Abstract
Buckwheat (Fagopyrum esculentum) is a member of the Polygonaceae family, cultivated as a cover crop to suppress or reduce weeds and improve soil health. In our field studies, buckwheat gave significant potato tuber protection from wireworm damage after two consecutive years of cropping. In this study, we identified the mechanism underlying the beneficial effect of buckwheat on wireworm suppression. Results show high wireworm numbers in buckwheat than other host plants in bioassays conducted under greenhouse and field conditions which reject the hypothesis that buckwheat has antifeedant activity. We found that newly hatched neonate wireworms feeding on either barley or buckwheat plants for 120 days, showed reduced body weight and head capsule size. The larvae feeding on buckwheat were 60% and 30% smaller than the ones feeding on barley. Survival was also impacted with 44% of the neonate larvae surviving on barley plants, and only 15% when feeding on buckwheat roots over 120 days. A similar bioassay with small to medium-sized wireworms showed higher mortality, lower weight gain and smaller head capsule size. Wireworms feeding on buckwheat were deformed and demonstrated irregular growth. In conclusion, this study revealed that buckwheat did not repel wireworms and they chose to feed on the roots despite it not being a good host. Long-term feeding on buckwheat roots caused reduced weight gain, abnormal growth, and reduced survival. This study provided a better understanding of how buckwheat functions as a biopesticide for wireworm control and its potential for use in an IPM program.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wireworms are the most common and widely distributed pests of cultivated crops with over 10,000 species worldwide (Brandl et al. 2017). Economic damage coupled with their wide host range makes wireworms a major concern to growers worldwide. Damage to root crops such as potato, rutabaga, carrot, onions, although cosmetic, can result in rejection by consumers and processors of an otherwise productive crop. In addition, feeding tunnels can serve as entry points for disease organisms causing additional losses in the field and storage facilities (Keiser et al. 2012). Wireworms, the larval stage of click beetles, are particularly difficult to control because of their long-life cycle (Parker and Howard 2001). Females lay their eggs singly or in clusters in the soil in late spring. The eggs hatch and the larvae begin feeding on the organic matter and roots of the crop. In the fall, the larvae feed voraciously before burrowing into the soil to overwinter and return to the surface in the spring where they once again begin feeding on the roots of their host plants. The time to complete larval development can vary between 2 and 5 years, depending on the species (Parker and Howard 2001). Feeding by wireworms in the fall prior to overwintering can result in root crops, such as potatoes, carrots, rutabaga, being severely damaged before harvest.
Prince Edward Island (PEI) is the largest potato-producing province in Canada. In 2015, the PEI agricultural industry estimated the yearly crop losses because of wireworm feeding on seeds and seedlings in the spring and on root vegetable crops in the fall to be over $7.2 million. Agriotes sputator is the most widely distributed and destructive species in PEI (Noronha personal observation; Vernon and Herk 2017).
The lack of efficacious insecticides for wireworm control led to a significant increase and spread of A. sputator in PEI (Noronha et al. 2007; van Herk et al. 2008; Vernon et al 2013). Alternative control measures to suppress populations have been studied such as, naturally occurring isolates of Metarhizium and Beauveria which were found to be effective in suppressing wireworms (Kabaluk et al. 2013), however, the impact of tillage intensity on wireworm density is inconsistent (Furlan et al. 2021; Morales-Rodriguez et al. 2022; Le Cointe et al. 2023), and mass trapping of male click beetles was found to be ineffective in reducing population (Sufyan et al. 2013). Planting Brown Mustard and Buckwheat as rotation crops in wireworm infested fields was effective in reducing wireworm populations and crop damage in the subsequent year (Noronha 2011). Crop rotation is a well-known practice for improving soil quality and building sustainable production systems.
Common buckwheat (Fagopyrum esculentum Moench) is a pseudo-cereal in the family Polygonaceae cultivated for its seed for human and animal consumption and as a cover crop and green manure. It originated from North or East Asia and cultivated for human consumption since at least 1000 BC (Wei 2018). Therefore, it is of great significance as a rotation crop. Buckwheat is grown as a crop and has several benefits for farmers, traditionally it plays a key role as a good preceding cover crop due to it being a phosphorus (P) scavenger, making P more available to crops during the following growth season (Possinger et al. 2013). It is also well known for its weed suppression provided by the production of allelopathic root exudates (Gfeller et al. 2018; Iqbal 2003). Plant root exudates are known to influence the chemical and biological conditions of the soil rhizosphere, palmitic acid and gallic acid derivatives were found by Kalinova et al. (2007) to be key components in root exudates from buckwheat. Kato-Noguchi et al. (2007) found that just one buckwheat seedling produced enough allelopathic exudate to inhibit the growth of lettuce. Not only do these root exudates from buckwheat impact plant growth but they can also inhibit the growth of soil-born pathogens (Abbasi et al 2018), making it a good rotation crop to grow in infested fields. Buckwheat is a useful source of pollen and nectar for insects and can attract natural predators to control crop pests (Amoabeng et al. 2019). A significant increase in 22 parasitoids in New Zealand was seen when buckwheat was grown in fields (Berndt et al. 2002). In nature, many plants can produce secondary metabolites that exhibit insecticidal activities (Amoabeng et al 2019). The extracts from buckwheat seeds showed insecticidal activity against Myzus persicae (Lee et al. 2000). Field studies with buckwheat can alter the behaviour of the Popillia japonica females and reduce the density of larvae in the soil when compared to clover and ryegrass indicating insecticidal activity (Szendrei and Isaacs 2006).
Our previous study successfully demonstrated significant potato tuber protection from wireworm damage by planting buckwheat for two consecutive years in highly infested fields (Noronha 2011). However, the potential mechanism underlying the beneficial effects of buckwheat on wireworm control remains poorly understood. According to Hawkins (1930), wireworms belonging to the genus A. mancus (Say) or Melanotus move deeper in the soil profile in the field planted with buckwheat. Therefore, the most common assumption for the effects of buckwheat on wireworm control was that plant root exudate functions as an insect antifeedant or feeding deterrent. This behaviour was also hypostasized by Bohorquez Ruiz et al. (2018). However, our observation in the field trial found a decrease in the number of wireworms and decreased damage to potato tubers by A. sputator wireworms following a buckwheat rotation crop.
We undertook this study to answer these questions and to understand the role of buckwheat in suppressing wireworm populations. We designed a series of field and laboratory experiments to clarify three questions: (1) Do buckwheat roots deter wireworm feeding? (2) What is the mode of action of buckwheat on wireworms (3) The feeding duration required to see the effects on wireworms feeding on buckwheat roots. In this study, we compared buckwheat to the most common rotation crops barley (Hordeum vulgare (var. Island)) red clover (Trifolium pretense (var wildcat)) and potatoes, all known to be good wireworm host plants.
Experimental methods
Wireworms used in the trials
Neonate larvae used in this study were one-day-old wireworms collected from newly hatched eggs laid by female click beetles under laboratory conditions (21 ± 2 °C, 16:8 (L:D) h, and 50 ± 10% relative humidity). For all other studies, wireworms collected using carrot bait traps from infested fields were maintained in 30 × 20 × 12.5 cm plastic containers filled with field-collected moist soil with fresh potato tubers as a food source. The containers were placed in an incubator at 6ºC; soil moisture was maintained at 40% by adding water and fresh potato tubers as food when required. Because of the difficulty in identifying wireworm instars, for our studies, we used head capsule size, which is a know method to determine instars in insects, and body weight to select wireworms. To choose a range of size, we randomly selected 78 larvae from our laboratory population and correlated the head capsule and body weight. Our results showed a positive correlation between body weight and head capsule size (Y = 16.277x − 5.8851 R2 = 06915). For consistency, when choosing larger wireworms for our greenhouse and laboratory studies, we selected wireworms within a certain range of head capsule size and body weight. Small-medium (head capsule 0.62–0.94 SD ± 0.1, weight 4–10 mg SD ± 1.9) wireworms from our laboratory population were used in the trials. Prior to the commencement of each trial, all wireworms were acclimatized to (20 °C) for a period of 72 h. This was done by removing the required number of wireworms from the rearing container and placing them in a new container with soil, similar to that used in the trials, and potato tubers as food source.
Crop varieties used in the trials
Wireworms feed on the roots of a wide variety of crops. In the trials discussed below, growing plants of four different crops were used as a food source. Varieties of the different crops used were Buckwheat (var. Mancan), Barley (var. Island), potato (var. russet burbank), and clover (var. wild cat). Barley and /or clover plants served as the control treatment in the trials.
Feeding preference of wireworms provided with three common rotation crops as a food source
Greenhouse trial
To determine the preference for buckwheat versus barley and clover as a food source, we used white plastic boxes, measuring 65 cm × 70 cm × 20 cm deep (GIMSE Underbed storage box). Each box was filled with 15 lbs of moist potting soil which produced approximately 10 cm soil depth, and fertilizer was evenly incorporated into all the soil. Within each box, four equal-sized compartments were established using vertical plastic dividers. The ensure viability of seeds, barley and buckwheat and clover seeds were pregerminated by placing them in the Petri dish with moist filter paper in the growth incubator set at 22 ± 2 °C, 16:8 (L:D) h, and 70% ± 10% relative humidity (RH) for three days. Germinated barley, or buckwheat plants (20 plants) were transplanted into separate randomly selected soil compartments in each box, and one gram of germinated clover seeds were seeded into the third compartment. The fourth compartment of each box contained only soil which served as an additional control. Plants were allowed to establish over two weeks after which the plastic dividers were removed. Twenty-five randomly selected small to medium-sized wireworms were released on top of the soil in the center of each box and were allowed to dig into the soil and move freely toward their food source. The wireworms were allowed to feed for an additional three weeks after which the aboveground plant parts were clipped, and the plastic dividers were once again inserted into each box to separate the soil into their original compartments. The soil along with the root mass of the plants in each compartment was collected and thoroughly checked for wireworms. Each bioassay consisted of four replicates and was repeated three times. A total of 300 larvae were used for this study.
Because red clover is a commonly grown rotation crop, we wanted to determine the choice of clover if the cropping area was larger. In this trial, the boxes, comparable in size to those in the trial above, were divided into two sections, and planted with buckwheat and clover at the field rate to examine the preference of wireworms between clover and buckwheat. The method used was similar to the one described above except that the boxes were divided into two compartments, clover was planted in one and buckwheat was planted in the other compartment at the recommended field rate. Twenty-five wireworms were released in the center of the box and allowed to move to their preferred food plant. After three weeks the soil and roots were removed and examined for wireworms. The numbers of wireworms in each crop were counted.
Field trial
The field trial was conducted with a slight modification of the greenhouse method stated above. Five micro plots consisting of 2 m × 2 m × 0.5 m deep bottomless wooden boxes were set up in the field at Harrington research farm (46.341493, − 63.156055). A floating Row cover (Veseys (AG-15 row cover)) was placed at the bottom, before filling the box (microplot) with soil, this was done to prevent naturally occurring wireworms from moving up into the soil within the microplot but, allowing rainwater to penetrate and prevent waterlogging. The microplot was divided into four sections by placing vertical partitions in the soil. Buckwheat, barley, and clover seeds were planted in 10 cm pots with three plants per pot in the greenhouse. One week after plant emergence, nine pots of each crop were transplanted in a randomly selected section within the microplot. After transplanting, the vertical partitions were removed, and 100 female and 100 male click beetles were released in the center of each microplot with cut apples and vials of 10% honey and water solution as adult food. A floating row was used to cover each microplot to prevent beetles from leaving or entering. Beetles were allowed to mate, feed and deposit eggs in the soil for a period of two months after which the row cover was removed and hatching neonate wireworms were allowed to choose among the different crops as their food source. After removing the row cover, the vertical partitions were reinstalled in each microplot, and nine carrot bait traps were placed in each section of the microplot every two weeks for a period of two months. The soil from the bait traps was carefully examined and all first year (neonates) wireworms were counted and recorded. The trial was conducted over a four-month period during the growing season (mid-June to October).
Effects of feeding on buckwheat roots on wireworm development—laboratory trials
Trial with neonate wireworms
A no-choice food test with neonate wireworms was conducted between 20th July, and 20th Nov. 2017. One hundred and twenty cups (ROPAK®) 7.5 cm in diameter and 8.5 cm in height were filled with moist potting soil and seeded with barley or buckwheat. The cups were placed in a growth chamber (Conviron™) at 21 °C, with a 16L:8D photo regime and approximately 75% RH and the plants were allowed to grow for four weeks. Two newly hatched wireworms were then gently transferred into each cup (N = 240 neonate larvae) and allowed to feed on the roots of the plants. After four months of feeding the soil from each cup was carefully checked for wireworms. Larval survival and the difference in development between larvae feeding on either barley or buckwheat for four months was determined by comparing the average body weight gained and the change in the width of the head capsule. Larval head capsule pictures were taken with the Olympus SZ-CTV Infinity 1 at 60X magnification, and the head capsule width was measured using QuickPHOTO Camera 3.1.
Trial with small to medium-sized wireworms
Buckwheat, barley, and potato plants were grown in potting soil in plastic cups (ROPAK®) at 7.5 cm diameter and 8.5 cm high. Wireworms (head capsule 0.62–0.94 SD ± 0.1, weight 4–10 mg SD ± 1.9), randomly selected from the laboratory wireworm population, were individually released in cups with a four-week-old plant and incubated in a growth chamber (Conviron™) at 21 °C, with a 16L:8D photo regime and approximately 75% RH. The plants received 50 ml of water everyday to maintain adequate moisture for both plants and insects. Every four weeks the wireworms were removed from the cup, the body weight and head capsule were measured and then transferred to a new cup with a plant. There were twenty replications for each treatment plant.
To further understand the impact of buckwheat on wireworms, a similar trial as above, with small to medium-sized wireworms (weight 4–10 mg SD ± 1.9) was conducted using buckwheat and potato plants. Sixty cups each of buckwheat or potato plants were planted and allowed to grow in a growth chamber for four weeks as mentioned previously. A hundred and twenty wireworms with body weight between 4 and 10 mg (SD ± 1.6) and head capsule size 0.64–0.97 (SD ± 0.08), were randomly selected from the population maintained in the laboratory and individually transferred into either four-week-old buckwheat or potato plants. The cups were placed in the growth chamber (Conviron) at 21 °C, with a 16L: 8D photo regime and approximately 75% RH. In this trail, the soil of each cup was checked every ten days for 80 days in total. The body weight and the head capsule width of all wireworms collected were measured and recorded. There were 60 replicates per treatment plant.
Statistical analysis
The food choice of wireworms between the different treatments on larval survival, body weight gain and head capsule were compared using a one-way analysis of variance (ANOVA). A two-way ANOVA was used to compare the body weight and head capsule over time, means were compared using Tukey’s HSD test using R statistical package (R Core Team 2018).
Results
Feeding preference of wireworms provided with three common rotation crops as a food source.
Greenhouse trial
In the greenhouse trial, when given the choice, wireworms preferred to move to buckwheat, followed by barley, clover and bare soil. Although a higher percentage of wireworms were found in the buckwheat treatment, overall, there was no statistically significant difference observed among all treatments (P = 0.66, df = 8) (Fig. 1).
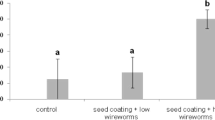
We found that when small medium-sized wireworms were provided with a choice between red clover and buckwheat, once again a significantly higher percentage of wireworms chose buckwheat as their food source instead of clover (P = 0.01, df = 4) (Fig. 2).
Field trial
The previous greenhouse trials provided information on the food plant preference of small to medium-sized wireworms. In this trial, we wanted to determine the preferred food choice of hatching neonate larvae after the first four months of their life cycle. Our results showed no significant difference between the total number of first year wireworms found in buckwheat, barley, clover and in bare soil (P = 0.40, df = 76) (Fig. 3). This lack of significance could be because all crops provided were host crops for the neonates and movement in search of food was not required.
Effects of feeding on buckwheat roots on wireworm development—laboratory trials
Trial with Neonate wireworms
In this study, we collected newly hatched wireworms and placed them on barley or buckwheat plants as food sources for a period of 120 days. Results showed that after feeding for 120 days on the roots of these plants mortality was significantly higher (P < 0.05) when feeding on the buckwheat roots as compared to barley roots. There was also a significant reduction (P < 0.05) of the average body weight of wireworms feeding on buckwheat when compared to those feeding on barley. Similarly, results showed a 30% reduction (P < 0.05) in the average size of the head capsule (0.95 (± 0.075) mm and 0.69 (± 0.024) mm for wireworms feeding on barley and buckwheat, respectively (Fig. 4). The wireworms despite starting at the same stage and size (newly hatched) show a significant reduction in growth when feeding exclusively on buckwheat roots when compared to the wireworms feeding on barley roots, the photographic difference in body size between the two groups is shown in (Fig. 5). There was a positive correlation between body weight and head capsule size (Fig. 6). A steeper slope in the buckwheat compared to the barley-feeding wireworms indicates a quicker increase in head capsule size compared to body size and may indicate an increase in the number of molts.
Trial with small medium-sized wireworms
In this study, we wanted to determine the impact on body weight and head capsule size of small to medium-sized wireworms feeding on buckwheat, barley, and potato plant roots over an extended period. Results showed a sharp increase in mortality after 4 weeks of feeding on buckwheat roots. Continued feeding resulted in a further increase in mortality of the experimental population reaching 65% after 12 weeks of feeding. Physical changes stabilized between 12 and 16 weeks followed by a further increase in mortality to 80% after 24 weeks of feeding. This stabilized period could be related to a period of non-feeding during molting. Similarly, results also showed little to no increase in average body weight during the first four weeks of feeding on buckwheat when compared to wireworms feeding on barley and potato. A slower increase in body weight was observed over the 24-week feeding period compared to potato and barley. A similar trend was observed in head capsule size with no change for both barley and buckwheat after the first four weeks of feeding, but continued feeding resulted in a constant decrease in head capsule size with the head capsule size being 20% smaller at the end of six months of feeding when compared to wireworms feeding on barley. In addition, the head capsule was deformed in the buckwheat fed wireworms when compared to the barley fed wireworms (Fig. 7).
To obtain more precise information on the developmental response of wireworms feeding on buckwheat, a second study was initiated with small medium-sized wireworms allowed to feed on potato and buckwheat plants for 72 days and checked every 10 days as opposed to every 4 weeks, as in the previous study. Results showed that wireworm weight started to decrease 23 days after feeding on buckwheat, this divergence continued and become greater as feeding on buckwheat continued resulting in a 25.3% decrease in body weight after feeding for 72 days on buckwheat when compared to potatoes (P ≤ 0.05, df = 1) (Fig. 8) However, the size of the head capsule showed an initial increase in average size of 4.2% when feeding on buckwheat as opposed to 0.9% on potatoes over the first 60 days after which the head capsule size of the wireworms feeding on potatoes continued to grow and surpassed the buckwheat feeding wireworms after day 60 days (P < 0.05, df = 1) (Fig. 8). We also found deformed wireworms in our trial with small to medium-sized wireworms feeding on buckwheat, primarily because it was easier to observe this phenomenon because of the larger body size compared to the neonate wireworms (Fig. 9).
Discussion and conclusion
Our study on the effects of buckwheat as a food source on wireworms has revealed interesting results. The mechanism underlying the effect of buckwheat on wireworm control was commonly regarded as a biofumigant or antifeedant (Hawkins 1930; Bohorquez Ruiz et al. 2018). However, this study revealed that wireworms were not repelled by buckwheat, and in contrast, most of the wireworms chose buckwheat as a food source despite having a choice of other acceptable food plants such as barley and clover. This information refutes the notion that suppression of wireworms found under field conditions was due to the repellency and food deprivation during the rotation crop year. Bohorquez Ruiz et al. (2018) found that wireworms were not deterred from buckwheat and no difference was found in host choice between buckwheat, barley, and wheat at germinating or flowering stage. In this study however, we did see slightly higher numbers of wireworms in the buckwheat which although not significantly different, suggests that there may be an attraction to buckwheat as a food source. The current study was conducted for a longer period which may be the reason why we found slightly more wireworms had selected buckwheat as their food source. This selection of buckwheat as a food source was also observed in our field microplot study with neonate larvae. We know from personal observations that neonate larvae can move 30 cm in 24 h toward a food source (unpublished data), making it possible for them to move and choose their preferred host. These results indicate that buckwheat does not have antifeedant prosperities and may be attractive to the wireworms. More detailed studies are required to verify this response. In our study, we also found wireworms in the bare soil control treatment. Wireworms are known to survive in bare soil feeding on organic matter in the soil and can survive without a food crop for extended periods of time (Evans and Gough 1942). A small percentage of wireworms were found to feed on decaying organic matter Wallinger et al. (2013). This behaviour may partly explain why in our study we found wireworms in the bare soil treatment used as a control. It was also difficult to keep the infiltration of roots from the crops in the other compartments into the bare soil section or wireworms were attracted to the carrot bait because of the low food supply in bare soil. These factors may explain why we found wireworms in the bare soil treatment.
This study also determines the mechanism of wireworm suppression that was observed in field trials. Laboratory- based studies used neonate larvae so that all individuals were starting to feed at the same larval instar on buckwheat and barley plants. Barley is known as a good host plant for wireworms and was used as the standard control. A significant decrease in survival after a period of 120 days corroborates the suppression of wireworms in the field reported by Noronha (2011). Not only did our study find a significant reduction in survival but also a significant decrease in body size and weight. Nijhout (1981) found that under suboptimal nutritional conditions growth is slower and weight gained is also lower for Manduca Sexta. The decrease in body size (weight an head capsule size) in this study indicates that buckwheat may be of low nutritional value for wireworms or have lower absorption qualities having a significant negative impact on body weight and size. A high correlation was found between body weight and head capsule size in this study. The overall head capsule size was significantly smaller in the wireworms feeding on buckwheat compared to the wireworms feeding on barley. The head capsule is an important aspect of insect growth and indicates molting. Grunert et al. (2015) found that when given a low-nutrient diet the number of larvae instars of Manduca sexta vary, with more molts at smaller increments are observed before threshold size is reached. They found that a 60% reduction in nutrients resulted in greater variability in growth rate and an increased number of larval instars. In this study when body weight was correlated with the head capsule width, we found more incremental levels in head capsule size when the wireworm was feeding on buckwheat before it reached the smallest head capsule size recorded in wireworms feeding on barley. We hypothesize that the phytochemicals found in buckwheat roots may influence the growth of wireworms feeding on the roots.
A similar pattern of growth and head capsule size was found in the small to medium-sized larvae. It however took four weeks before this delay in development to become visible. The negative effects on growth may take longer to manifest and become visible in larger wireworms because of prior feeding on a nutritious host for a significant duration of their life cycle before feeding on buckwheat. Bohorquez Ruiz et al. (2018) found a difference of 7.79% in the weight of medium to large size larvae over a 21-day period. However, in the present study the average body weight of 60 medium-sized wireworms increased by 28.7% when feeding on buckwheat plants for 23 days which is slightly less than the 30.3% found in the ones feeding on potato plant roots. This difference might be because of the difference in size and initial weight of wireworms used in our study (4 and 10 mg) compared to (10 or 17 mg) in Bohorquez Ruiz et al. (2018) study. In addition, we also found that the effects of feeding on buckwheat were more discernible when feeding continued beyond 21 days which was the feeding time frame used in Bohorquez Ruiz et al. (2018) study. This discrepancy may be because, in larger larvae, a longer feeding period on food source contains physiology altering phytochemicals is required to overcome the benefits of feeding on nutritious food during the earlier instars or being larger wireworms nearing their maximum size and weight consumption of plant roots was reduced. More studies are required with larger wireworms to identify these pathways and confirm this hypothesis.
In our trial with small to medium-sized wireworms feeding on buckwheat, we observed developmental setbacks resulting in larvae being deformed or having difficulty molting. Unsuccessful molting when feeding on buckwheat indicates the presence of phytochemicals in the roots that are capable of inhibiting normal growth. Larval deformation and inhibition of normal growth results in eventual mortality, which would explain why we found 80% mortality in our trials. The buckwheat seed extract was found to have insecticidal activity against Myzus persicae (Lee et al. 2000), indicating that buckwheat has the potential to have detrimental effects on insects. In addition to phytochemicals, a low nutrient diet impacted development and resulted in an increased number of molts Grunert et al. (2015). We found that within a 13-day period medium-sized wireworms feeding on buckwheat molt twice compared to only once in the wireworms feeding on potatoes (unpublished data). The lower nutritional quality of buckwheat together with phytochemicals may be responsible for unsuccessful molting leading to lower survival of wireworms feeding on buckwheat. Plant secondary metabolites such as alkaloids, terpenoids, phenols, glycosides, flavonoids, can have insecticidal activities toxicity, repellency, attraction, oviposition, or feed deterrence towards insect pests (Koul et al. 2008). These defensive phytochemicals may not be produced by the plant in large quantities, but toxicity may be the result of a mixture of several compounds resulting in synergistic toxicity (Koul 2016). The mechanism of this synergism involves the ability of one compound in the mixture inhibiting the detoxification or absorption of others in the insect gut (Singh et al. 2009). This type of synergistic phytotoxicity may explain why we found reduced survival, weight and growth in wireworms feeding on buckwheat. Our results also showed that when feeding on buckwheat began earlier in the life cycle, at the neonate stage, growth and survival were impacted within the first four months but for the medium-sized wireworms that had fed on a good food source previously, a longer duration of feeding on buckwheat was required to see the negative effects on the development. This would explain why decreased damage and numbers of wireworms were found after growing buckwheat in the field for one growing season (Noronha 2011). Szendrei and Isaacs (2006) found a significant decrease in survival of Popillia japonica larvae under a buckwheat crop compared to ryegrass. Like wireworms, larvae of P. japonica live in the soil and feed on the roots and organic matter of plants. The decrease in the population of P. japonica suggests that the negative effects of feeding on buckwheat on development may not be confined to wireworms alone.
In conclusion, we found that buckwheat does not repel and may be attractive to wireworms. We also found that despite its attractiveness it is not a very nutritious food source and results in lower body weight, increased detrimental effects on development resulting in lower survival. However, we also found that wireworms need to feed for an extended period for these detrimental effects to occur and that this time may be longer for larger sized larvae. These results have serious implications for establishing an IPM program to reduce wireworm populations in farmers’ fields. When grown as a rotation crop in infested fields, wireworms will be feeding on the roots of the crop over the entire growing season, planting to harvest. In addition, the natural suppression of weeds by buckwheat prevents the presence of other good host plant roots. Our results show that this continuous feeding on the buckwheat roots over the growing season will negatively impact the development, growth and survival of wireworms and result in a decrease in the wireworm population. Noronha (2011) field trials showed that planting buckwheat reduced wireworm populations and crop damage in the subsequent year, verifying the detrimental effects of buckwheat roots on wireworms. In addition to the crop being a food source over the entire growing season, the recent practice of leaving crop stubble in the field to prevent soil erosion will prolong the time for wireworms to feed on viable buckwheat roots causing a further reduction in the field population.
In our study, the acceptance of buckwheat as a food source when given a choice shows that buckwheat does not repel wireworms, this information is important as it provides an opportunity to study the impact of intercropping buckwheat on wireworm suppression. Intercropping is a suggested strategy to control wireworms in infested fields (Vernon et al. 2017) and the potential for using buckwheat in this way would be especially beneficial for organic farmers. Further studies to verify the phytochemicals present in the root of buckwheat that are responsible for decreased wireworm survival in the field and the impact of intercropping buckwheat to reduce wireworm populations are needed.
Data availability
Data is available from the corrosponding author upon request.
References
Abbasi PA, Renderos W, Fillmore S (2018) Soil incorporation of buckwheat as a pre-plant amendment provides control of Rhizoctonia damping-off and root rot of radish and Pythium damping-off and root rot of cucumber. Canadian Journal of Plant Pathology 41:24–34. https://doi.org/10.1080/07060661.2018.1559224
Amoabeng BW, Johnson AC, Gurr GM (2019) Natural enemy enhancement and botanical insecticide source: a review of dual use companion plants. Applied Entomology and Zoology 54:1–19. https://doi.org/10.1007/s13355-018-00602-0
Berndt LA, Wratten SD, Hassan PG (2002) Effects of buckwheat flowers on leafroller (Lepidoptera: Tortricidae) parasitoids in a New Zealand vineyard. Agricultural and Forest Entomology 4:39–45. https://doi.org/10.1046/j.1461-9563.2002.00126.x.S2CID85231915
Bohorquez Ruiz YL, Scott IM, McNeil JN (2018) The buckwheat effect: a biopesticide for wireworm? Journal of Economic Entomology 112:625–632. https://doi.org/10.1093/jee/toy366
Brandl MA, Schumann M, Przyklenk M, Vidal PA, S, (2017) Wireworm damage reduction in potatoes with an attract-and-kill strategy using Metarhizium brunneum Journal of Pest Science 90:479–493. https://doi.org/10.1007/s10340-016-08240-x
Evans AC, Gough HC (1942) Observations on some factors influencing growth in wireworms of the genus Agriotes esch. Ann Appl Biol 29:168–175. https://doi.org/10.1111/j.1744-7348.1942.tb07584.x
Furlan l, Milosavljević I, Chiarini F, Benvegnù I (2021) Effects of conventional versus no-tillage systems on the population dynamics of elaterid pests and the associated damage at establishment of maize crops. Crop Protection 149:105751. https://doi.org/10.1016/j.cropro.2021.105751
Gfeller A, Glauser G, Etter C, Signarbieux C, Wirth J (2018) Fagopyrum esculentum alters its root exudation after Amaranthus retroflexus recognition and suppresses weed growth. Frontiers in Plant Science 9:1–13. https://doi.org/10.3389/fpls.2018.00050
Grunert LW, Clarke JW, Ahuja C, Eswaran H, Nijhout HF (2015) A quantitative analysis of growth and size regulation in Manduca sexta: the physiological basis of variation in size and age at metamorphosis. PLoS One1 10:0127988. https://doi.org/10.1371/journal.pone.0127988
Hawkins JH (1930) Wireworm control in maine. Journal of Economic Entomology 23:349–352. https://doi.org/10.1093/jee/23.2.349
Iqbal Z (2003) Allelopathic activity of buckwheat: isolation and characterization of phenolics. Weed Science 51:657–662. https://doi.org/10.1614/0043-1745
Kabaluk T, Janmaat A, Sheedy C, Goettel M, and Noronha C (2013) Agriotes spp. L., wireworms and click beetles (Coleoptera: Elateridae) in Biological control programmes in Canada 2001–2012 (Wallingford: CABI) 72– 82. https://doi.org/10.1079/9781780642574.0072
Kalinova J, Vrchotova N, Triska J (2007) Exudation of Allelopathic substances in buckwheat (Fagopyrum esculentum Moench). Journal of Agricultural and Food Chemistry 55:6453–6459. https://doi.org/10.1021/jf070795u
Kato-Noguchi H, Sugimotoo H, Yamada M (2007) Buckwheat seedlings may inhibit other plant growth by Allelopathic substances. Environmental Control in Biology 45:27–32. https://doi.org/10.2525/ecb.45.27
Keiser A, Häberli M, Stamp P (2012) Quality deficiencies on potato (Solanum tuberosum L.) tubers caused by Rhizoctonia solani, wireworms (Agriotes ssp.) and slugs (Deroceras reticulatum, Arion hortensis) in different farming systems. Field Crops Res 128:147–155. https://doi.org/10.1016/j.fcr.2012.01.004
Koul O (2016) The handbook of naturally occurring insecticidal toxins. CABI, Oxfordshire
Koul O, Walia S, Dhaliwal G (2008) Essential oils as green pesticides: potential and constraints. Biopestic Int 4:63–84
Le Cointe R, Plantegenest M, Poggi S (2023) Wireworm management in conservation agriculture. Bioevaluation of Subabul(Leucaena leucocephala) Proteinase Inhibitors on Helicoverpa armigera. https://doi.org/10.1007/s11829-023-09966-9
Lee H-S, Choi G-J, Cho K-Y, Lee S-G, Ahn Y-J (2000) Fungicidal and insecticidal activities of various grain extracts against five insect pests and six phytopathogenic fungi. The Korean J Pestic Sci 4:7–14
Morales-Rodriguez A, Wichman D, Wanner KW (2022) Effects of tillage and fallow rotation on wireworm populations and damage to cereal grain crops in Montana. Crop Forage Turfgrass Manag 8:20193. https://doi.org/10.1002/cft2.20193
Nijhout FH (1981) Physiological control of molting in insects. Amer Zool 21:631–640. https://doi.org/10.1093/icb/21.3.631
Noronha C (2011) Crop rotation: a management tool for wireworms in potatoes. IOBC/WPRS Bull 66:467–471
Noronha C, Smith M, Vernon RS (2007) Efficacy of seed-piece or in-furrow insecticide treatments against wireworm in potatoes 2007 Pest Management Research Report 2007
Parker WE, Howard JJ (2001) The biology and management of wireworms (Agriotes spp.) on potato with particular reference to the U.K. Agricultural and Forest Entomology 3:85–98. https://doi.org/10.1046/j.1461-9563.2001.00094.x
Possinger AR, Byrne LB, Breen NE (2013) Effect of buckwheat (Fagopyrum esculentum) on soil-phosphorus availability and organic acids. J Plant Nutri Soil Sci 176:16–18. https://doi.org/10.1002/jpln.201200337
R Core Team (2018) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Singh R, Koul O, Rup PJ, Jindal J (2009) Toxicity of some essential oil constituents and their binary mixtures against Chilo partellus Swinehoe (Lepidoptera: Pyralidae). International Journal of Tropical Insect Science 29:93–101. https://doi.org/10.1017/S1742758409990087
Sufyan M, Daniel N, Lorenzo F (2013) Effect of male mass trapping of Agriotes species on wireworm abundance and potato tuber damage. Bulletin of Insectology 66(1):135–1422013. https://doi.org/10.1016/j.cropro.2021.105751
Szendrei Z, Isaacs R (2006) Ground covers influence the abundance and behavior of Japanese beetles. Environmental Entomology 35(3):789–796. https://doi.org/10.1603/0046-225X-35.3.789
van Herk WG, Vernon RS, Tolman JH, Saavedra HO (2008) Mortality of a wireworm, Agriotes obscurus (Coleoptera: Elateridae), after topical application of various insecticides. Journal of Economic Entomology 101:375–383. https://doi.org/10.1093/jee/101.2.375
Vernon R, van Herk W (2017) Wireworm and Flea Beetle IPM in potatoes in Canada: implications for managing emergent problems in Europe. Potato Research 60:285. https://doi.org/10.1007/s11540-018-9355-6
Vernon RS, van Herk WG, Clodius M, Harding C (2013) Further studies on wireworm management in Canada: damage protection versus wireworm mortality in potatoes. Journal of Economic Entomology 106(2):786–799. https://doi.org/10.1603/EC12180
Wallinger C, Staudacher K, Schallhart N, Peter E, Dresch P, Juen A, Traugott M (2013) The effect of plant identity and the level of plant decay on molecular gut content analysis in a herbivorous soil insect. Mol Ecol Res 13:75–83. https://doi.org/10.1111/1755-0998.12032
Wei Y (2018) Buckwheat remains from the late Neolithic site of Donghuishan, Ganshu province, China. Cereal Chemistry 2019:1–6. https://doi.org/10.1002/cche.10130
Acknowledgements
We would like to acknowledge the technical assistance of Courtney ward, Danielle Younker, Heather Simpson, Jenna Cahill, and Holly McKenna with the field, greenhouse, and laboratory trials. We would also like to thank the Harrington farm crew for their assistance.
Funding
Open access funding provided by Agriculture & Agri-Food Canada. Funding for this project was provided by Agriculture and Agri-Food Canada.
Author information
Authors and Affiliations
Contributions
CN provided the concept, funding, and resources and supervision. CN wrote the manuscript; SL provided the original rough draft. SL contributed to design of the study and led the execution of the greenhouse field and laboratory trials with NMG’s assistance. MDB contributed to the design and execution of the laboratory neonate trials. SL and MDB provided the pictures and SL, MDB and NMG contributed to the analysis and graphs.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Handling Editor: Giselher Grabenweger.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Noronha, C., Liu, S., Bahar, M.H. et al. Buckwheat (Fagopyrum esculentum): its impact on wireworm development and survival. Arthropod-Plant Interactions 17, 429–440 (2023). https://doi.org/10.1007/s11829-023-09982-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11829-023-09982-9