Abstract
Phytophagous insects employ various sensory cues in the host plant location such as visual, olfactory, gustatory, and tactile stimuli, which are perceived by sensory systems and integrated in higher brain centres to trigger the correct behavioural responses. In the present study, the host location process of the oligophagous species Chnootriba elaterii (melon ladybird) is investigated in controlled conditions using both a Y-Tube olfactometer and an open Y-Track olfactometer. Olfactory and visual cues from the host plant act synergistically to lead the ladybirds towards the host plant. Females of C. elaterii are not able to discriminate between host and non-host plants using either olfactory or visual cues alone. Visual cues, particularly those associated with the colour green, are of higher relative importance compared to olfactory cues in the host location process. Green dummy plants made of cardboard represent strong supernormal stimuli for C. elaterii females. The results of the present study can shed light on the host location process of Coccinellidae and can help to develop visual or chemical traps which can be useful in monitoring and controlling this important crop pest of the Mediterranean Basin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During the long antagonistic co-evolution between phytophagous insects and host plants, insects have developed sensory receptors to detect plant cues and choose suitable hosts (Visser 1986; Bernays and Chapman 1994; Stenberg and Ericson 2007; Missbach et al. 2014). Herbivorous insects typically employ various sensory modalities, including visual, olfactory, gustatory, and tactile senses, which are integrated in higher brain centres to trigger their behaviour (Prokopy and Owens 1978; Saxena and Goyal 1978; Harris and Miller 1988; Bernays and Chapman 1994; Schoonhoven et al. 2005; Fernandez and Hilker 2007; Carrasco et al. 2015; Piersanti et al. 2020). Chemical and visual cues play a crucial role at both long and short distances in host selection, while mechanical and gustatory aspects are important when herbivores land on the host (Bernays and Chapman 1994; Bruce et al. 2005; Schoonhoven et al. 2005; Fernandez and Hilker 2007; Salerno et al. 2018; Saitta et al. 2022). Olfaction, in particular, is widely recognized as a crucial factor in host plant selection for phytophagous insects. Herbivorous insects detect plant volatile organic compounds (VOCs) through olfactory sensilla primarily located on their antennae (Chapman 1998; De Bruyne and Baker 2008; Hao et al. 2020; Piersanti et al. 2020; Sevarika et al. 2020). In many insect species, olfactory cues alone can elicit a behavioural response even over long distances (Visser 1986; Bernays and Chapman 1994; Stenberg and Ericson 2007). Consequently, research on olfactory stimuli has received more attention than that regarding visual stimuli in phytophagous insects as well as in their predators and parasitoids (Prokopy and Owens 1983; Visser 1986; Stenberg and Ericson 2007; Chiappini et al. 2012). In some cases, attraction to a host plant enhances when visual cues are added to olfactory cues (review in Anton and Cortesero (2022)). Interactions between colours and volatiles play a role in the attraction of Drosophila suzukii Matsumura (Diptera: Drosophilidae) towards its host plant (Bolton et al. 2021). Ambrosia beetles use visual and olfactory cues acting synergistically during searching behaviour of their host trees (Campbell and Borden 2009). Attraction towards visual cues like plant shapes and colours combined with host plant odours has been recorded in the melon fly Bactrocera cucurbitae Coquillet (Diptera: Tephritidae) (Piñero et al. 2006). Prokopy and Owens (1983) reported numerous examples where both chemical and visual stimuli operate simultaneously or sequentially. In sporadic cases, visual stimuli alone are sufficient to guide insect behaviour, such as the herbivorous chrysomelid Altica engstroemi Sahlberg (Coleoptera: Chrysomelidae), which does not respond to plant VOCs in Y-tube olfactometer tests (Stenberg and Ericson 2007).
Vision in insects is mediated by their main visual organ, the compound eyes. Studies on insect photoreceptors have been conducted in various species, primarily in Lepidoptera, Odonata, Diptera, and Coleoptera (Van Der Kooi et al. 2021). Generally, radiation below 300 nm can damage the photoreceptor pigment of the visual sensory system, whereas wavelengths above 730 nm lack the energy to generate a chemical pigment response in the eyes (Prokopy and Owens 1983; Song and Lee 2018).
Despite the ecological and economic significance of phytophagous Coleoptera, few studies have explored the synergistic effects of olfactory and visual stimuli in host plant selection. Among Coccinellidae, Epilachnini are considered serious insect pests for Solanaceae and Cucurbitaceae (Stenberg and Ericson 2007; Tomaszewska and Szawaryn 2016), causing significant crop losses in Poaceae, Urticaceae, and Fabaceae as well (Park and Yoon 1991; Beyene et al. 2007; Zhang and Ou 2010). The melon ladybird Chnootriba elaterii (Rossi) (Coleoptera: Coccinellidae) is one of the most destructive oligophagous ladybirds feeding on various cultivated and wild Cucurbitaceae in organic crops, primarily targeting leaves, flowers, fruits, and stems (Liotta 1964; Ali and Saeady 1980; Akandeh et al. 2011) and is widespread in Mediterranean regions like Southern Europe, North Africa, Arabian Peninsula, and Iran (Liotta 1964; Akandeh et al. 2011; Al-Saggaff et al. 2012). Both larvae and adults of the melon ladybird can cause significant damage to economically important crops such as melon (Cucumis melo L.), watermelon (Citrullus lanatus (Thunb) Matsumura and Nakai), snake cucumber (Cucumis sativus L.), and Armenian cucumber (Cucumis melo var. flexuosus (L.) Naudin. Several studies have investigated the preferences of the melon ladybird among Cucurbitaceae species (Ali and Saeady 1980; El-Abdin and Siragelnour 1991; Akandeh et al. 2011; Awadalla et al. 2011; Bayhan and Bayhyan 2017; Piersanti et al. 2023), but none have explored in detail the cues involved in host selection process and in particular the role of both visual and olfactory cues. In a recent study on the chemical ecology of the melon ladybird, Piersanti et al. (2022) investigated the electroantennographic response of C. elaterii to VOCs emitted by Cucurbitaceae. Such investigation demonstrated the capability of both males and females to detect many of the tested chemical compounds but, when adult host preferences among seven Cucurbitaceae species were further investigated through dual-choice chamber tests and Y-Tube olfactometer bioassays (Piersanti et al. 2023), contradictory results were observed. This finding has cast doubt on the exclusive role of olfaction in the host plant selection by the melon ladybird, suggesting that visual cues may also be involved in eliciting a proper behavioural response. Consequently, the present study aimed to investigate in behavioural bioassays involving females of C. elaterii the following aspects: (i) The ability to approach the host plant using olfactory and/or visual stimuli in olfactometers; (ii) The ability to approach the host plant and dummy plants of different colours using olfactory and/or visual cues.
Materials and methods
Insects
Chnootriba elaterii adults were collected on plants of Ecballium elaterium (L.) A. Richard (Cucurbitaceae) in Perugia, (Italy) (43° 07′ 41.7″ N 12° 21′ 43.3″ E), in July 2022. Insects were kept inside a white net cage (30 × 30 × 30 cm) (Type 80.301, Vermandel, Hulst, the Netherlands) in a culture room under controlled conditions (25 ± 2 °C, 45 ± 15% RH, photoperiod 16L:8D using lamps 3350 lm; 4000 K). The different developmental stages (eggs, larvae, and adults) were maintained in separate cages with a maximum of 30 individuals per cage. Cucumis melo var. inodorus (L.) Naudin plants provided ad libitum were used as feed. Six plants with five healthy fully expanded leaves were placed in each cage and were replaced every two days. Adults of both sexes were maintained in the same cage (sex ratio of 50%) and mated females (2 weeks old) were used for the tests. Mated females were used because Coccinellidae females can be highly selective in choosing an optimal site for oviposition (Salerno et al. 2022) and mated coccinellids exhibit a more complete and higher response compared to unmated (Fouad 2021). The sex of ladybirds was determined under stereomicroscope (Optika SFX-33, Ponteranica, Italy) according to literature (Dieke 1947; Tomaszewska and Szawaryn 2016). For each bioassay, a mated female of C. elaterii starved for 12 h in the laboratory conditions was used.
Plants
Cucumis melo var. inodorus as host plant and Vicia faba var. minor L. (Fabaceae) as non-host plants were used in bioassays. Plants were obtained by sowing commercial seeds (Rosi sementi, Italy) into individual plastic pots (Bamaplast, Italy) (8 × 8 × 9) with topsoil (Patzer Einheitserde, Manna Italia, Bolzano, Italy). Plants were grown in a controlled climate chamber (25 ± 2 °C, 45 ± 15% RH, photoperiod 14L:10D) for approximately 30 days with a photosynthetic photon fluence rate 200 μmol m−2 s−1 placed above the foliage and water was supplied by sub-irrigation. Healthy plants, about 1 month old, with 5–6 fully expanded leaves were used as olfactory and visual stimuli in the bioassays.
Dummy plants
The dummy plants (DP) were prepared by assembling plastic-coated iron wire and six square cardboards, arranged alternately simulating the leaves of the melon plant with the age of 1 month (Fig. 1); the area of each cardboard square was similar to that of the true leaf (50 × 50 mm). Total dummy height (220 mm) and width (130 mm) were similar to those of the host plant. DP with different colours (white, black, green, yellow, red, blue) were used in the open Y-Track olfactometer. The colours were chosen in consideration of the typical trichromatic insect vision (UV, blue, and green) and the colours perceived by some insect species (red, black, and yellow) (Van Der Kooi et al. 2021). White DP were used as control.
Colour measurements
The quantitative detection of the colours was measured according to Bolletta et al. (2022) employing a Minolta CM-2022 portable spectrophotometer (d/8° geometry; Minolta Co., Ltd. Osaka, Japan) in the CIE-Lab space (illuminant A and 10° standard observer). The measurements were conducted in a dark room placing the instrument directly in contact with melon leaves or cardboard squares of coloured DP. We measured the reflectance spectra between 400 and 700 nm. For melon plants, three leaves of different plants were measured while for DP each sample was read two times at different points to obtain an average value for all the measured parameters.
Y-tube olfactometer
The Y-tube olfactometer consists of a polycarbonate plate (10 mm thick) (LexanTM), with a Y-shaped space milled into the centre (stem 185 mm long; arms 125 mm long at 60° angle; internal section 20 mm), sandwiched between two glass plates (10 mm thick). A 7-mm-diameter hole drilled through the polycarbonate plate into the end of each arm allowed air tube connections, and a similar hole drilled into the end of the stem permitted the airflow outlet. Compressed medical-grade air (79% nitrogen and 21% oxygen by volume), regulated by flow metres (Matheson) and humidified by bubbling through bi-distilled water (Drechsel bottle 125 ml), flowed through both arms, creating an air stream of 70 ml min–1 per arm. The olfactometer arena was surrounded by a cardboard screen to minimize disturbance and was illuminated by four cool white fluorescent tubes (3350 lm; 4000 K) above the device.
Before entering the olfactometer arms, each air stream, controlled by flow metres, passed through a cylindrical glass chamber (Ø = 12 cm; h = 52 cm; 5.8 l) (Steroglass S.r.l., San Martino in Campo, Italy) containing the plant as odour source. The plant pot was wrapped with aluminium foil to minimize the odour from the soil. Two plants of different species were located in the two glass chambers to produce alternative olfactory stimuli in the two arms of the olfactometer. For each bioassay, females were introduced into the olfactometer at the entrance of the central stem and the insect position (stem, right arm, or left arm) was recorded for 5 min using EthoWatcher® (Florianópolis, Brazil). Every six replicates, odour sources (plants of C. melo and V. faba) were replaced with new ones, and the glass chambers were washed with (in sequence) 10 ml of hexane (Sigma-Aldrich, St. Louis, USA), 10 ml of acetone (Carlo Erba, Cornaredo, Italy), 50 ml of distilled water, and baked overnight (120 °C). The olfactometer was cleaned and the odour sources were changed to alternate arms (to prevent any directional biases) every five replicates. The glass plates were then cleaned with hexane, acetone, and distilled water. The polycarbonate part of the olfactometer was cleaned with a laboratory detergent and rinsed with hot tap water (approximately 90 °C for 5 min) and distilled water. After washing, the polycarbonate plate was inverted. The possible presence of bias was tested by running controls with both air streams passing through empty glass chambers (n = 18). No significant difference in the time spent by the ladybirds (t = 1.17; d.f. = 17; P = 0.294) in each of the two arms in the control tests was recorded. Twenty-seven replicates testing olfactory cues from C. melo (Host plant) vs olfactory cues from V. faba (not host plant) were performed between 07.00 and 15.00 h (CEST), and the bioassay room was maintained at 25 ± 1.6 °C, 50–60% RH.
Open Y-Track olfactometer
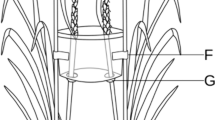
The open Y-Track olfactometer was made of a brass rod (diameter 1.6 mm) according to Visser and Piron (1998). The stem was 220 mm long and the two arms 150 mm long forming an angle of 45° (Fig. 2). The olfactometer was inclined at 22° respect to the horizontal plane. Above the olfactometer, four cool white fluorescent tubes (− 3350 lm; 4000 K) were placed. The end of each arm was surrounded by a glass tube (50 mm long and 30 mm i.d.) to allow air flows to reach the Y-junction. Compressed purified air (79% nitrogen and 21% oxygen by volume), humidified by bubbling through bi-distilled water (Drechsel bottle 125 ml), and regulated by flow metres (Matheson), with an air stream of 70 ml min–1 per arm flowed through two glass chambers (5 l) (Steroglass, Italy) where the odour source (plants) was placed. Glass chambers were connected to the glass tubes by silicon tubes. The olfactometer was surrounded by white cardboard to prevent any visual interference. The glass chambers (containing the olfactory cues) were hidden by a white cardboard and were not visible to tested insects while the plants or dummy plants used as visual cues were placed at a distance of 20 mm from the end of each arm and visible for the tested insects. Females were released at the base of the brass rod central stem using a pen brush. The first choice of the insect (right arm or left arm) was recorded. Every ten replicates, odour sources (plants of C. melo and/or V. faba) were replaced with new ones, and the glass chambers and glass tubes were washed as above reported. The olfactometer was cleaned after each replicate using a cotton ball soaked with acetone to eliminate any insect track, and the odour sources were changed between the two arms (to prevent any directional biases) every five replicates. The possible presence of bias was tested by running controls with both air streams passing through empty glass chambers (n = 42). No significant difference in the first choice of the insects (right arm or left arm) (z = 0.31; n = 42; P = 0.758) in the control tests was recorded. All tests were performed between 07.00 and 15.00 h (CEST) and the bioassay room was maintained at 25 ± 1.6 °C, 50–60% RH.
Around thirty females were tested for each bioassay. The following bioassays were carried out:
-
To evaluate the ladybird ability to move towards a host plant (C. melo) or a non-host plant (V. faba) using olfactory and/or visual cues:
-
V. faba (olfactory and visual cues) vs C. melo (olfactory and visual cues) (n = 50);
-
V. faba (olfactory cues) vs C. melo (olfactory cues) (n = 30);
-
V. faba (visual cues) vs C. melo (visual cues) (n = 45);
-
Air (absence of visual and olfactory cues) vs C. melo (olfactory and visual cues) (n = 35);
-
Air (absence of visual and olfactory cues) vs C. melo (olfactory cues) (n = 30);
-
Air (absence of visual and olfactory cues) vs C. melo (visual cues) (n = 70);
-
C. melo (olfactory cues) vs. C. melo (olfactory and visual cues) (n = 30);
-
C. melo (visual cues) vs. C. melo (olfactory and visual cues) (n = 50).
-
-
To evaluate the ladybird ability to move towards the host plant and dummy plants (DP) of different colours (white or green) using olfactory cues from a host plant (C. melo) and/or visual cues from the DP and/or the host plant.
-
White DP (visual cues) vs. green DP (olfactory and visual cues) (n = 30);
-
White DP (visual cues) vs. white DP (olfactory and visual cues) (n = 31);
-
Green DP (visual cues) vs. green DP (olfactory and visual cues) (n = 40);
-
White DP (olfactory and visual cues) vs. green DP (olfactory and visual cues) (n = 40);
-
C. melo (olfactory and visual cues) vs. green DP (olfactory and visual cues) (n = 30);
-
White DP (visual cues) vs. green DP (visual cues) (n = 70);
-
Air (absence of visual and olfactory cues) vs green DP (visual cues) (n = 30);
-
C. melo (visual cues) vs. green DP (visual cues) (n = 30).
-
-
To evaluate the ladybird ability to move towards dummy plants (DP) of different colours (white, green, red, blue, yellow, black) using olfactory cues from a host plant (C. melo) associated to visual cues from the different DPs:
-
White DP vs. green DP (n = 40);
-
White DP vs. red DP (n = 50);
-
White DP vs. blue DP (n = 17);
-
White DP vs. yellow DP (n = 25);
-
White DP vs. black DP (n = 15);
-
Green DP vs. yellow DP (n = 30).
-
Statistical analysis
The walking behaviour of C. elaterii was described in the Y-Tube olfactometer by the residence time, i.e. the time spent by the insect in each olfactometer arm, while in the open Y-Track olfactometer by the first choice, i.e. the olfactometer arm the insect chose first. Females that did not make a choice after 5 min were considered not responding and were discarded from the analysis. Their number was very low (less than 3%). For the analysis, the logarithmic transformation of the ratio between the residence time in the treatment arm versus the residence time in the control arm was calculated. This transformation (log ratio) ensured that only one measure per insect was later analysed (Rondoni et al. 2017, 2022). Generalized linear models (GLMs) with Gaussian error distribution for residence time data or with binomial error distribution for first choice data were fitted to test differences in choice between the two arms. Analyses were conducted in the R statistical environment, version 4.0.3 (R Core Team 2021).
Results
Y-Tube olfactometer
Y-tube olfactometer experiments carried out to test the ability of C. elaterii females to recognize host and non-host plants using exclusively plant volatiles showed that the time spent by ladybirds in the arm with C. melo (72.2 ± 14.3 s mean ± SE) was not significantly different from the time spent in the arm with V. faba (40.3.3 ± 10.3 s mean ± SE) (t = 1.83; d.f. = 26; P = 0.075).
Open Y-Track olfactometer
In the bioassays to evaluate the ladybird ability to move towards the host plant using olfactory and/or visual cues from a host plant (C. melo) and from a non-host plant (V. faba) in open Y-Track olfactometer, C. elaterii females were significantly attracted towards C. melo in comparison with V. faba when both olfactory and visual cues were supplied (z = 2.75), while no significant difference was recorded in the ladybird response to C. melo and V. faba when only olfactory (z = 0.73) or only visual (z = 0.74) cues were provided (Fig. 3). Comparing the absence of visual and olfactory cues (air) with the host plant C. melo, C. elaterii were significantly attracted towards C. melo when both olfactory and visual cues were supplied (z = 3.02), while no significant difference was recorded when only olfactory (z = 0.73) or only visual (z = 0.24) cues from C. melo were provided (Fig. 3). When both olfactory and visual cues of C. melo were provided in comparison with only olfactory cues, females were significantly attracted (z = 2.17) while when compared with only visual cues, females were not significantly attracted (z = 0.73) (Fig. 3).
Comparing white and green DP, C. elaterii females were significantly attracted to green DP + olfactory cues (from C. melo) in comparison with white DP without olfactory cues (z = 2.13), while no significant differences were recorded when white DP + olfactory cues (from C. melo) were compared with white DP without olfactory cues (z = 0.54) or with green DP without olfactory cues (z = 0.32) (Fig. 4). When white and green DP were compared as visual cues associated with olfactory cues from C. melo, females preferred green DP (z = 2.74) and the preference towards green DP + olfactory cues from C. melo was confirmed also when these last were compared with C. melo plant as visual stimulus + olfactory cues (z = 2.45) (Fig. 4). Comparing only visual cues, green DP attracted C. elaterii against white DP (z = 2.36) and against air (absence of visual and olfactory cues) (z = 2.13), while no significant differences occurred between the ladybirds moving towards green DP and towards C. melo plant (z = 0.37) (Fig. 4).
Using white DP compared with green, red, blue, yellow, and black coloured DP (in all cases associated with olfactory cues from C. melo) C. elaterii females were significantly attracted towards green (z = 2.74), and yellow (z = 2.45) DPs but not towards blue (z = 0.17), red (z = 1.95) and black (z = 0.84) DPs (Fig. 5). Comparing the ladybird response towards olfactory (from C. melo) and visual cues from green and yellow DPs, no significant difference was observed (z = 0.73) (Fig. 5).
Wavelengths of DP and host plant leaves
Spectrophotometer measurements revealed the following peaks of reflectance for the different DP squares: 440 nm for white DP, 520 nm for green, 700 nm for red, 460 for blue, 610 for yellow, and 550 for melon leaves (Fig. 6).
Discussion
Synergistic effect of visual and chemical cues in C. elaterii host plant selection
The data from this study demonstrate that females of C. elaterii lack the ability to discriminate between host and non-host plants based solely on olfactory or visual stimuli. However, the presence of visual cues from the host plant, combined with volatiles, is necessary to trigger a response in ladybirds. In our bioassays, when in Y-Track olfactometer, only air was compared with a combination of olfactory and visual stimuli from the host plant or with only olfactory or only visual stimuli, C. elaterii females required both olfactory and visual stimuli to make a choice. These findings align with the hypothesis proposed by Piersanti et al. (2023) that olfactory cues alone do not guide C. elaterii females in the host-finding process.
In Coleoptera, as in other insects, olfaction is a major driver used to locate host plants (Campos and Peña 1995; Bolter et al. 1997; Park et al. 2004; Jackson et al. 2005), but in many insect species, it has been demonstrated that the searching for host plants or prey is positively affected by the simultaneous presence of olfactory and visual stimuli (Vaishampayan et al. 1975; Saxena and Goyal 1978; Pellmyr and Patt 1986; Obata 1986; Harris and Miller 1988; Todd et al. 1990; Hesler and Sutter 1993; Raguso and Willis 2002; Blackmer and Canas 2005; Fernandez and Hilker 2007; Patt and Sétamou 2014), providing a degree of redundancy advantageous in complex environments (Carrasco et al. 2015). The perception of visual cues can modify the response to volatiles, as evidenced in Oreina cacaliae (Schrank) (Coleoptera: Chrysomelidae), where a positive response to volatiles emitted by the host plant occurs when insects can see the plants (Kalberer et al. 2001).
Given the significant contribution of olfactory and visual cues observed in C. elaterii in our bioassays, we investigated their relative importance in ladybird host plant selection. In open Y-Track olfactometer tests, we compared both olfactory and visual stimuli of the host plant C. melo versus only visual or only olfactory cues. The results of these bioassays showed that, in the comparison of both olfactory plus visual cues versus only olfactory cues, females of C. elaterii significantly preferred the arm where both stimuli were present. This indicates that olfaction alone is not sufficient to elicit a behavioural response. However, when comparing both olfactory plus visual cues versus only visual cues, females of C. elaterii were not able to make a choice between the two olfactometer arms. This result is interesting because it highlights the higher relative importance of visual cues compared to olfactory ones in the host location of the melon ladybird. In this regard, it is fascinating to note that, as reported above, Stenberg and Ericson (2007) demonstrated that visual cues in the phytophagous chrysomelid A. engstroemi override the role of olfaction in the host-finding process; this is an unusual feature for the species belonging to this beetle family because, reviewing 20 years of literature related to host selection in chrysomelids, all 19 studied species were guided by olfactory cues, and none of these investigations reported visual cues involved in host location (Stenberg and Ericson 2007). The authors hypothesized that “the use of visual cues in host-finding may have evolved among chrysomelids with limited dispersal ability in persistent habitats”, where host plants are abundant, dominate the structures of vegetation, and their presence is predictable in space and time. Given the similarities between A. engstroemi and C. elaterii biology and habitat, we consider the assumptions made by Stenberg and Ericson (2007) for Chrysomelidae to be valid also for Epilachninae. Indeed, C. elaterii is a phytophagous ladybird with limited dispersal ability (Liotta 1964), overwinters in proximity to the host plant and searching for new habitats, differently from other insects (Toepfer et al. 2006; Ferracini et al. 2023) is probably not a recurrent activity for this pest. Furthermore, considering the plant community structures, the wild cucurbit E. elaterium is abundant in some uncultivated spaces and, due to its perennial character, dominates the vegetation, with its presence predictable in space and time (Blank et al. 2019). Further studies on the main cues used in host location behaviour of different species of Coccinellidae are necessary to clarify this hypothesis.
Green colour and C. elaterii host selection
The visual detection of plants varies at different distances, and for plant species, different features such as dimensions (size), plant silhouette (shape), hue contrast, and colours may elicit a different insect response (Prokopy and Owens 1983; Scherer and Kolb 1987; Papaj and Prokopy 1989; Harmon et al. 1998; Drew and Prokopy 2003; Schoonhoven et al. 2005). Given the great relative importance of visual cues in the host location of the melon ladybird, we investigated the importance of plant visual features such as plant silhouette and colour, by preparing dummy plants (DP) of the host plants made with coloured cardboard. When we compared white and green DP with or without olfactory cues of the host plant, C. elaterii females were significantly more attracted to green DP plus olfactory cues compared with white DP without olfactory cues. When we compared two white or two green DP (one with olfactory cues and the other without olfactory cues), ladybirds did not show any preferences. These results confirm that (1) insects use the synergic effect of both olfactory and visual stimuli in host selection, (2) the colour, particularly green, has great visual importance for C. elaterii, and (3) visual cues have a higher relative importance compared with olfactory ones in the host location of the melon ladybird.
Insect attraction towards certain coloured arenas or coloured traps has been shown in numerous investigations (Prokopy and Owens 1983; Obata 1986; Van der Ent and Visser 1991; Hesler and Sutter 1993; Szentesi et al. 2002; Hausmann et al. 2004). For example, when the apple blossom weevil was tested in a dual-choice arena providing the main components of the visual spectrum (green, blue, and UV versus black or one colour vs another), Anthonomus pomorum (L.) (Coleoptera: Curculionidae) females chose UV, green, and blue over black (Hausmann et al. 2004). Furthermore, Hesler and Sutter (1993) found in the corn rootworm beetle Diabrotica barberi Smith & Lawrence (Coleoptera: Chrysomelidae) that yellow traps captured the greatest number of this important pest. In our experiments, C. elaterii females are attracted towards the green colour of the DP, while white is not recognized as an attractive stimulus, even when both DPs are coupled with host plant olfactory cues. Stenberg and Ericson (2007), in field investigations, found herbivorous ladybirds both on host plants and green DPs. In the recent review by Van Der Kooi et al. (2021) on insect colour perception, updating the current information regarding insect spectral sensitivities, Coleoptera are reported to be particularly sensitive to ultraviolet and green (with some sporadic examples of species sensitive to red). In particular in Coccinellidae the spectral sensitivity of the compound eyes of both sexes of Coccinella septumpunctata L. was measured electrophysiologically at wavelengths of 350–700 nm. Both sexes in each group showed two peaks of sensitivity, one at 365 nm (ultraviolet) and a second at 500 nm (green) (Herndon et al. 1990), in agreement with our results.
Green DP colour as a supernormal stimulus for C. elaterii females
In our bioassays, when we compared the responsiveness of C. elaterii females to two types of visual stimuli (host plant vs green DP), both associated with host plant olfactory cues, ladybirds significantly preferred green DP, thus revealing the green DP as a supernormal stimulus. When comparing white DP or air (absence of stimuli) vs green DP without olfactory cues, melon ladybirds significantly preferred green DP even when olfactory cues were absent. When we compared the host plant and green DP without olfaction, insects did not show any preference, probably because two strong visual cues are given without the support of olfaction. These results are coherent with the fact that, even if previous experiments performed with plants proved that insects necessarily need both olfactory and visual stimuli to select a host plant, considering the relative weight of visual stimuli the green DP acts as a superstimulus. The supernormal stimuli (or superstimuli) refer to any stimulus that elicits a response stronger than the stimulus for which it evolved (Staddon 1975) and are often associated with the highest relative rewards (Kral 2016). This applies not only to reproductive success but also to food rewards, as happens in flower-visiting behaviour (Kral 2016). In green plants, this probably occurs because, in the insect-visible spectrum, the maximum emitting peak energy reflected by many kinds of plant foliage lies between 540 and 560 nm (Shull 1929; Prokopy 1972), but also other colours may elicit great attractiveness. For example, some thrips are strongly stimulated by the blue wavelength (Lopez-Reyes et al. 2022), while Prokopy and Owens (1983) suggest that, in some herbivorous insects, yellow constitutes a supernormal stimulus because emitted by foliage. Indeed, in some cases, the most attractive object may not be that which mimics the natural visual stimulus but one that embodies the supernormal stimuli (Prokopy 1972; Staddon 1975). For this reason, herbivore insect perception is subjected to be manipulated for monitoring or control (Prokopy 1975; Prokopy and Coli 1978; Prokopy and Hauschild 1979; Lie and Bakke 1981; Campbell and Borden 2006; Lopez-Reyes et al. 2022). For instance, tests comparing commercial traps suggest that the capture of Diabrotica virgifera virgifera Le Conte (Coleoptera: Chrysomelidae) varied both with colour and chemical attractants (Hesler and Sutter 1993); similar results are found also by Campbell and Borden (2006) in three bark beetles that preferred attractant-baited black (host-simulating) traps and avoided attractant-baited white (non-host simulating) traps.
In our experiments the difference in ladybird response between the host plant and green DP may be explained because the reflectance spectrum of a leaf of C. melo plant shows a peak of reflectance (550 nm) slightly different in comparison with the reflectance spectrum of the green cardboard of the DP which is 520 nm, closer to the optimal green wavelength generally perceived by Coccinellidae (Herndon et al. 1990). Indeed, it is known that plant reflection spectra are clearly influenced by chlorophyll, carotenoid absorption (Pearman 1966), leaf age, and hairiness (Shull 1929).
Attractiveness of C. elaterii females towards different colours
In consideration that the only available information on Coccinellidae visual spectrum concerns Coccinella septempunctata L. (Coleoptera: Coccinellidae) (Herndon et al. 1990) and no information is available on the visual spectrum of C. elaterii, we performed further bioassays to assess the attractiveness of C. elaterii females to different colours. We compared five coloured DPs (green, red, blue, yellow, black) versus white DP with olfactory cues of the host plant associated. Females showed significant preferences for green (as previous results suggested), and yellow in comparison with white DP, while no significant preferences were detected in ladybird preference between white DP and blue or black or red DP. In addition, to highlight any colour preference of the melon ladybird, we made comparisons among green and yellow DP. As expected, the comparison between two attractive coloured DPs did not elicit any significant preferences.
Trichromatic vision in insects allows them to perceive mainly ultraviolet, blue, and green (Prokopy and Owens 1983; Briscoe and Chittka 2001; Van Der Kooi et al. 2021), but also red in Lepidoptera (Bernays and Chapman 1994), yellow in many Coleoptera (Van der Ent and Visser 1991; Hesler and Sutter 1993; Adedipe and Park 2010), and black in Scolytidae (Campbell and Borden 2006). Our results showing attraction towards green colour are in agreement with the visual spectrum of C. septempuntata (Herndon et al. 1990) and, concerning the attraction towards green and yellow, are in agreement with some behavioural studies on the predaceous harlequin ladybird Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae) (Obata 1986; Mondor and Warren 2000; Adedipe and Park 2010). On the other hand, many investigations were done to assess the visual ability of the Colorado potato beetle Leptinotarsa decemlineata Say (Coleoptera: Chrysomelidae) in host plant-seeking behaviour and also in this chrysomelid, yellow and green are the main detected colours (Zehnder and Speese III, 1987; Van der Ent and Visser 1991; Lönnendonker 1993; Otálora‐Luna and Dickens 2011).
Plant features such as colour influence not only adult insects feeding behaviour but also the preference for the oviposition site (Harris and Rose 1990). The choice of a suitable site for oviposition is a crucial aspect for pregnant ladybirds which can be highly selective with the oviposition site preference (Salerno et al. 2022). In this context, Lorenzetti et al. (1997) found more adult aphidophagous coccinellids on corn-stressed plants, suggesting that the yellow colour of foliage could be related with the presence of aphids. Some studies reported high trap captures of Coleomegilla maculata De Geer (Coleoptera: Coccinellidae) and C. septempunctata on adhesive yellow traps, while Hippodamia parenthesis (Say) and Hippodamia convergens Guérin-Méneville (Coleoptera: Coccinellidae) did not show any colour preferences (Capinera and Walmsley 1978; Maredia et al. 1992; Udayagiri et al. 1997) suggesting some differences regarding the importance of colour between different ladybird species. Iperti and Prudent (1986) showed in paired choice tests that Adalia bipunctata (L.) (Coleoptera: Coccinellidae) preferred to oviposit on surfaces with particular colours, and the order of preference was red, green, yellow, and blue.
Limitations
Further morphological, electrophysiological, and behavioural studies are necessary to highlight the importance of colour in Coccinellidae. In addition, our study has been performed in laboratory conditions and should be validated through investigations in the field. It would be very interesting also to deepen the knowledge regarding the host plant location of C. elaterii in relation with its limited dispersal ability and to compare it with other Coccinellidae species. Our study refers to the visual spectrum of C. septempunctata but C. elaterii visual spectrum should be analysed. We limited our study to females but similar investigations concerning males can be interesting.
Conclusions
The data collected in the present study shed light on the plant host-finding behaviour of female C. elaterii and the interplay between olfactory and visual stimuli from the host plant which is represented by C. melo. Females of C. elaterii demonstrate an inability to discriminate between host and non-host plants using either olfactory or visual cues alone. The Y-Track olfactometer bioassays reveal that females require a combination of olfactory and visual cues to make a choice. Visual cues, particularly those associated with the colour green, are of higher relative importance compared to olfactory cues in the host location process. Green colour from cardboard in particular represents for C. elaterii females a supernormal stimulus which is highly attractive: green dummy plants act as supernormal stimuli, attracting C. elaterii females even in the absence of olfactory cues, probably in relation with the optimal green wavelength perceived by Coccinellidae. Further research both in controlled condition and in the field is recommended to explore colour preferences and their implications in the broader context of Coccinellidae behaviour.
Deepening our understanding of how insect pests perceive signals from their environment is crucial for developing effective and sustainable methods for their control. The present results provide a first basis for the development of biological control strategies against this and other insect pests using combinations of visual and chemical traps.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Adedipe F, Park Y (2010) Visual and olfactory preference of Harmonia axyridis (Coleoptera: Coccinellidae) adults to various companion plants. J Asia Pac Entomol 13:319–323. https://doi.org/10.1016/j.aspen.2010.07.004
Akandeh M, Shishehbor P, Mosadegh MS, Khajezadeh Y (2011) Sampling program and host preference of melon ladybeetle, Epilachna chrysomelina (F.) on some hosts of cucurbit family in Ahvaz, Southwest Iran. Iran J Plant Prot Sci 42:1–10
Ali MA, Saeady AAI (1980) Influence of temperature, photoperiod, and host-plant on the bionomics of the melon ladybird Epilachna crysomelina.
Al-Saggaff AI, Mahyoub JA, Al-Digail SA et al (2012) A Study on the abundance of egg production by Epilachna chrysomellina Fabricius (Coccinellidae: Coleoptera) and its relation with temperature and relative humidity. Biosci Biotechnol Res Asia 9:623–628. https://doi.org/10.13005/bbra/1041
Anton S, Cortesero AM (2022) Plasticity in chemical host plant recognition in herbivorous insects and its implication for pest control. Biology (basel) 11:1843. https://doi.org/10.3390/biology11121842
Awadalla S, Abd-Wahab H, Abd El-Baky N, Abdel-salam S (2011) Host plant preference of the melon ladybird beetle Epilachna chrysomelina (F.) (COLEOPTERA: COCCINELLIDAE) on different cucurbit vegetables in Mansoura Region. J Plant Prot Pathol 2:41–47. https://doi.org/10.21608/jppp.2011.84653
Bayhan E, Bayhyan SÖ (2017) Determination of some biological parameters Henosepilachna elaterii Rossi (COLEOPTERA: COCCINELLIDAE) on different watermelon cultivars. Middle East J Sci 3:1–8. https://doi.org/10.23884/mejs.2017.3.1.01
Bernays EA, Chapman RF (1994) Host-plant selection by phytophagous insects. Chapman and Hall, New York
Beyene Y, Hofsvang T, Azerefegne F (2007) Population dynamics of tef Epilachna (Chnootriba similis Thunberg)(Coleoptera, Coccinellidae) in Ethiopia. Crop Prot 26:1634–1643. https://doi.org/10.1016/j.cropro.2007.01.005
Blackmer JL, Canas LA (2005) Visual cues enhance the response of Lygus hesperus (Heteroptera: Miridae) to volatiles from host plants. Environ Entomol 34:1524–1533. https://doi.org/10.1603/0046-225X-34.6.1524
Blank L, Birger N, Eizenberg H (2019) Spatial and temporal distribution of Ecballium elaterium in almond orchards. Agronomy 9:751
Bolletta V, Pauselli M, Pomente C et al (2022) Dietary olive leaves improve the quality and the consumer preferences of a model sheep cheese. Int Dairy J 134:105464. https://doi.org/10.1016/j.idairyj.2022.105464
Bolter C, Dicke M, van Loon J et al (1997) Attraction of Colorado potato beetle to herbivoredamaged plants during herbivory and after its termination. J Chem Ecol 23:1003–1023
Bolton LG, Piñero JC, Barrett BA (2021) Olfactory cues from host-and non-host plant odor influence the behavioral responses of adult Drosophila suzukii (Diptera: Drosophilidae) to visual cues. Environ Entomol 50:571–579. https://doi.org/10.1093/ee/nvab004
Briscoe AD, Chittka L (2001) The evolution of color vision in insects. Annu Rev Entomol 46:471–510
Bruce TJ, Wadhams LJ, Woodcock CM (2005) Insect host location: a volatile situation. Trends Plant Sci 10:269–274. https://doi.org/10.1016/j.tplants.2005.04.003
Campbell SA, Borden JH (2006) Integration of visual and olfactory cues of hosts and non-hosts by three bark beetles (Coleoptera: Scolytidae). Ecol Entomol 31:437–449. https://doi.org/10.1111/j.1365-2311.2006.00809.x
Campbell SA, Borden JH (2009) Additive and synergistic integration of multimodal cues of both hosts and non-hosts during host selection by woodboring insects. Oikos 118:553–563. https://doi.org/10.1111/j.1600-0706.2009.16761.x
Campos M, Peña A (1995) Response of Phloeotribus scarabaeoides (Coleoptera, Scolytidae) to ethylene in an olfactometer. Experientia 51:77–79
Capinera JL, Walmsley MR (1978) Guérin-Méneville. J Econ Entomol 71:926–927
Carrasco D, Larsson M, Anderson P (2015) Insect host plant selection in complex environments. Curr Opin Insect Sci 8:1–7
Chapman R (1998) Chemoreception. In: Chapman R (ed) The insects: structure and function, 4th edn. Cambridge University Press, New York, NY, USA, pp 610–652
Chiappini E, Salerno G, Berzolla A et al (2012) Role of volatile semiochemicals in host location by the egg parasitoid Anagrus breviphragma. Entomol Exp Appl 144:311–316
De Bruyne M, Baker TC (2008) Odor detection in insects: volatile codes. J Chem Ecol 34:882–897
Dieke G (1947) Ladybeetles of the genus Epilachna (sens. lat.) in Asia, Europe, and Australia. Smithson Misc Collect 106:
Drew R, Prokopy R (2003) Attraction of fruit flies of the genus Bactrocera to colored mimics of host fruit. Entomol Exp Appl 107:39–45. https://doi.org/10.1046/j.1570-7458.2003.00039.x
El-Abdin AMZ, Siragelnour BG (1991) Biological aspects, food preference and chemical control of the cucurbit beetle, Henosepilachna elaterii (Rossi) (Coleoptera; Coccinellidae). Arab J Plant Prot 9:103–110
Fernandez P, Hilker M (2007) Host plant location by Chrysomelidae. Basic Appl Ecol 8:97–116. https://doi.org/10.1016/j.baae.2006.05.001
Ferracini C, Saitta V, Rondoni G, Rollet I (2023) Variables affecting the pine processionary moth flight: a survey in the North-Western Italian Alps. Forests 14:31. https://doi.org/10.3390/F14010031
Fouad HA (2021) Responses of the predatory species, Coccinella undecimpunctata L. (Coleoptera: Coccinellidae), to the volatiles from its prey, Aphis craccivora Koch. and Vicia faba plant. Egypt J Biol Pest Control 31:1–15
Hao YN, Sun YX, Liu CZ (2020) Functional morphology of antennae and sensilla of Hippodamia variegata (Coleoptera: Coccinellidae). PLoS ONE 15:e0237452. https://doi.org/10.1371/journal.pone.0237452
Harmon JP, Losey JE, Ives AR (1998) The role of vision and color in the close proximity foraging behavior of four coccinellid species. Oecologia 115:287–292. https://doi.org/10.1007/S004420050518
Harris M, Miller J (1988) Host-acceptance behaviour in an herbivorous fly, Delia antiqua. J Insect Physiol 34:179–190. https://doi.org/10.1016/0022-1910(88)90048-0
Harris M, Rose S (1990) Chemical, color, and tactile cues influencing oviposition behavior of the Hessian fly (Diptera: Cecidomyiidae). Environ Entomol 19:303–308. https://doi.org/10.1093/ee/19.2.303
Hausmann C, Samietz J, Dorn S (2004) Visual orientation of overwintered Anthonomus pomorum (Coleoptera: Curculionidae). Environ Entomol 55:1410–1415. https://doi.org/10.1603/0046-225X-33.5.1410
Herndon R, Everett R, Flanders R (1990) Spectral sensitivity of the compound eye of Coccinella septempunctata (Coleoptera: Coccinellidae). Ann Entomol Soc Am 83:817–819. https://doi.org/10.1093/aesa/83.4.817
Hesler L, Sutter G (1993) Effect of trap color, volatile attractants, and type of toxic bait dispenser on captures of adult corn rootworm beetles (Coleoptera: Chrysomelidae). Environ Entomol 22:743–750. https://doi.org/10.1093/ee/22.4.743
Iperti G, Prudent P (1986) Effect of the substrate properties on the choice of oviposition sites by Adalia bipunctata. In: Hodek I (ed) Ecology of Aphidophaga. Junk Publishers, Dordrecht, pp 143–149
Jackson D, Sorensen K, Sorenson C, Stow R (2005) Monitoring cucumber beetles in sweetpotato and cucurbits with kairomone-baited traps. J Econ Entomol 98:159–170
Kalberer NM, Turlings TCJ, Rahier M (2001) Attraction of a leaf beetle (Oreina cacaliae) to damaged host plants. J Chem Ecol 27:647–661
Kral K (2016) Implications of insect responses to supernormal visual releasing stimuli in intersexual communication and flower-visiting behaviour: a review. Eur J Entomol 113:429. https://doi.org/10.14411/eje.2016.056
Lie R, Bakke A (1981) Practical results from the mass trapping of Ips typographus in Scandinavia. In: Management of Insect Pests with Semiochemicals: Concepts and Practice. pp 175–181
Liotta G (1964) Contributo alla conoscenza della biologia dell’Epilachna chrysomelina F. Sicilia (Col. Coccinellidae). Palermo
Lönnendonker U (1993) Features effective in course control during object fixation by walking Colorado beetles. Comp Physiol A 172:741–747
Lopez-Reyes K, Armstrong KF, Van Tol RW et al (2022) Colour vision in thrips (Thysanoptera). Philos Trans R Soc B 377:20210282. https://doi.org/10.1098/rstb.2021.0282
Lorenzetti F, Arnason JT, Philogène R, Hamilton RI (1997) Evidence for spatial niche partitioning in predaceous aphidophaga: use of plant colour as a cue. Biocontrol 42:49. https://doi.org/10.1007/BF02769879
Maredia KM, Gage SH, Landis DA, Wirth TM (1992) Visual response of Coccinella septempunctata (L.), Hippodamia parenthesis (Say), (Coleoptera: Coccinellidae), and Chrysoperla carnea (Stephens), (Neuroptera: Chrysopidae) to colors. Biol Control 2:253–256
Missbach C, Dweck HK, Vogel H, et al (2014) Evolution of insect olfactory receptors. Elife
Mondor EB, Warren JL (2000) Unconditioned and conditioned responses to colour in the predatory coccinellid, Harmonia axyridis (Coleoptera: Coccinellidae). Eur J Entomol 97:463–468
Obata S (1986) Mechanisms of prey finding in the aphidophagous ladybird beetle, Harmonia axyridis [Coleoptera: Coccinellidae]. Entomophaga 31:303–311. https://doi.org/10.1007/BF02373340
Otálora-Luna F, Dickens JC (2011) Spectral preference and temporal modulation of photic orientation by Colorado potato beetle on a servosphere. Entomol Exp Appl 138:93–103
Papaj DR, Prokopy RJ (1989) Ecological and evolutionary aspects of learning in phytophagous insects. Annu Rev Entomol 34:315–350. https://doi.org/10.1146/ANNUREV.EN.34.010189.001531
Park HC, Yoon IB (1991) A taxonomic revision of subfamily Epilachninae in Korea (Coleoptera: Coccinellidae). Entomol Res Bull 17:81–92
Park IK, Lee SG, Shin SC et al (2004) Feeding and attraction of Agelastica coerulea (Coleoptera: Chrysomelidae) to Betulaceae plants. J Econ Entomol 97:1978–1982
Patt JM, Sétamou M (2014) Olfactory and visual stimuli affecting host plant detection in Homalodisca coagulata (Hemiptera: Cicadellidae). Environ Entomol 36:142–150
Pearman GI (1966) The reflection of visible radiation from leaves of some western Australian species. Aust J Biol Sci 19:97–104
Pellmyr O, Patt JM (1986) Function of olfactory and visual stimuli in pollination of Lysichiton americanum (Araceae) by a staphylinid beetle. Madrono 47–54
Piersanti S, Rebora M, Ederli L et al (2020) Role of chemical cues in cabbage stink bug host plant selection. J Insect Physiol 120:103994. https://doi.org/10.1016/J.JINSPHYS.2019.103994
Piersanti S, Saitta V, Rebora M, Salerno G (2022) Olfaction in phytophagous ladybird beetles: antennal sensilla and sensitivity to volatiles from host plants in Chnootriba elaterii (Coleoptera Coccinellidae). Arthropod Plant Interact. https://doi.org/10.1007/s11829-022-09923-y
Piersanti S, Saitta V, Rebora M, Gianandrea S (2023) Adult host preference and larval performance in an ologophagous insect (Chnootriba elaterii). Physiol Entomol 48:171
Piñero JC, Jácome I, Vargas R, Prokopy RJ (2006) Response of female melon fly, Bactrocera cucurbitae, to host-associated visual and olfactory stimuli. Entomol Exp Appl 121:261–269
Piñero JC, Souder SK, Vargas RI (2017) Vision-mediated exploitation of a novel host plant by a tephritid fruit fly. PLoS ONE 12:e0174636
Prokopy R (1972) Response of apple maggot flies to rectangles of different colors and shades. Environ Entomol 1:720–726
Prokopy R (1975) Apple maggot control by sticky red spheres. J Econ Entomol 68:197–198
Prokopy RJ, Hauschild KI (1979) Comparative effectiveness of sticky red spheres and Pherocon® AM standard traps for monitoring apple maggot flies in commercial orchards. Environ Entomol 8:696–700
Prokopy RJ, Coli WM (1978) Selective traps for monitoring Rhagoletis mendax flies. Prot Ecol
Prokopy R, Owens E (1978) Visual generalist with visual specialist phytophagous insects: host selection behaviour and application to management. Entomol Exp Appl 24:609–620
Prokopy RJ, Owens ED (1983) Visual detection of plants by herbivorous insects. Annu Rev Entomol 28:337–364. https://doi.org/10.1146/ANNUREV.EN.28.010183.002005
R Core Team (2021) R: A language and environment for statistical computing. R Foundation for Statistical Computing
Raguso RA, Willis MA (2002) Synergy between visual and olfactory cues in nectar feeding by naıve hawkmoths, Manduca sexta. Anim Behav 64:685–695
Rondoni G, Ielo F, Ricci C, Conti E (2017) Behavioural and physiological responses to prey-related cues reflect higher competitiveness of invasive vs. native ladybirds. Sci Rep 7:3716
Rondoni G, Chierici E, Giovannini L et al (2022) Olfactory responses of Trissolcus mitsukurii to plants attacked by target and non-target stink bugs suggest low risk for biological control. Sci Rep 12:1880
Saitta V, Rebora M, Piersanti S et al (2022) Effect of leaf trichomes in different species of Cucurbitaceae on attachment ability of the melon ladybird beetle Chnootriba elaterii. InSects 13:1123
Salerno G, Rebora M, Gorb E, Gorb S (2018) Attachment ability of the polyphagous bug Nezara viridula (Heteroptera: Pentatomidae) to different host plant surfaces. Sci Rep 8:1–14
Salerno G, Rebora M, Piersanti S et al (2022) Oviposition site selection and attachment ability of Propylea quatuordecimpunctata and Harmonia axyridis from the egg to the adult stage. Wiley Online Libr 47:20–37. https://doi.org/10.1111/phen.12368
Saxena KN, Goyal S (1978) Host-plant relations of the citrus butterfly Papilio demoleus L.: orientational and ovipositional responses. Entomol Exp Appl 24:1–10. https://doi.org/10.1111/J.1570-7458.1978.TB02750.X
Scherer C, Kolb G (1987) Behavioral experiments on the visual processing of color stimuli in Pieris brassicae L. (Lepidoptera). J Comp Physiol A 160:645–656. https://doi.org/10.1007/BF00611937
Schoonhoven L, Van Loon J, Dicke M (2005) Insect-plant biology. Oxford University Press, Oxford
Sevarika M, Rondoni G, Conti E, Romani R (2020) Antennal sensory organs and glands of the harlequin ladybird, Harmonia axyridis. Entomol Exp Appl 169:111–124. https://doi.org/10.1111/eea.12948
Shull CA (1929) A spectrophotometric study of reflection of light from leaf surfaces. Bot Gaz 87:583–607
Song BM, Lee CH (2018) Toward a mechanistic understanding of color vision in insects. Front Neural Circuits 12:16. https://doi.org/10.3389/fncir.2018.00016
Staddon JER (1975) A note on the evolutionary significance of “supernormal” stimuli. Am Nat 109:541–545
Stenberg J, Ericson L (2007) Visual cues override olfactory cues in the host-finding process of the monophagous leaf beetle Altica engstroemi. Entomol Exp Appl 125:81–88. https://doi.org/10.1111/j.1570-7458.2007.00597.x
Szentesi A, Weber DC, Jermy TIBOR (2002) Role of visual stimuli in host and mate location of the Colorado potato beetle. Entomol Exp Appl 105:141–152
Todd JL, Phelan PL, Nault LR (1990) Interaction between visual and olfactory stimuli during host-finding by leafhopper, Dalbulus maidis (Homoptera: Cicadellidae). J Chem Ecol 16:2121–2133
Toepfer S, Levay N, Kissd J (2006) Adult movements of newly introduced alien Diabrotica virgifera virgifera (Coleoptera: Chrysomelidae) from non-host habitats. Bull Entomol Res 96:327–335
Tomaszewska W, Szawaryn K (2016) Epilachnini (Coleoptera: Coccinellidae) - a revision of the world genera. J Insect Sci. https://doi.org/10.1093/jisesa/iew082
Udayagiri S, Mason CE, Pesek JD Jr (1997) Coleomegilla maculata, Coccinella septempuncta (Coleoptera: Coccinellidae), Chrysoperla carnea (Neuroptera: Chrysopidae), and Macrocentrus grandii (Hymenoptera: Bracondiae) trapped on colored sticky traps in corn habitats. Environ Entomol 26:983–988
Vaishampayan SM, Waldbauer GP, Kogan M (1975) Visual and olfactory responses in orientation to plants by the greenhouse whitefly, Trialeurodes vaporariorum (HOMOPTERA: ALEYRODIDAE). Entomol Exp Appl 18:412–422. https://doi.org/10.1111/J.1570-7458.1975.TB00418.X
Van der Ent L, Visser J (1991) The visual world of the Colorado potato beetle. In: Experimental and Applied Entomology of the Netherlands Entomological Society. pp 80–85
Van Der Kooi CJ, Stavenga DG, Arikawa K et al (2021) Evolution of insect color vision: from spectral sensitivity to visual ecology. Annu Rev Entomol 66:435–461
Visser JH (1986) Host odor perception in phytophagous insects. Annu Rev Entomol 31:121–144. https://doi.org/10.1146/ANNUREV.EN.31.010186.001005
Visser J, Piron P (1998) An open Y-track olfactometer for recording of aphid behavioural responses to plant odours. In: Netherlands entomological society (ed) The section experimental and applied section entomology. pp 41–46
Zehnder G, Speese J III (1987) Assessment of color response and flight activity of Leptinotarsa decemlineata (Say)(Coleoptera: Chrysomelidae) using window flight traps. Environ Entomol 16:1199–1202
Zhang X, Ou X (2010) Fauna of some species of phytophagous ladybirds Epilachninae (Coleoptera: Coccinellidae) in Yunnan Province. Entomotaxonomia 32:53–60
Acknowledgements
We would like to thank Giulia Petroni and Giorgia Carboni Marri for a relevant contribution to data collection, and Bricofer Group S.p.A. for supporting the research with a free supply of some equipment. We are very grateful to Gabriele Rondoni for his suggestions for data statistical analysis and to Viviana Bolletta and the Research Unit of Animal Science of the Department of Agriculture, Food and Environmental Science of the University of Perugia for the spectrophotometer measurements.
Funding
Open access funding provided by Università degli Studi di Perugia within the CRUI-CARE Agreement. The authors did not receive support from any organization for the submitted work.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. VS conducted the experiments and collected data. GS analysed data. VS and MR wrote the manuscript. All authors have read, commented on, and approved the final manuscript. GS supervised.
Corresponding author
Ethics declarations
Competing interests
The authors have no conflict of interests in this contribution.
Additional information
Handling Editor: Danny Haelewaters.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Saitta, V., Rebora, M., Piersanti, S. et al. Visual and chemical cues in the host plant selection of the melon ladybird Chnootriba elaterii (Coleoptera: Coccinellidae). Arthropod-Plant Interactions (2023). https://doi.org/10.1007/s11829-023-10018-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11829-023-10018-5