Abstract
Medical imaging is an invaluable resource in medicine as it enables to peer inside the human body and provides scientists and physicians with a wealth of information indispensable for understanding, modelling, diagnosis, and treatment of diseases. Reconstruction algorithms entail transforming signals collected by acquisition hardware into interpretable images. Reconstruction is a challenging task given the ill-posedness of the problem and the absence of exact analytic inverse transforms in practical cases. While the last decades witnessed impressive advancements in terms of new modalities, improved temporal and spatial resolution, reduced cost, and wider applicability, several improvements can still be envisioned such as reducing acquisition and reconstruction time to reduce patient’s exposure to radiation and discomfort while increasing clinics throughput and reconstruction accuracy. Furthermore, the deployment of biomedical imaging in handheld devices with small power requires a fine balance between accuracy and latency. The design of fast, robust, and accurate reconstruction algorithms is a desirable, yet challenging, research goal. While the classical image reconstruction algorithms approximate the inverse function relying on expert-tuned parameters to ensure reconstruction performance, deep learning (DL) allows automatic feature extraction and real-time inference. Hence, DL presents a promising approach to image reconstruction with artifact reduction and reconstruction speed-up reported in recent works as part of a rapidly growing field. We review state-of-the-art image reconstruction algorithms with a focus on DL-based methods. First, we examine common reconstruction algorithm designs, applied metrics, and datasets used in the literature. Then, key challenges are discussed as potentially promising strategic directions for future research.





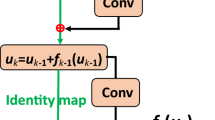
Reprinted by permission from Springer Nature (Thaler et al. 2018)






Similar content being viewed by others
References
Adler J, Öktem O (2018) Learned primal-dual reconstruction. IEEE Trans Med Imaging 37(6):1322–1332
Aghasi A, Abdi A, Nguyen N, Romberg J (2017) Net-trim: convex pruning of deep neural networks with performance guarantee. In: Advances in neural information processing systems, pp 3177–3186
Alford S, Robinett R, Milechin L, Kepner J (2018) Pruned and structurally sparse neural networks. arXiv preprint arXiv:1810.00299
Alom MZ, Taha TM, Yakopcic C, Westberg S, Sidike P, Nasrin MS, Hasan M, Van Essen BC, Awwal AA, Asari VK (2019) A state-of-the-art survey on deep learning theory and architectures. Electronics 8(3):292
Al-Shakhrah I, Al-Obaidi T (2003) Common artifacts in computerized tomography: a review. Appl Radiol 32(8):25–32
Antun V, Renna F, Poon C, Adcock B, Hansen AC (2019) On instabilities of deep learning in image reconstruction-does AI come at a cost? arXiv preprint arXiv:1902.05300
Arridge S, Maass P, Öktem O, Schönlieb C-B (2019) Solving inverse problems using data-driven models. Acta Numerica 28:1–174
Assili S (2018) A review of tomographic rconstruction techniques for computed tomography. arXiv preprint arXiv:1808.09172
Baikejiang R, Zhang W, Li C (2017) Diffuse optical tomography for breast cancer imaging guided by computed tomography: a feasibility study. J X-ray Sci Technol 25(3):341–355
Beattie B (2018) Improvements in the robustness and accuracy of bioluminescence tomographic reconstructions of distributed sources within small animals. PhD thesis, Columbia University
Beck A, Teboulle M (2009) A fast iterative shrinkage-thresholding algorithm for linear inverse problems. SIAM J Imaging Sci 2(1):183–202
Bennett L, Simon W (2013) Cardiac atlas project standard challenge-MICCAI 2013 grand challenge, 2013. http://masiweb.vuse.vanderbilt.edu/workshop2013/index.php/
Bhadra S, Zhou W, Anastasio MA (2020) Medical image reconstruction with image-adaptive priors learned by use of generative adversarial networks. In: Medical imaging 2020: physics of medical imaging, vol 11312, p 113120V. International Society for Optics and Photonics
Biobank U (2014) About uk biobank. https://www.ukbiobank.ac.uk/about-biobank-uk. Accessed 17 June 2020.
Boublil D, Elad M, Shtok J, Zibulevsky M (2015) Spatially-adaptive reconstruction in computed tomography using neural networks. IEEE Trans Med Imaging 34(7):1474–1485
Boyd S, Parikh N, Chu E, Peleato B, Eckstein J et al (2011) Distributed optimization and statistical learning via the alternating direction method of multipliers. Found Trends® Mach Learn 3(1):1–122
Boyd N, Jonas E, Babcock HP, Recht B (2018) Deeploco: fast 3D localization microscopy using neural networks. BioRxiv 267096
Braun H, Turaga P, Spanias A, Katoch S, Jayasuriya S, Tepedelenlioglu C (2019) Reconstruction-free compressive vision for surveillance applications. Synth Lect Signal Process 14(1):1–100
Cai C, Deng K, Ma C, Luo J (2018) End-to-end deep neural network for optical inversion in quantitative photoacoustic imaging. Opt Lett 43(12):2752–2755
Cardoen B, Yedder HB, Sharma A, Chou KC, Nabi IR, Hamarneh G (2019) ERGO: efficient recurrent graph optimized emitter density estimation in single molecule localization microscopy. IEEE Trans Med Imaging, 39(6):1942–1956
Chambolle A, Pock T (2011a) A first-order primal-dual algorithm for convex problems with applications to imaging. J Math Imaging Vis 40(1):120–145
Chambolle A, Pock T (2011b) A first-order primal-dual algorithm for convex problems with applications to imaging. J Math Imaging Vis 40(1):120–145
Chen Y, Yang Z, Hu Y, Yang G, Zhu Y, Li Y, Chen W, Toumoulin C et al (2012) Thoracic low-dose CT image processing using an artifact suppressed large-scale nonlocal means. Phys Med Biol 57(9):2667
Chen H, Zhang Y, Kalra MK, Lin F, Chen Y, Liao P, Zhou J, Wang G (2017a) Low-dose CT with a residual encoder-decoder convolutional neural network. IEEE Trans Med Imaging 36(12):2524–2535
Chen H, Zhang Y, Zhang W, Liao P, Li K, Zhou J, Wang G (2017b) Low-dose CT via convolutional neural network. Biomed Opt Express 8(2):679–694
Cheng JY, Chen F, Alley MT, Pauly JM, Vasanawala SS (2018) Highly scalable image reconstruction using deep neural networks with bandpass filtering. arXiv preprint arXiv:1805.03300
Cui J, Gong K, Guo N, Kim K, Liu H, Li Q (2019) CT-guided PET parametric image reconstruction using deep neural network without prior training data. In: Medical imaging 2019: physics of medical imaging, vol 10948, p 109480Z. International Society for Optics and Photonics
Dabov K, Foi A, Katkovnik V, Egiazarian K (2007) Image denoising by sparse 3-D transform-domain collaborative filtering. IEEE Trans Image Process 16(8):2080–2095
Daubechies I, Defrise M, De Mol C (2004) An iterative thresholding algorithm for linear inverse problems with a sparsity constraint. Commun Pure Appl Math J Issued Courant Inst Math Sci 57(11):1413–1457
Despres P, Jia X (2017) A review of GPU-based medical image reconstruction. Physica Medica 42:76–92
Dong B, Shen Z (2015) Image restoration: a data-driven perspective. In: Proceedings of the international congress of industrial and applied mathematics (ICIAM), pp 65–108. Citeseer
Fan Q, Witzel T, Nummenmaa A, Van Dijk KR, Van Horn JD, Drews MK, Somerville LH, Sheridan MA, Santillana RM, Snyder J et al (2016) MGH-USC human connectome project datasets with ultra-high b-value diffusion MRI. Neuroimage 124:1108–1114
Feng J, Sun Q, Li Z, Sun Z, Jia K (2018) Back-propagation neural network-based reconstruction algorithm for diffuse optical tomography. J Biomed Opt 24(5):051407
Fessler JA (2017) Medical image reconstruction: a brief overview of past milestones and future directions. arXiv preprint arXiv:1707.05927
Gabay D, Mercier B (1976) A dual algorithm for the solution of nonlinear variational problems via finite element approximation. Comput Math Appl 2(1):17–40
Gao H, Yu H, Osher S, Wang G (2011) Multi-energy CT based on a prior rank, intensity and sparsity model (prism). Inverse Probl 27(11):115012
Gates AJ, Ahn Y-Y (2017) The impact of random models on clustering similarity. J Mach Learn Res 18(1):3049–3076
Geffrin J-M, Sabouroux P, Eyraud C (2005) Free space experimental scattering database continuation: experimental set-up and measurement precision. Inverse Probl 21(6):S117
Georgeson G, Safai M (2017) Portable X-ray backscattering imaging system including a radioactive source, May 23. US Patent 9,658,173
Geyer RC, Klein T, Nabi M (2017) Differentially private federated learning: a client level perspective. arXiv preprint arXiv:1712.07557
Gilad-Bachrach R, Dowlin N, Laine K, Lauter K, Naehrig M, Wernsing J (2016) Cryptonets: Applying neural networks to encrypted data with high throughput and accuracy. In: International conference on machine learning, pp 201–210
Goceri E, Goceri N (2017) Deep learning in medical image analysis: recent advances and future trends. International Conferences Computer Graphics, Visualization, Computer Vision and Image Processing (CGVCVIP 2017), Portugal.
Goceri E, Gooya A (2018) On the importance of batch size for deep learning. In: International conference on mathematics. Istanbul, Turkey
Gong K, Catana C, Qi J, Li Q (2018) Pet image reconstruction using deep image prior. IEEE Trans Med imaging 38(7):1655–1665
Gong K, Catana C, Qi J, Li Q (2019) Direct patlak reconstruction from dynamic pet using unsupervised deep learning. In: 15th International meeting on fully three-dimensional image reconstruction in radiology and nuclear medicine, vol 11072, p 110720R. International Society for Optics and Photonics
Goodfellow I, Pouget-Abadie J, Mirza M, Xu B, Warde-Farley D, Ozair S, Courville A, Bengio Y (2014) Generative adversarial nets. In: Advances in neural information processing systems, pp 2672–2680
Gottschling NM, Antun V, Adcock B, Hansen AC (2020) The troublesome kernel: why deep learning for inverse problems is typically unstable. arXiv preprint arXiv:2001.01258
Guo H, Qiu C, Vaswani N (2014) An online algorithm for separating sparse and low-dimensional signal sequences from their sum. IEEE Trans Signal Process 62(16):4284–4297
Guo J, Qi H, Xu Y, Chen Z, Li S, Zhou L (2016) Iterative image reconstruction for limited-angle CT using optimized initial image. Comput Math Methods Med 2016 2016:1–9
Gupta H, Jin KH, Nguyen HQ, McCann MT, Unser M (2018) CNN-based projected gradient descent for consistent CT image reconstruction. IEEE Trans Med Imaging 37(6):1440–1453
Häggström I, Beattie BJ, Schmidtlein CR (2016) Dynamic PET simulator via tomographic emission projection for kinetic modeling and parametric image studies. Med Phys 43(6Part1):3104–3116
Häggström I, Schmidtlein CR, Campanella G, Fuchs TJ (2019) Deeppet: a deep encoder-decoder network for directly solving the PET image reconstruction inverse problem. Med Image Anal 54:253–262
Harrison RL (2010) Monte carlo simulation of emission tomography and other medical imaging techniques. In: AIP conference proceedings, vol 1204, pp 126–132. AIP
He K, Zhang X, Ren S, Sun J (2016) Deep residual learning for image recognition. In: Proceedings of the IEEE conference on computer vision and pattern recognition, pp 770–778
Holden S, Sage D (2016) Imaging: super-resolution fight club. Nat Photonics 10(3):152
Hoshi Y, Yamada Y (2016) Overview of diffuse optical tomography and its clinical applications. J Biomed Opt 21(9):091312
Hosseini SAH, Yaman B, Moeller S, Hong M, Akçakaya M (2019) Dense recurrent neural networks for inverse problems: History-cognizant unrolling of optimization algorithms. arXiv preprint arXiv:1912.07197
Huang Y, Preuhs A, Lauritsch G, Manhart M, Huang X, Maier A (2019b) Data consistent artifact reduction for limited angle tomography with deep learning prior. In: International workshop on machine learning for medical image reconstruction, pp 101–112. Springer
Huang Z, Wang N (2018) Data-driven sparse structure selection for deep neural networks. In: Proceedings of the European conference on computer vision (ECCV), pp 304–320
Huang Q, Yang D, Yi J, Axel L, Metaxas D (2019a) FR-Net: joint reconstruction and segmentation in compressed sensing cardiac MRI. In: International conference on functional imaging and modeling of the heart, pp 352–360. Springer
Hyun CM, Kim HP, Lee SM, Lee S, Seo JK (2018) Deep learning for undersampled MRI reconstruction. Phys Med Biol 63(13):135007
Jhamb TK, Rejathalal V, Govindan V (2015) A review on image reconstruction through MRI k-space data. Int J Image Graph Signal Process 7(7):42
Jin KH, Unser M, Yi KM (2019) Self-supervised deep active accelerated MRI. arXiv preprint arXiv:1901.04547
Jin KH, McCann MT, Froustey E, Unser M (2017) Deep convolutional neural network for inverse problems in imaging. IEEE Trans Image Process 26(9):4509–4522
Kang E, Koo HJ, Yang DH, Seo JB, Ye JC (2019) Cycle-consistent adversarial denoising network for multiphase coronary CT angiography. Med Phys 46(2):550–562
Kearney V, Ziemer BP, Perry A, Wang T, Chan JW, Ma L, Morin O, Yom SS, Solberg TD (2020) Attention-aware discrimination for MR-to-CT image translation using cycle-consistent generative adversarial networks. Radiol Artif Intell 2(2):e190027
Khan A, Sohail A, Zahoora U, Qureshi AS (2020) A survey of the recent architectures of deep convolutional neural networks. Artif Intell Rev. https://doi.org/10.1007/s10462-020-09825-6
Knoll F, Hammernik K, Zhang C, Moeller S, Pock T, Sodickson DK, Akcakaya M (2020) Deep-learning methods for parallel magnetic resonance imaging reconstruction: a survey of the current approaches, trends, and issues. IEEE Signal Process Mag 37(1):128–140
Lan H, Zhou K, Yang C, Cheng J, Liu J, Gao S, Gao F (2019) Ki-gan: knowledge infusion generative adversarial network for photoacoustic image reconstruction in vivo. In: International conference on medical image computing and computer-assisted intervention, pp 273–281. Springer
Latorre-Carmona P, Traver VJ, Sánchez JS, Tajahuerce E (2019) Online reconstruction-free single-pixel image classification. Image Vis Comput 86:28–37
Lebed E, Lee S, Sarunic MV, Beg MF (2013) Rapid radial optical coherence tomography image acquisition. J Biomed Opt 18(3):036004
Li D, Du C, He H (2020a) Semi-supervised cross-modal image generation with generative adversarial networks. Pattern Recogn 100:107085
Liang Z-P (2007) Spatiotemporal imaging with partially separable functions. In: 2007 4th IEEE international symposium on biomedical imaging: from nano to macro, pp 988–991. IEEE
Liang D, Cheng J, Ke Z, Ying L (2020) Deep magnetic resonance image reconstruction: inverse problems meet neural networks. IEEE Signal Process Mag 37(1):141–151
Liang K, Yang H, Kang K, Xing Y (2018) Improve angular resolution for sparse-view CT with residual convolutional neural network. In: Medical imaging 2018: physics of medical imaging, vol 10573, p 105731K. International Society for Optics and Photonics
Li D, Li S, Zhu M, Gao Q, Bian Z, Huang H, Zhang S, Huang J, Zeng D, Ma J (2020b) Unsupervised data fidelity enhancement network for spectral ct reconstruction. In: Medical imaging 2020: physics of medical imaging, vol 11312, p 113124D. International Society for Optics and Photonics
Lin Z (2016) A review on low-rank models in data analysis. Big Data Inf Anal 1(2&3):139–161
LowDoseCT (2014) Low dose CT grand challenge. https://www.aapm.org/GrandChallenge/LowDoseC/
Lucas A, Iliadis M, Molina R, Katsaggelos AK (2018) Using deep neural networks for inverse problems in imaging: beyond analytical methods. IEEE Signal Process Mag 35(1):20–36
Lung Cancer Alliance. Give a scan, Fact Sheet No. 282, 2017. http://www.giveascan.org. Accessed 20 Feb 2020
Luo G, Zhao N, Jiang W, Cao P (2019) MRI reconstruction using deep bayesian inference. arXiv preprint arXiv:1909.01127
McCann MT Unser M (2019) Algorithms for biomedical image reconstruction. arXiv preprint arXiv:1901.03565
McCann MT, Jin KH, Unser M (2017) Convolutional neural networks for inverse problems in imaging: a review. IEEE Signal Process Mag 34(6):85–95
Mendrik AM, Vincken KL, Kuijf HJ, Breeuwer M, Bouvy WH, De Bresser J, Alansary A, De Bruijne M, Carass A, El-Baz A et al (2015) MRbrains challenge: online evaluation framework for brain image segmentation in 3T MRI scans. Comput Intell Neurosci 2015:1
Meng M, Li S, Yao L, Li D, Zhu M, Gao Q, Xie Q, Zhao Q, Bian Z, Huang J et al (2020) Semi-supervised learned sinogram restoration network for low-dose ct image reconstruction. In: Medical imaging 2020: physics of medical imaging, vol 11312, p 113120B. International Society for Optics and Photonics
Moslemi V, Erfanian V, Ashoor M (2020) Estimation of optimized timely system matrix with improved image quality in iterative reconstruction algorithm: a simulation study. Heliyon 6(1):e03279
Mridata. mridata.org. http://mridata.org. Accessed 30 Nov 2019
Nehme E, Weiss LE, Michaeli T, Shechtman Y (2018) Deep-storm: super-resolution single-molecule microscopy by deep learning. Optica 5(4):458–464
Nesterov YE (1983) A method for solving the convex programming problem with convergence rate o (\({\rm 1/k}^{\wedge }\) 2). Dokl Akad Nauk SSSR 269:543–547
Oksuz I, Clough J, Ruijsink B, Puyol-Antón E, Bustin A, Cruz G, Prieto C, Rueckert D, King AP, Schnabel JA (2019) Detection and correction of cardiac MRI motion artefacts during reconstruction from k-space. In: International conference on medical image computing and computer-assisted intervention, pp 695–703. Springer
Otazo R, Candès E, Sodickson DK (2015) Low-rank plus sparse matrix decomposition for accelerated dynamic mri with separation of background and dynamic components. Magn Reson Med 73(3):1125–1136
Ouyang J, Chen KT, Gong E, Pauly J, Zaharchuk G (2019) Ultra-low-dose PET reconstruction using generative adversarial network with feature matching and task-specific perceptual loss. Med Phys 46(8):3555–3564
Pap G, Lékó G, Grósz T (2019) A reconstruction-free projection selection procedure for binary tomography using convolutional neural networks. In: International conference on image analysis and recognition, pp 228–236. Springer
Paul G, Cardinale J, Sbalzarini IF (2013) Coupling image restoration and segmentation: a generalized linear model/bregman perspective. Int J Comput Vis 104(1):69–93
Pelt DM, Batenburg KJ (2013) Fast tomographic reconstruction from limited data using artificial neural networks. IEEE Trans Image Process 22(12):5238–5251
Qin C, Schlemper J, Caballero J, Price AN, Hajnal JV, Rueckert D (2018) Convolutional recurrent neural networks for dynamic MR image reconstruction. IEEE Trans Med Imaging 38(1):280–290
Rajagopal A, Stier N, Dey J, King MA, Chandrasekaran S (2019) Towards deep iterative-reconstruction algorithms for computed tomography (CT) applications. In: Medical imaging 2019: physics of medical imaging, vol 10948, p 1094856. International Society for Optics and Photonics
Ravishankar S, Ye JC, Fessler JA (2019) Image reconstruction: From sparsity to data-adaptive methods and machine learning. arXiv preprint arXiv:1904.02816
Rivest RL, Adleman L, Dertouzos ML et al (1978) On data banks and privacy homomorphisms. Found Secure Comput 4(11):169–180
Rodríguez P (2013) Total variation regularization algorithms for images corrupted with different noise models: a review. J Electr Comput Eng 2013:10
Ronneberger O, Fischer P, Brox T (2015) U-net: convolutional networks for biomedical image segmentation. In: International conference on medical image computing and computer-assisted intervention, pp 234–241. Springer
Rykkje A, Carlsen JF, Nielsen MB (2019) Hand-held ultrasound devices compared with high-end ultrasound systems: a systematic review. Diagnostics 9(2):61
Sandino CM, Cheng JY, Chen F, Mardani M, Pauly JM, Vasanawala SS (2020) Compressed sensing: From research to clinical practice with deep neural networks: shortening scan times for magnetic resonance imaging. IEEE Signal Process Mag 37(1):117–127
Sbalzarini IF (2016) Seeing is believing: quantifying is convincing: computational image analysis in biology. In: Focus on bio-image informatics, pp 1–39. Springer
Schlemper J, Caballero J, Hajnal JV, Price AN, Rueckert D (2018) A deep cascade of convolutional neural networks for dynamic MR image reconstruction. IEEE Trans Med Imaging 37(2):491–503
Schlemper J, Caballero J, Hajnal JV, Price A, Rueckert D (2017) A deep cascade of convolutional neural networks for MR image reconstruction. In: International conference on information processing in medical imaging, pp 647–658. Springer
Schweiger M, Arridge SR (2014) The toast++ software suite for forward and inverse modeling in optical tomography. J Biomed Opt 19(4):040801
Sharma A, Hamarneh G (2019) Missing MRI pulse sequence synthesis using multi-modal generative adversarial network. IEEE Trans Med Imaging. 39(4):1170–1183
Shen G, Dwivedi K, Majima K, Horikawa T, Kamitani Y (2019) End-to-end deep image reconstruction from human brain activity. Front Comput Neurosci 13:21
Shokoufi M, Golnaraghi F (2016) Development of a handheld diffuse optical breast cancer assessment probe. J Innov Opt Health Sci 9(02):1650007
Singh R, Digumarthy SR, Muse VV, Kambadakone AR, Blake MA, Tabari A, Hoi Y, Akino N, Angel E, Madan R et al (2020) Image quality and lesion detection on deep learning reconstruction and iterative reconstruction of submillisievert chest and abdominal CT. Am J Roentgenol. 214(3):566–573.
Singh V, Tewfik AH, Ress DB (2015) Under-sampled functional MRI using low-rank plus sparse matrix decomposition. In: 2015 IEEE international conference on acoustics, speech and signal processing (ICASSP), pp 897–901. IEEE
St-Yves, G, Naselaris T (2018) Generative adversarial networks conditioned on brain activity reconstruct seen images. In: 2018 IEEE international conference on systems, man, and cybernetics (SMC), pp 1054–1061. IEEE
Sun Y, Xia Z, Kamilov US (2018) Efficient and accurate inversion of multiple scattering with deep learning. Opt Express 26(11):14678–14688
Sun Y, Wohlberg B, Kamilov US (2019c) An online plug-and-play algorithm for regularized image reconstruction. IEEE Trans Comput Imaging 5(3):395–408
Sun X, Choi J, Chen C-Y, Wang N, Venkataramani S, Srinivasan VV, Cui X, Zhang W, Gopalakrishnan K (2019b) Hybrid 8-bit floating point (HFP8) training and inference for deep neural networks. In: Advances in neural information processing systems, pp 4901–4910
Sun L, Fan Z, Ding X, Huang Y, Paisley J (2019a) Joint CS-MRI reconstruction and segmentation with a unified deep network. In: International conference on information processing in medical imaging, pp 492–504. Springer
Sun Y, Kamilov US (2018) Stability of scattering decoder for nonlinear diffractive imaging. arXiv preprint arXiv:1806.08015
Sun J, Li H, Xu Z et al (2016) Deep ADMM-Net for compressive sensing MRI. In: Advances in neural information processing systems, pp 10–18
Thaler F, Hammernik K, Payer C, Urschler M, Štern D (2018) Sparse-view CT reconstruction using wasserstein GANs. In: International workshop on machine learning for medical image reconstruction, pp 75–82. Springer
Ulyanov D, Vedaldi A, Lempitsky V(2018) Deep image prior. In: Proceedings of the IEEE conference on computer vision and pattern recognition, pp 9446–9454
Vandenberghe S, D’Asseler Y, Van de Walle R, Kauppinen T, Koole M, Bouwens L, Van Laere K, Lemahieu I, Dierckx R (2001) Iterative reconstruction algorithms in nuclear medicine. Comput Med Imaging Graph 25(2):105–111
Waibel D, Gröhl J, Isensee F, Kirchner T, Maier-Hein K, Maier-Hein L (2018) Reconstruction of initial pressure from limited view photoacoustic images using deep learning. In: Photons plus ultrasound: imaging and sensing 2018, vol 10494, p 104942S. International Society for Optics and Photonics
Wang G (2016) A perspective on deep imaging. IEEE Access 4:8914–8924
Wang G, Kalra M, Murugan V, Xi Y, Gjesteby L, Getzin M, Yang Q, Cong W, Vannier M (2015) Vision 20/20: simultaneous CT-MRI—next chapter of multimodality imaging. Med Phys 42(10):5879–5889
Wang G, Ye JC, Mueller K, Fessler JA (2018) Image reconstruction is a new frontier of machine learning. IEEE Trans Med Imaging 37(6):1289–1296
Wang H, Wu N, Cai Y, Ren L, Zhao Z, Han G, Wang J (2019) Optimization of reconstruction accuracy of anomaly position based on stacked auto-encoder neural networks. IEEE Access 7:116578–116584
Wang Z, Simoncelli EP, Bovik AC (2003) Multiscale structural similarity for image quality assessment. In: The thrity-seventh asilomar conference on signals, systems & computers, vol 2, pp 1398–1402. IEEE
Wang S, Su Z, Ying L, Peng X, Zhu S, Liang F, Feng D, Liang D (2016) Accelerating magnetic resonance imaging via deep learning. In: 2016 IEEE 13th international symposium on biomedical imaging (ISBI), pp 514–517. IEEE
Webb A, Kagadis GC (2003) Introduction to biomedical imaging. Med Phys 30(8):2267–2267
Wells WM III, Viola P, Atsumi H, Nakajima S, Kikinis R (1996) Multi-modal volume registration by maximization of mutual information. Med Image Anal 1(1):35–51
Wen B, Ravishankar S, Pfister L, Bresler Y (2019) Transform learning for magnetic resonance image reconstruction: from model-based learning to building neural networks. arXiv preprint arXiv:1903.11431
Wolterink JM, Leiner T, Viergever MA, Išgum I (2017) Generative adversarial networks for noise reduction in low-dose CT. IEEE Trans Med Imaging 36(12):2536–2545
Wu D, Kim K, Li Q (2019) Computationally efficient deep neural network for computed tomography image reconstruction. Med Phys 46(11):4763–4776
Wu S, Gao Z, Liu Z, Luo J, Zhang H, Li S (2018) Direct reconstruction of ultrasound elastography using an end-to-end deep neural network. In: International conference on medical image computing and computer-assisted intervention, pp 374–382. Springer
Würfl T, Hoffmann M, Christlein V, Breininger K, Huang Y, Unberath M, Maier AK (2018) Deep learning computed tomography: learning projection-domain weights from image domain in limited angle problems. IEEE Trans Med Imaging 37(6):1454–1463
Xu J, Gong E, Pauly J, Zaharchuk G (2017) 200x low-dose PET reconstruction using deep learning. arXiv preprint arXiv:1712.04119
Xu Q, Yu H, Mou X, Zhang L, Hsieh J, Wang G (2012) Low-dose X-ray CT reconstruction via dictionary learning. IEEE Trans Med Imaging 31(9):1682–1697
Yang G, Yu S, Dong H, Slabaugh G, Dragotti PL, Ye X, Liu F, Arridge S, Keegan J, Guo Y et al (2017a) DAGAN: deep de-aliasing generative adversarial networks for fast compressed sensing MRI reconstruction. IEEE Transactions on Medical Imaging 37(6):1310–1321
Yang B, Ying L, Tang J (2018) Artificial neural network enhanced bayesian pet image reconstruction. IEEE Trans Med imaging 37(6):1297–1309
Yang Y, Sun J, Li H, Xu Z (2017b) ADMM-Net: a deep learning approach for compressive sensing MRI. CoRR, arXiv:abs/1705.06869
Yedder HB, BenTaieb A, Shokoufi M, Zahiremami A, Golnaraghi F, Hamarneh G (2018) Deep learning based image reconstruction for diffuse optical tomography. In: International workshop on machine learning for medical image reconstruction, pp 112–119. Springer
Yedder HB, Shokoufi M, Cardoen B, Golnaraghi F, Hamarneh G (2019) Limited-angle diffuse optical tomography image reconstruction using deep learning. In: International conference on medical image computing and computer-assisted intervention, pp 66–74. Springer
Yoon YH, Khan S, Huh J, Ye JC (2018) Efficient b-mode ultrasound image reconstruction from sub-sampled RF data using deep learning. IEEE Trans Med Imaging 38(2):325–336
Yoo J, Sabir S, Heo D, Kim KH, Wahab A, Choi Y, Lee S-I, Chae EY, Kim HH, Bae YM et al. (2020) Deep learning diffuse optical tomography. IEEE Trans Med Imaging. 39(4):877–887
Zbontar J, Knoll F, Sriram A, Muckley MJ, Bruno M, Defazio A, Parente M, Geras KJ, Katsnelson J, Chandarana H et al (2018) FastMRI: an open dataset and benchmarks for accelerated MRI. arXiv preprint arXiv:1811.08839
Zhang Y, Wang Y, Zhang W, Lin F, Pu Y, Zhou J (2016a) Statistical iterative reconstruction using adaptive fractional order regularization. Biomed Opt Express 7(3):1015–1029
Zhang Y, Xi Y, Yang Q, Cong W, Zhou J, Wang G (2016b) Spectral CT reconstruction with image sparsity and spectral mean. IEEE Trans Comput Imaging 2(4):510–523
Zhang H, Zeng D, Zhang H, Wang J, Liang Z, Ma J (2017) Applications of nonlocal means algorithm in low-dose X-ray CT image processing and reconstruction: a review. Med Phys 44(3):1168–1185
Zhang H, Dong B (2019) A review on deep learning in medical image reconstruction. arXiv preprint arXiv:1906.10643
Zhang Q, Liang D (2020) Visualization of fully connected layer weights in deep learning ct reconstruction. arXiv preprint arXiv:2002.06788
Zhao B, Haldar JP, Christodoulou AG, Liang Z-P (2012) Image reconstruction from highly undersampled (k, t)-space data with joint partial separability and sparsity constraints. IEEE Trans Med Imaging 31(9):1809–1820
Zhou B, Lin X, Eck B (2019) Limited angle tomography reconstruction: synthetic reconstruction via unsupervised sinogram adaptation. In: International conference on information processing in medical imaging, pp 141–152. Springer
Zhu B, Liu JZ, Cauley SF, Rosen BR, Rosen MS (2018) Image reconstruction by domain-transform manifold learning. Nature 555(7697):487
Zhu L, Liu Z, Han S (2019) Deep leakage from gradients. In: Advances in neural information processing systems, pp 14747–14756
Acknowledgements
We thank the Natural Sciences and Engineering Research Council of Canada (NSERC) for partial funding.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ben Yedder, H., Cardoen, B. & Hamarneh, G. Deep learning for biomedical image reconstruction: a survey. Artif Intell Rev 54, 215–251 (2021). https://doi.org/10.1007/s10462-020-09861-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10462-020-09861-2