiol 30(7):3951–3959. https://doi.org/10.1007/s00330-020-06724-w
Chen H et al (2017) ALow-dose CT via convolutional neural network. Biomed Opt Express 8(2):679. https://doi.org/10.1364/BOE.8.000679
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gothwal, R., Tiwari, S. & Shivani, S. Computational Medical Image Reconstruction Techniques: A Comprehensive Review. Arch Computat Methods Eng 29, 5635–5662 (2022). https://doi.org/10.1007/s11831-022-09785-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11831-022-09785-w