Abstract
The first data on the interaction between a phytophagous hoverfly and holoparasitic broomrape plants in Europe is reported. Description of the new species Eumerus larvatus sp. nov. Aracil, Grković et Pérez-Bañón is presented from specimens feeding within the fleshy hypogeal stems of Cistanche phelypaea in different localities of Southeast of Iberian Peninsula. Both, adult and preimaginal morphology are studied, including also a molecular analysis based on COI gene sequences. The molecular data, together with the diagnostic morphological characteristics of the adult, indicate that the species belongs to the Eumerus tricolor species-group. This paper presents the first in-depth and detailed description of the preimaginal morphology of a representative of this species group, based on scanning electron microscopy (SEM) images.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Eumerus Meigen 1822 (Diptera: Syrphidae) is one of the most diverse genera of flower flies (also named hoverflies), comprising around 280 different species worldwide (Souba-Dols et al. 2020). At least, 170 species of those are known from the Palearctic region (Chroni et al. 2018), having its highest diversity in the Mediterranean Basin (Ricarte et al. 2008). Eumerus belongs, together with the genus Merodon Meigen, 1803 to a monophyletic lineage of hoverflies (Ståhls et al. 2003; Mengual et al. 2015; Young et al. 2016). Although phylogenetic relationships within the genus remain unclear, Eumerus tricolor species-group and several other species-groups have been revealed and supported by both molecular and adult morphological markers (Chroni et al. 2017; Grković et al. 2017, 2021; Ricarte et al. 2018; Gilasian et al. 2020; Malidžan et al. 2022).
Despite the abundance of some species, relevant data related to the diversity, habitat preferences and natural history of most Eumerus are still unknown (Chroni et al. 2018). One of the main gaps is the information related to larval biology, including also morphological descriptions, as less than 10% of the larvae have been described to date (Ricarte et al. 2017; Souba-Dols et al. 2020). Considering available information involving hosts plants and larval feeding sources, we may conclude that the genus is unbelievably diverse and polyphagous compared with the closely related Merodon, that is strictly phytophagous. Eumerus larvae has been reported feeding on bulbs, fruits and stems or swollen roots, among other vegetable tissues from a wide range of host plants as Asparagaceae, Cactaceae, Campanulaceae or Solanaceae (25 families in total), with Amaryllidaceae and Asphodelaceae being the most frequently associated with (Souba-Dols et al. 2020; Choi et al. 2021).
Orobanchaceae (i.e. Cistanche spp.) has been also associated with larval feeding habits of some Eumerus species: E. amnophilus Paramonov, 1927, E. arnoldii Stackelberg 1952, E. cistanchei Efflatoun, 1926, E. compertus Villeneuve, 1924, E. mucidus Bezzi, 1921 and E. turcmenorum Paramonov, 1927 (Souba-Dols et al. 2020), but until now this plant-interaction was unknown from Europe (Speight et al. 2021). The broomrapes of the genus Cistanche includes approximately 25 species of holoparasitic plants that frequently parasitize roots of Amaranthaceae (including Chenopodiaceae) in desert and semi-desert habitats of Eurasia and North Africa (Piwowarczyk et al. 2019). These plants are characterized by a big, fleshy, and hypogeal stem with scattered scales covering it. The aim of this study is to report the first interaction of broomrape-phytophagous hoverfly for Europe and the description of a new species of Eumerus. The first data about its life cycle, morphological description of larvae and pupae and molecular information are provided as well.
Methodology
Collecting site and sampling procedure
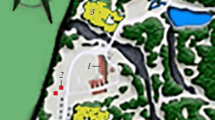
Field work was organized considering the Iberian geographical distribution of the potential host-plant species i.e. Cistanche phelypaea (L.) Cout., (see Piwowarczyk et al. 2016), which included two well recognized subspecies (C. phelypaea subsp. phelypaea and C. phelypaea subsp. lutea (Desf.) Fern. Casas & M. Laínz). A total of nine points were sampled, which are mostly distributed in the two well-established areas of the host-plant (Fig. 1). The survey was done on (i) five localities with mainly semi-arid and semi-desert environments located in the eastern part and, (ii) four areas conformed by wetlands, lagoons and coastal salt marshes near the shore coast, which are mostly located in the western part. Sampling period was done from March to June of the years 2021 and 2022.
Distribution maps and habitat types. A Distribution map of Cistanche phelypaea, based on Piwowarczyk et al. 2016). B Distribution map of sampling points of the present study. C Habitat type of western Iberian Peninsula. D Habitat type of most eastern Iberian Peninsula. Filled circle presence of C. phelypaea; filled square sampling point without Eumerus larvatus sp. nov.; filled triangle sampling point with E. larvatus sp. nov
Larvae were reared in a rearing chamber under controlled conditions of 25 ± 5 °C of temperature and 50–60% of relative humidity, feeding on the same plant tissue in which they were found, until pupation and adult emergence. Adults were killed by freezing and pinned for its preservation and taxonomic description.
Morphological studies
Adult morphology
The morphological characters used in the descriptions and drawings are based on the terminology established by Thompson (1999). Terminology referring to male genitalia follows Doczkal (1996). Colour characters are described from dry-mounted specimens. Male genitalia were extracted from specimens using standard methods for studying male hoverfly genitalia, described by Grković et al. (2015). Photographs were taken with a Nikon Coolpix D7100 digital camera attached to a Nikon SMZ 745T (Nikon Corporation, Tokyo, Japan) stereomicroscope and then processed in CombineZ 1.0 software (Hadley 2012) and Adobe Photoshop CS3 v. 10.0 software (Adobe Systems, San Jose, CA, USA).
Institutional abbreviations where the material is deposited:
CEUA = Colección Entomológica de la Universidad de Alicante, Alicante, Spain.
CNC = Canadian National Collection of Insects, Arachnids and Nematodes, Ottawa, Canada.
CSCA = California State Collection of Arthropods, Sacramento, CA, USA.
FSUNS = Department of Biology and Ecology, Faculty of Sciences, University of Novi Sad, Serbia.
MNCN = Colección de Entomología del Museo Nacional de Ciencias Naturales, Madrid, Spain.
MZH = Zoological Museum, Finnish Museum of Natural History, University of Helsinki, Helsinki, Finland.
ZFMK = Zoologisches Forschungsmuseum Alexander Koenig, Bonn, Germany.
Preimaginal morphology
Terminology used for larval and pupal descriptions follows Rotheray (1991, 1993), head skeleton description was done following Courtney et al. (2000), Rotheray and Gilbert (2008) and Rotheray (2019). The morphological analysis was performed following the methodology stated by Aracil et al. (2022).
At least 30 third instar larvae were preserved by immersion in cold water to extend them, and then heated slowly for about four minutes to kill them. Debris adhered to the larval integument and puparium were removed by placing the specimens in an ultrasonic cleaner for a few minutes. Subsequently, the larvae were washed and preserved in 70% alcohol and the pupae in plastic microcapsules. The head skeleton was extracted from the puparia and from third-instar larvae and cleared by an immersion in 10% hot potassium hydroxide (KOH) for 15 min. The resulting suspension was neutralized by immersing the structure in 98% CH3COOH. After the clearing process the structures were preserved in glycerine.
The micromorphology of third instar larvae and puparium was studied using cryo-scanning techniques coupled to a Field Emission Scanning Electron Microscopy (cryo-FESEM) in the first case and scanning electron microscope (S3000N Hitachi) using variable-pressure (or low vacuum) mode for the second. Third instar larvae, puparia, head skeleton and adult mouthparts morphology were analysed using a stereomicroscope (Leica M205C), and pictures were taken using a camera adapted to it (Leica DFC450). Dimensions of preserved specimens were measured using ImageJ informatics tool v. 1.52 (Schneider et al. 2012) based on the pictures previously obtained.
Molecular analysis
The analysis includes representative species of the major Eumerus genus species groups. A total of 26 hoverfly specimens were analysed, including two taxa that served as outgroups: Xanthograma citrofasciatum (De Geer 1776) and Archimicrodon sp. The newly generated sequences (ISL8-ISL11) of the four larvae found were analysed together with the previously generated sequences set of data published by Grković et al. (2015, 2017, 2021), Chroni et al. (2018), and Ricarte et al. (2018). All examined samples with the GenBank accession numbers are listed in Supplementary Table 1. DNA voucher specimens are deposited in the insect collections of the FSUNS, the MZH, and CEUA (located at the Department of Environmental Sciences & Natural Resources, Alicante, Spain).
Genomic DNA extractions were performed on the abdomen of the larvae, based on the sodium dodecyl sulfate (SDS) extraction protocol described by Chen et al. (2010). The 5′-end of mtDNA COI (cytochrome c oxidase subunit I) gene was amplified using LCO1-490 and HCO-2198 primer pair (Folmer et al. 1994). The PCR reactions were carried out according to Kočiš Tubić et al. (2018). Purification of the obtained PCR products was performed by the Exonuclease I and FastAP Thermosensitive Alkaline Phosphatase (ThermoScientific, Lithuania) following the manufacturer’s instructions. Clean products were sequenced in forward direction by the Macrogen EZ-Seq service (Macrogen Europe, Amsterdam, the Netherlands).
The sequences of 5′-end of COI gene were edited for base-calling errors using BioEdit v. 7.2.5. (Hall 1999) and adjusted manually. The sequence alignment was made by the Clustal W algorithm (Thompson et al. 1994), as implemented in BioEdit 7.2.5. (Hall 1999). The alignment was straightforward as no indels (insertions or deletions) were found. A Maximum-Likelihood (ML) tree (with 1000 bootstrap replicates) for the analysed sequences matrix was constructed using MEGA version 7.0 (Kumar et al. 2016), with the GTR + G + I substitution model, as the best determined. The tree was rooted on Archimicrodon sp.
Results
Larvae of Eumerus larvatus sp. nov. were found feeding within the stems of Cistanche phelypaea at four semiarid localities across the south-eastern region of the Iberian Peninsula, but no larvae were found during the survey in the south-western distribution of the host-plant (Fig. 1; Table 1). Only three adults were observed in the field, during the second year of sampling period (one male and two females). It is noteworthy that the presence of adults took place at the beginning of spring (10.iii.22), an unusually early season for the activity of the genus Eumerus in Spain (own data). The occurrence of wild adults coincided with the presence of eggs and small larvae in the host-plant (Aracil et al. in prep).
Taxonomy
Eumerus larvatus Aracil, Grković et Pérez-Bañón sp. nov.
Type localities
Barranc de Pina in Agost (Alicante province) (38° 26′ 12.5″ N, 0° 36′ 31.4″ W), la Algüeda dry riverbed, close to Albatera (Alicante province) (38° 13′ 20.64″ N, 0° 53′ 32.362″ W); Sorbas (Almería province) (37° 3′ 17.87″ N, 2° 12′ 8.125″ W); two localities in Tabernas (Almería province) (37° 0′ 6.44″ N, 2° 27′ 30.04″ W; 37° 1′ 16.60″ N, 2° 25′ 48.39″ W, respectively).
Type material
HOLOTYPE: ♂ SPAIN: Agost // Barranc de Pina// Leg: C.Pérez// 10.iii.22 // [WELAGCM001- CEUA].
PARATYPES: SPAIN: Albatera // la Algüeda dry riverbed // Leg: A. Aracil and C. Pérez// ♂ 20.iv.21 P:30.iv.21—A: 14.v.21// genitalia extracted [ELACM001-CEUA]. SPAIN: Tabernas // Oasys MiniHollywood // Leg: A. Aracil and C. Pérez // iv.21 (all specimens): ♂ P: 07.vi.21—A: 20.vi.21 [ELTCM001-CEUA]; ♂ P: 22–24.vi.21—A: 07.vi.21 [ELTCM002-CEUA]; ♂ P: 30.iv.21—A: 14.v.21// genitalia extracted [ELTCM003-CEUA]; ♀ P: 01–03.v.21—A: 17.v.21 [ELTCF001-CEUA]. SPAIN: Sorbas // Peñoncillo dry riverbed// Leg: A. Aracil and C. Pérez// iv.21 (all specimens): ♀ P: 03.v.21—A: 17.v.21 [ELSCF001-CEUA]; ♂ P: 03.v.21—A: 17.v.21 [ELSCM001-CEUA]; ♀ P: 15–17.v.21—A: 30.v.21 [ELSCF002-CEUA]; ♀ P: 03.v.21—A: 18.v.21 [ELSCF003-CEUA]; ♂ P: 15–17.v.21—A: 31.v.21 [ELSCM002-CEUA]; ♂ P: 03.v.21—A: 17.v.21// genitalia extracted [ELSCM003-CEUA]; ♀ P: 04.v.21—A: 19.v.21 [ELSCF004-CEUA]; ♀ P: 07.vi.21—A: 20.vi.21 [ELSCF005-CEUA]; ♂ P: 18–19.v.21—A: 02.vi.21 [ELSCM004-FSUNS]; ♂ P: 07.v.21—A: 24.v.21 [ELSCM005-FSUNS]; ♀ P: 04.vi.21—A: 18.vi.21 [ELSCF006-FSUNS]; ♀ P: 18–19.v.21—A: 02.vi.21 [ELSCF007-FSUNS]. SPAIN: Agost // Barranc de Pina// Leg: A. Aracil and A. Juan// iv-v.21 (all specimens) ♂ P: 01–03.v.21—A: 16.v.21 [ELAGCM001-CEUA]; ♂ P: 07.v.21—A: 17.v.21 [ELAGCM002-CEUA]; ♂ P: 14.vi.21—A: 23.vi.21 [ELAGCM003-MZH]; ♂ P: 29–30.iv.21—A: 13.v.21 [ELAGCM004-CEUA]; ♂ P: 01–03.v.21—A: 18.v.21 [ELAGCM005-CEUA]; ♂ P: 29–30.iv.21—A: 14.v.21 [ELAGCM006-ZFMK]; ♂ P: 07.v.21—A: 19.v.21 [ELAGCM007-MNCN]; ♂ P: 07.vi.21—A: 20.vi.21 [ELAGCM008-CSCA]; ♂ P: 22–24.v.21—A: 08.vi.21 [ELAGCM009-CEUA]; ♂ P: 05.v.21—A: 23.v.21 [ELAGCM010-CEUA]; ♂ P: 22.iv.21—A: 07.v.21 [ELAGCM011-CEUA]; ♂ P: 25–26.iv.21—A: 09.v.21 [ELAGCM012-CNC]; ♂ P: 25–26.iv.21—A: 09.v.21 [ELAGCM013-CEUA]; ♂ P: 22–23.iv.21—A: 06.v.21 [ELAGCM014-FSUNS]; ♂ P: 15–17.v.21—A: 31.v.21 [ELAGCM015-FSUNS]; 2♀ P: 23–24.iv.21—A: 09.v.21 [ELAGCF001-002-CEUA]; ♀ P: 23–24.iv.21—A: 09.v.21 [ELAGCF003-MZH]; 2♀ P: 18–20.iv.21—A: 04.v.21 [ELAGCF004-005-CEUA];♀ P: 05–07.v.21—A: 21.v.21 [ELAGCF006-ZFMK]; ♀ P: 25–26.iv.21—A: 11.v.21 [ELAGCF007-MNCN]; ♀ P: 29–30.iv.21—A: 14.v.21 [ELAGCF008-CSCA];♀ P: 29–30.iv.21—A: 14.v.21 [ELAGCF009-CEUA] 2♀ P: 27–28.iv.21—A: 13.v.21 [ELAGCF010-011-CEUA];♀ P: 25–26.iv.21—A: 10.v.21 [ELAGCF012-CNC]; ♀ P: 21.iv.21—A: 06.v.21 [ELAGCF013-CEUA];♀ P: 22–24.vi.21—A: 07.vi.21 [ELAGCF014-CEUA]; ♀ P: 15–17.v.21—A: 31.v.21 [ELAGCF015-CEUA];♀ P: 22–24.v.21—A: 10.vi.21 [ELAGCF016-CEUA]; ♀ P: 31.v.21—A: 14.vi.21 [ELAGCF017-CEUA]; ♀ P: 15–17.v.21—A: 31.v.21 [ELAGCF018-FSUNS]; ♀ P: 18–20.v.21—A: 03.v.21 [ELAGCF019-FSUNS]. SPAIN: Agost // Pina Gorge// Leg: C. Pérez// 10.iii.22: ♀ [WELAGCF001-CEUA]; ♂ [WELAGCM002-CEUA].
Description
Male
Body length (n = 10) 9–10 mm (Fig. 2A, B). Wing length: 7 mm.
Head Holoptic, eye contiguity 5–6 facets long; covered in dense, white moderately long pilosity, longer anteriorly than in lateral parts; posterior margin bare. Vertex, occiput, frons and face black, densely punctuated except on the facial carina. Face flattened in lateral view, covered in mixed, dense curved, brownish-black in upper and whitish long pilosity in lower part; silvery pollinose narrowly near the eye margin. Frons covered in dense curved, mixed, brownish-black above the antennae and white pilosity in lateral and upper parts, shorter compared to pilosity of face and vertex; silvery pollinose in upper corner and near the eye margin. Vertex unraised, covered in long, predominantly brownish-black pilosity, except in small anterior part; with small line of silvery pollinose macula on posterior margin. Ocellar triangle slightly longer than wide, placed medially; posterior ocellus very close to eye margin. Occiput covered in very long whitish pilosity (Figs. 2B and 3A). Basoflagellomere brown, basally reddish, small, square, striated with one transverse arc sulcus, extending from almost dorsal margin to ventral margin of basoflagellomere and 2–3 short longitudinal wrinkles; on the outer side apically with concave ellipsoidal fossette, placed slightly ventrally; arista black; pedicel short, about two times wider than long with black pilosity, ventral much longer than dorsal (Fig. 3C).
Thorax Scutum, scutellum and pleurae black, densely punctuated; pilosity on mesonotum dense, long and whitish, sometimes with few black pile intermixed on notopleuron and on scutum medially; postpronotum with triangular pollinose maculae not extending into longitudinal vittae (Fig. 2A, B). Posterior margin of scutellum nodular. Pleurae unpollinose, covered in curved predominantly whitish pilosity; on anepimeron with mixed brown-black pilosity. Legs predominantly black covered in black pilosity; pro and meso-femur with long curved pilosity posteriorly, on mesofemur longer than the width of the mesofemur; metafemur moderately swollen, with curved ventral pilosity much longer than dorsal, long as the width of the metafemur; ventro-apically with a row of very long 6–9 spinae in anterior row and 3–5 spinae in posterior row (Fig. 3B). Wing transparent, covered in very small microtrichia, basally sparse and denser towards the apex and with large bare areas in cells R, BM, CuP and alula; wing veins yellowish to brown; calypter white yellow; haltere translucent yellow (Fig. 2B).
Abdomen Terga black, second tergum sometimes with barely visible brown markings dorso-laterally; terga 2–3 with pairs of large white pollinose maculae not reaching the lateral margins; maculae on second tergum almost parallel to posterior margin of tergum, maculae on terga 3–4 are oblique, oval and wider at inner end; terga covered in short predominantly white pilosity, black on anterior and posterior medial parts of terga 2–3 and anteriorly on fourth tergum; pilosity on second tergum laterally longer. Sterna black; first sternum with sparse long black curved pile; second sternum covered in long dense curved predominantly whitish pilosity with some black pile intermixed; pilosity on sterna 3–4 short, black and dense, longer on lateral margins of fourth sternum. Pilosity of genital capsule black, moderately long (Fig. 2A, B).
Genitalia Posterior surstyle lobe of epandrium simple, square-shaped, on outer side apicoventrally with longer strong setae, laterally, dorsally and in inner side with longer and slightly thinner pilosity, inner accessory lobe covered in very dense and short pilosity, cerci simple, small (Fig. 4A); hypandrium robust, in apical part narrowed resembling to a bottle, base of theca with a pair of lateral folded wings; ejaculatory apodeme smaller; hamus elongated, narrow; ctenidia situated subapically (Fig. 4B).
Female
Body length: 10–11 mm. Wing length: 7–8 mm (Fig. 2C, D). Similar to the male except for normal sexual dimorphism and for the following characteristics: Frons narrow (one fifth the width of the head), covered in dense moderately long white pilosity; pilosity on vertex longer, mostly whitish but in the area in front of and on the ocellar triangle brownish-black and in the area of posterior of ocellar triangle with few or more black pile (Fig. 3D). Basoflagellomere small but slightly larger than in male, brown, ventrally expanded, striated with one transverse arc sulcus, extending from almost dorsal margin to ventral margin of basoflagellomere and 4–5 short longitudinal wrinkles; fossette small, oval (Fig. 3F). Brown lateral markings on second tergum more visible than in male; posterior half of fourth tergum covered in brownish-black pilosity. Pilosity on sterna shorter than in male, pile on fourth tergum completely short.
Differential diagnosis
Species belongs to the Eumerus tricolor group by radially striated basoflagellomere, apico-laterally with distinctly bordered fossette (Fig. 3C, F); katepisternum completely pilose; in male genitalia (epandrium) anterior surstyle lobe undeveloped and posterior surstyle lobe simple (Fig. 4A). In E. larvatus sp. nov. all terga completely black with a pair of large white pollinose maculae on terga 2–4 (Fig. 2B), metafemur with conspicuously long and needle-like postero-ventral setae (Fig. 3B), male genitalia with simple, square-shaped posterior surstyle lobe of epandrium (Fig. 4A); eyes holoptic, densely whitish pilose (Fig. 2B); antenna small, dark-brownish, square (Fig. 3C), slightly enlarged in females (Fig. 3F); legs black, both in male and female with brownish-black pilosity (Fig. 3B, E); scutum and scutellum black, covered in predominantly whitish pilosity. It can be distinguished from other species without red markings on terga by the following characters: from E. richteri Stackelberg, 1960 by dark coloured antenna which is yellow in E. richteri, significantly raised vertex in E. richteri in contrast to flat vertex of E. larvatus sp. nov. which is especially noticeable in females, two very conspicuous broad pollinose vittae on the scutum of E. richteri in contrast to E. larvatus sp. nov. whose scutum is pollinose only in the anterior part forming a pair of triangles, and by holoptic eyes which are separated in male of E. richteri for about the width of two ocelli. There is also an obvious difference in the shape of male genitalia of these two species. From E. arctus van Steenis, 2021 and E. crispus Vujić et Grković, 2021 can be distinguished by longer ventral pilosity and longer apicoventral spines on meta femur.
Compared to E. compertus and E. mucidus, whose larvae also develop in Cistanche, abdomen is completely black, while according to original descriptions of mentioned species at least tergum 2 is with reddish coloration laterally. Moreover legs are, at least at the connections, yellowish while in E. larvatus sp. nov are completely black; leg pilosity is mostly black in E. larvatus sp. nova and predominantly white, yellowish or greyish in these other species. Differs from similar E. turcmenorum by completely dark terga in females and quite different epandrium in male genitalia (see: Mutin and Barkalov 2018, p. 17, Fig. 22).
Preimaginal morphology
Larvae overall characters
Length 12.52 ± 0.3 mm, greatest height 3.96 ± 0.1 mm, and greatest width 4.24 ± 0.1 mm (N = 30). “Short-tailed” larvae, mouthhooks external and sclerotized, mandibular lobes slightly sclerotized, external, and fused to the mandibles. Light brown, becoming darker at the anal segment (Fig. 5A). Sub-cylindrical in cross section, slightly tapered anteriorly and rounded posteriorly in dorsal view and truncated (not tapered) in lateral view. Anal segment with one pair of well-developed lappets, located dorsally, above the PRP, sclerotized, dark brown and pointed apically (Fig. 5B). Integumental vestiture well developed with short rounded, blunt, and slightly sclerotized spicules; being bulkier in the prothorax. Segmental sensilla not very pronounced, translucent, bearing two or three short setae, lacking fleshy papillae at the base.
Head skeleton
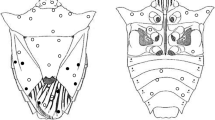
Mandibles hook-shaped apically, external, very sclerotised and robust. Small protuberance in the inner part of mouthhook, reminiscence of accessory teeth. Big hole in the posterior part, filled with muscles (Figs. 6B, 7A). Mandibular lobes external, partially fused to the mouth-hooks and sclerotized. Formed by five ventral ridges, reaching up to top and between four and five dorsal ridges covering half of the structure. Dorsal and ventral ridges alternately arranged. Each ridge bearing short and rounded bristles, between 5 and 15 in the dorsal ones and between 15 and 25 in the ventral ones (Fig. 6C). Basal sclerite elongated, twice as long as broad, dorsal cornu curved and tapered apically (dome shape), not covering the whole length of ventral cornu, giving a sub-elliptical appearance. Intermediate sclerite and dorsal bridge sclerotized and fused between each other, forming an elongated structure that fuses posteriorly with the vertical plate, not so sclerotized. Cibarium located at the base of the ventral cornu, bearing 9 transversal ridges across all the structure. Posterior end of ventral cornu bearing sclerotized plaques, forming the mortar and small sclerotized sclerite at the upper part of ventral cornu, the pestle (Fig. 6A).
Pseudocephalon and thorax
Dorsal lip divided in two lobes, smooth and lacking any ornamentation, presence of a group of short spicules (between 5 and 10) in the joining between dorsal lip and mandibular lobe (Fig. 7C). Lateral lips not developed, mostly smooth but with some short spicules scattered on the surface. Ventral lip totally smooth (Figs. 7B, 8A, B). Dorsal surface of the prothorax with five longitudinal grooves. Antenno-maxillary organs are well-developed, placed between the dorsal lip and the dorsal surface of the prothorax. They consist in two pairs of cylindrical-shaped structures placed on top of two not well-developed fleshy projections. Antenna and maxillary palpus clearly identified, several satellite sensilla present on top (Figs. 8A, C). Anterior fold of the prothorax with the same vestiture than the rest of the dorsal surface, except the region up to the second sensilla of the prothorax, with more scattered and slightly sharper spicules. Anterior larval spiracles are a pair of semi-circular in apical view, short and small spiracles, plain at the apex, located at the dorsal surface of the prothorax. They present between six and nine elongated spiracular openings arranged radially on the surface, facing the dorsal part of the body (Fig. 9B). Mesothoracic prolegs absent (Fig. 7A).
Abdomen
Primordia of pupal spiracles present at the dorsal surface of the first abdominal segment (Fig. 5B). Absence of prolegs. Pairs of raised and more sclerotized domes as locomotory organs, almost fused on the abdominal segments 1–6 and fused in seventh abdominal segment, lacking crochets but with the ornamental spicules more developed, slightly more pointed and densely aggregated (Fig. 10C). Dorsally, abdominal segment 1–6 bearing three folds, segmental sensilla 1–4th present in the second fold; on the seventh abdominal segment only two folds present, bearing segmental sensilla 1–4th on second fold. Anal segment very short, dorsally presenting one single fold ended in one pair of sclerotized lappets pointed at the apex (horn-shaped), bearing double setae at the posterior part of the base (third segmental sensilla) (Fig. 10A).
Posterior respiratory process (PRP)
Blackish-brown, elliptical in cross section, not visible from dorsal view (much shorter than wide) (Fig. 5A). Spiracular surface smooth, slightly wrinkled, getting more densely wrinkled at the base, without an annular groove. Spiracular plate 1.4 times wider than long (0.442 ± 0.08 mm wide, N = 5), three pairs of sinuous spiracular openings around two central scars are present; spiracular openings clearly separated from each other. Four pairs of branched inter-spiracular setae emerge from the spiracular plate, close to the second inter-spiracular setae a small hole without setae is present, presumably acting as a sensory organ (Fig. 9C).
Chaetotaxy
Prothorax (P) with eleven pairs of sensilla, mesothorax (Ms) and metathorax (Mt) with eight pairs of sensilla. Abdominal segments 1–7 with ten pairs of sensilla, anal segment (A8) with eight pairs of sensilla and one pair of lappets (Fig. 11).
Map of the chaetotaxy of the third instar larva of Eumerus larvatus sp. nov. in lateral view showing the positions of sensilla groups. P prothorax; Ms mesothorax; Mt metathorax; A1 and A7 abdominal segments; A8 anal segment; Sp anterior spiracle; Ll lateral lip, Lo locomotory organs; Ps primordia of pupal spiracles; Lpt Lappet. Black dot indicates sensilla bearing seta, black circle indicates sensilla without seta and ten-pointed star indicates double sensillae
Puparium overall description
It is sub-cylindrical in cross-section, flattened ventrally, with the anterior end tapered and the posterior “truncated”. It presents a dark brown colour. The length including PRP is 8.87 ± 0.2 mm, maximum width is 4.03 ± 0.1 mm and maximum height is 3.80 ± 0.1 mm (N = 22) (Fig. 9A). Pupal spiracles are projected from the middle of the upper part of the operculum, being separated by a distance about four-five the length of one spiracle.
Pupal spiracles
Short, 198.1 ± 7.41 µm long and 181.1 ± 5.38 µm (N = 20), conical in shape, brown in colour (Fig. 9D). Big and rounded tubercles, on top of 1–2 layers present covering whole lateral surfaces and apex, distributed in 2–3 rows (Fig. 9E). Dorsal and ventral areas not bearing tubercles. 5–8 oval spiracular openings on each tubercle, white and slightly domed ridges between each opening. Granulated spiracular surface, becoming reticulated in the area between tubercles (Fig. 9D, E).
Molecular data
The final dataset of analysed sequences of 5′-end of COI gene comprised 570 base pairs, with 137 parsimony informative characters. The conducted Maximum Likelihood analysis yielded a tree with two main clades (Fig. 12). One clade consisted of taxa belonging to the E. tricolor group (13 taxa) with the second comprising representative species of the other analysed Eumerus species groups. The monophyly of the E. tricolor group was resolved with high bootstrap support (100 BS). The four sequences of immature specimens (larvae) labelled as ISL8-ISL11 assigned to the E. tricolor species group clade. Within the clade, the specimens of the newly described taxa E. larvatus sp. nov. formed a subclade, clearly separated from the previously described Spanish species E. azabense Ricarte et Marcos-García, 2018, as well as from the other species of E. tricolor species group, with a 100 bootstrap value of support.
Maximum Likelihood tree based on the General Time Reversible model. A discrete Gamma distribution was used to model evolutionary rate differences among sites (5 categories (+ G, parameter = 1.2376)). The rate variation model allowed for some sites to be evolutionarily invariable ([+ I], 53.65% sites). Bootstrap support values (≥ 50) are depicted near nodes
Biology
Several larvae were found feeding on each stem of Cistanche phelypaea, and all specimens presumably had the same level of development and age. Pupae were found in the ground, surrounding the stems. Larvae were feeding within fresh stem tissue, most stems were found fresh, with big rotten parts, derived from larval feeding (Fig. 13B–D). Conversely, pupae were found in the ground, surrounding the stems. Puparia reared in captivity took around 15 days to emerge after pupation. Time that larvae took to pupate was very variable, between eight days and more than a month; since the age and stage of the larvae captured was unknown, it was not possible to accurately estimate the time of preimaginal development. However, an in-depth study of the life cycle of the species in natural conditions and in captivity is currently ongoing (Aracil et al. in prep.).
Host plant and Eumerus larvatus sp. nov. third instar larvae. A Cistanche phelypaea subsp. lutea inflorescence. B Cistanche phelypaea subsp. lutea digged plant. C Cistanche phelypaea subsp. lutea stem with Eumerus larvatus sp. nov. larvae feeding on it. D Cistanche phelypaea subsp. lutea inflorescence stem with Eumerus larvatus sp. nov. larvae feeding on it. Ft fresh tissue; Lv larvae; Rt rotten tissue
Etymology
The specific epithet (larvatus, -a, -um), refers to the difficulty of finding adults of this species in the wild, which were mostly found in the larval stage i.e. “hidden as larva”. Moreover, the original meaning of the word larva is “ghost, spectre”, prior to the current use established by Linnaeus; this is related also with this species, referring to the difficulty of finding adults in the field.
Discussion
Eumerus larvatusas member of Eumerus tricolor species-group
The results obtained in this paper indicate, unequivocally, that Eumerus larvatus sp. nov. belongs to the monophyletic tricolor species-group defined by Chroni et al. (2017) [see also Grković et al. (2017, 2021), Ricarte et al. (2018), Gilasian et al. (2020)].
Morphologically, adults show some similarities with the long-pilose eye species of this group as E. ovatus, E. sinuatus, E. niveitibia, E. azabense and E. richteri. However, it is quite different from the mentioned species by some unique characteristics: very long and slender postero-ventral setae of metafemur, long black pilosity all over the body, absence of red coloration on the abdomen and very small dark basoflagellomere (Ricarte et al. 2018; Grković et al. 2021). Male genitalia also present different morphology, being obvious in the specific case of E. richteri (Grković et al. 2021). It also presents morphological similarities with other species with black coloured terga i.e. E. arctus, E. crispus, E. compertus, E. turcmenorum and E. mucidus. From E. arctus and E. crispus it can be distinguished by the ventral pilosity and the presence of apicoventral spines on hind femur; in addition, E. arctus presents very different morphology of posterior surstyle lobe of epandrium in male genitalia (Grković et al. 2021). Regarding the other three species (E. compertus, E. turcmenorum and E. mucidus) some differences can be stated, first the abdomen, being completely black in E. larvatus sp. nov. while with some reddish spots in, at least, tergum 2 in the other species. Secondly, the coloration of the blasoflagellomere, which is completely dark brown in both males and females of E. larvatus sp. nov. but more pale and orangish in the other species. Above all, coloration of legs and legs pilosity differs deeply: completely black with black pilosity in E. larvatus sp. nov. while in the other species present some yellowish areas, at least in the connection parts of the legs with predominantly whitish, greyish, or yellowish pilosity (Mutin and Barkalov 2018; Piwowarczyk and Mielczarek 2018).
This morphological uniqueness has been also supported by molecular data of the COI gene information, which clearly separates this new described species from the rest of species of the tricolor group. However, no species of the tricolor group associated with Cistanche has been sequenced, so it is not possible to use this feature to differentiate E. larvatus within this group of species.
Unfortunately, the information related with larval stages and plant-host interaction of tricolor species-group is scarce. Thus, Arzone (1971) published a brief description of preimaginal stages of E. tricolor (Fabricius, 1798) and Waitzbauer (1976) described and figured the larva and pupa of E. compertus. Later, Munk (2000) presented general morphology of eggs and first/second instar larvae of E. sabulonum (Fallen, 1817). Lastly, Krivosheina and Krivosheina (2021) described and figured E. ammophilus.
The overall preimaginal morphology of the species described in this paper is shared with all known species belonging to tricolor group (Arzone 1971; Waitzbauer 1976; Munk 2000; Krivosheina and Krivosheina (2021), however, some diagnostic differences can be established between them. First E. tricolor present a horse-shoe shaped spiracular openings in the posterior respiratory process, with transversal prolongations (see Fig. 13 in Arzone 1972) while in E. larvatus sp. nov. present convoluted spiracular openings with thin outline (Fig. 9C). Secondly, head skeleton of E. compertus is characterized by having a very long dorsal cornu, exceeding the length of ventral cornu (see Fig. 5 in Waitzbauer 1976; Stuke 2000), contrary to E. larvatus sp. nov. that has quite short dorsal cornu, not covering the whole length of ventral cornu. Moreover, Stuke (2000) described for E. compertus an anal segment plate, formed by the fusion of integumental spicules in the dorsal area of the segment; this structure is not present in E. larvatus sp. nov., in which the spicules remain independent between them in the mentioned area (Fig. 10A). On the other hand, E. amnophilus can be mainly distinguished from E. larvatus sp. nov. in the shape of the anal segment, the fifth sensilla is located at the same height than the posterior respiratory process, the fold in which is placed is much more concave and elongated, reaching PRP (see Figs. 3, 4 in Krivosheina and Krivosheina 2021); while in E. larvatus sp. nov. the fifth sensilla is placed in a fold not elongated and, therefore, it is located below the posterior respiratory process (Fig. 10B). Unfortunately, E. sabulonum cannot be used for comparison because the description made by Munk (2000) is not based on last instar larvae.
Preliminary morphological delimitation of Eumerus species-groups based on preimaginal features
As already mentioned, several species groups have been established using morphological and molecular characters, but the phylogenetic relationships within the genus Eumerus are not resolved. Unfortunately, although a lot of effort has been made to describe preimaginal stages of this genus during the last decade, the morphology of only 19 species is currently known, including the present description. Consequently, further analysis including in-deep studies of the preimaginal morphology are needed to clarify the taxonomic classification of the different species-groups.
Nevertheless, two main groupings can be preliminary established based on preimaginal morphology. In one hand, most of the known species are characterised by the presence of cylindrical anterior respiratory processes with two spiracular openings on top, reduced mouth-hooks with well-developed and fleshy mandibular lobes with comb-like bristles (Fig. 14) and an elongated PRP (Rotheray and Gilbert 1999). On the other hand, a second group of species has been recently proposed based on the presence of: semi-circular and flat anterior respiratory processes with multiple spiracular openings one pair of horn-shape sclerotized dorsal lappets, anal segment truncated with one pair of horn-shape sclerotized dorsal lappets, and a very short PRP slightly invaginated not visible from dorsal view (Krivosheina and Krivosheina 2021). Considering the available preimaginal descriptions, and the information presented in this paper, this group is probably formed exclusively by true phytophagous species, very close to the larval trophic habits of the bulb-feeding larvae of the genus Merodon. All of them characterised by the presence of well-developed and sclerotized mouth-hooks with reduced mandibular lobes that are not fleshy but quite sclerotized, bearing few ridges with very short bristles (Fig. 7) are congruent with this type of specialised feeding.
The four species of tricolor group with preimaginal stages described share the morphology of this second morphological group, together with unknown species named as Eumerus sp. (Krivosheina and Krivosheina 2021) probably also belong to tricolor-species group. On the other hand, we also found another morphological aspect shared between E. larvatus sp. nov. and E. compertus which is interesting to be highlighted: the shape of the pupal spiracles. This character has not been studied in other species of tricolor group. Moreover E. sogdianus, presented by Krivosheina and Krivosheina (2021), also shares the same characteristics of the above species but it does not belong to tricolor group. Therefore, more research is needed to determine which are the diagnostical characteristics of tricolor group. Unfortunately, as stated before, the available information related to preimaginal morphology of genus Eumerus is still very scarce and fragmented. However, it is a valuable first step in advancing the taxonomic classification of the larvae of the genus Eumerus.
Understanding insect–plant relationship
Relative to insect–plant relationship, Souba-Dols et al. (2020) stated that, despite that there are many polyphagous species inside the genus Eumerus, all the ones feeding on Cistanche plants are not known to be feeding on any other plant species and are, consequently, monophagous. The morphology of some of this Eumerus species is already described and, as it has been mentioned, it differs substantially among them, not only in the general body morphology but also in the head skeleton. This suggests that the biology and the feeding requirements of each species might be different. Hence, more studies in the relation of Eumerus and Cistanche are strongly needed to confirm this potential monophagy and understand why such different species can develop in the same plant (Aracil et al. in prep). On the other hand, the morphology of mouthhooks and mandibular lobes of E. larvatus sp. nov. and all the other close species belonging to the tricolor-group, are close to other putative phytophagous genera such as Merodon. The structure of the head skeleton is a useful character for inferring the larval feeding habits of the flower fly larvae (Campoy et al. 2020) and in this case it is also concordant with the phytophagous activity that was observed both in nature and during laboratory rearing.
Finally, considering the results of our field survey, the species described in this paper is, apparently endemic, only present in localities of the southeast of the Iberian Peninsula (Fig. 1B). Considering the spread distribution of the boomrape Cistanche genus in North Africa, it is reasonable to think that this species might be present as well there, nevertheless no records have been mentioned up to now and field surveys in that area are strongly needed to check the distribution.
However, analysing the distribution of the species in the Iberian Peninsula, it has not been found in the sampling of Cistanche phelypaea from west of the Iberian Peninsula. According to Piwowarczyk et al. (2016), the two well-differentiated geographical areas correspond to two subspecies: (i) C. phelypaea subsp. lutea grows in the eastern part whereas (ii) C. phelypaea subsp. phelypaea appear along the western area. There are some morphological differences between them, mainly related with the corolla and the leaves, but also related with the general habit, as the typical subspecies correspond to graceful plants whereas C. phelypaea subsp. lutea shows robust stems (Piwowarczyk et al. 2016). This last character would mean a higher food availability for syrphid larvae. Additionally, habitats of both populations are very different: the eastern populations of C. phylepaea subsp. lutea are mainly typical of semi-arid and semi-desert environments (Fig. 1C) relatively close to Mediterranean coast, while the western population habitat are wetlands, lagoons, and coastal salt marshes immediately close to shore coast of the Atlantic Sea (Fig. 1D). Strikingly, one locality of eastern population of the host-plant show a similar habitat to the west locations and were found growing in coastal salt marshes, and any larvae of Eumerus larvatus sp. nov. were found (Table 1). More studies are needed to understand the insect–plant relationship of the genus Eumerus with broomrapes and other host-plants, including detailed studies of life cycle (Aracil et al. in prep.) and the potential influence of floral volatiles and physicochemical properties related with the interaction between and broomrapes and the plants that parasitise (Toth et al. 2016; Alia et al. 2021).
References
Alia F, Chouikh A, Djahra AB, Bousbia Brahim A, Nani S, Tliba A (2021) Comparative study of some physicochemical and biological properties of effect host species variation on the relationship Saharan parasitic plant Cistanche violacea (Desf.) Beck. Not Sci Biol 13(4):11054. https://doi.org/10.15835/nsb13411054
Aracil A, Ačanski J, Pérez-Bañón C, Šikoparija B, Miličić M, Campoy A, Radenković S, Vujić A, Radišić P, Rojo S (2022) Characterization of preimaginal developmental stages of two cryptic South African species of the Merodon planifacies complex (Diptera: Syrphidae: Eristalinae: Merodontini), with differentiation through morphometry analysis. Arthropod Struc Dev 70:101187. https://doi.org/10.1016/j.asd.2022.101187
Arzone A (1971) Reperti biologici su Eumerus tricolor Meigen, nocivo alle coltivazioni di Tragopogon porrifolius L. in Piemonte (Diptera, Syrphidae). Annali della Facoltà di Scienze Agrarie della Università degli Studi di Torino, vol VII. (Centro di entomologia alpina e forestale del consiglio nazionale delle ricerche).
Campoy A, Aracil A, Pérez-Bañón C, Rojo S (2020) An in-depth study of the larval head skeleton and the external feeding structures related with the ingestion of food particles by the eristaline flower flies Eristalis tenax and Eristalinus aeneus. Entomol Exp Appl 168:783–798. https://doi.org/10.1111/eea.12974
Chen H, Rangasamy M, Tan SY, Wang H, Siegfried BD (2010) Evaluation of five methods for total DNA extraction from western corn rootworm beetles. PLoS ONE 5(8):6. https://doi.org/10.1371/journal.pone.0011963
Choi DS, Park DG, Lee YB, Hong KJ (2021) A new species of the genus Eumerus (Diptera: Syrphidae) infesting roots of Campanulaceae crops in South Korea. J Asia-Pac Entomol 24(1):402–408. https://doi.org/10.1016/j.aspen.2021.01.008
Chroni A, Djan M et al (2017) Molecular species delimitation in the genus Eumerus (Diptera: Syrphidae). Bull Entomol Res 107(1):126–138. https://doi.org/10.1017/S0007485316000729
Chroni A, Grković A, Ačanski J, Vujić A, Radenković S, Veličković N, Petanidou T (2018) Disentangling a cryptic species complex and defining new species within the Eumerus minotaurus group (Diptera: Syrphidae), based on integrative taxonomy and Aegean palaeogeography. Contrib Zool 87(4):197–225
Courtney GW, Sinclair BJ, Meier R (2000) Morphology and terminology of Diptera larvae. In: Papp L, Darvas B (eds) Contributions to a Manual of Palearctic Diptera. Science Herald, Budapest, pp 85–161
Doczkal D (1996) Description of two new species of the genus Eumerus Meigen (Diptera, Syrphidae) from Corsica. Volucella 2:3–19
Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R (1994) DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol 3:294–299
Gilasian E, van Steenis J, Parchami-Araghi M (2020) Review of the Eumerus tricolor species group (Diptera: Syrphidae) in Iran, with description of six new species. Eur J Taxon 722:152. https://doi.org/10.5852/ejt.2020.722.1139
Grković A, Vujić A, Radenković S, Chroni A, Petanidou T (2015) Diversity of the genus Eumerus Meigen (Diptera, Syrphidae) on the eastern Mediterranean islands with description of three new species. Ann Soc Entomol Fr 51(4):361–373. https://doi.org/10.1080/00379271.2016.1144483
Grković A, Vujić A, Chroni A, van Steenis J, Djan M, Radenković S (2017) Taxonomy and systematics of three species of the genus Eumerus Meigen, 1822 (Diptera: Syrphidae) new to southeastern Europe. Zool Anz 270:176–192. https://doi.org/10.1016/j.jcz.2017.10.007
Grković A, van Steenis J, Miličić M, KočišTubić N, Djan M, Radenković S, Vujić A (2021) Taxonomic revision of the highly threatened Eumerus tricolor species group (Diptera: Syrphidae) in Southeast Europe, with insights into the conservation of the genus Eumerus. Eur J Entomol 118:368–393. https://doi.org/10.14411/eje.2021.039
Hadley A (2012) CombineZP 1.0 (computer software for image stacking). https://combinezp.software.informer.com/download/#downloading
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/ NT. Nucl Acids Symp Ser 41:95–98
Kočiš Tubić N, Ståhls G, Ačanski J, Djan M, Obreht Vidaković D, Hayat R, Khaghaninia S, Vujić A, Radenković S (2018) An integrative approach in the assessment of species delimitation and structure of the Merodon nanus species group (Diptera: Syrphidae). Org Divers Evol 18(4):479–497. https://doi.org/10.1007/s13127-018-0381-7
Krivosheina NP, Krivosheina NG (2021) New Data on the Larvae of the Hover-Fly Genus Eumerus Meigen, 1822 (Diptera, Syrphidae). Entomol Rev 101(2):162–173. https://doi.org/10.1134/S0013873821020020
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33(7):1870–1874. https://doi.org/10.1093/molbev/msw054
Malidžan S, Grković A, Kočiš Tubić N, Radenković S, Vujić A (2022) A new species of Eumerus from Montenegro, belonging to newly established torsicus species group (Diptera: Syrphidae). Zool Anz 297:71–78. https://doi.org/10.1016/j.jcz.2022.02.001
Mengual X, Ståhls G, Rojo S (2015) Phylogenetic relationships and taxonomic ranking of pipizine flower flies (Diptera: Syrphidae) with implications for the evolution of aphidophagy. Cladistics 31(5):491–508. https://doi.org/10.1111/cla.12105
Munk T (2000) Eumerus sabulonum Fallen, 1817 Syrphidae, Diptera breeds in Jasione montana L. Flora Og Fauna 1061:19–22
Mutin VA, Barkalov AV (2018) New data on the hover-flies of the genus Eumerus (Diptera: Syrphidae) from Russia. Far East Entomol 363:11–20
Piwowarczyk R, Mielczarek Ł (2018) First report of Eumerus mucidus (Diptera: Syrphidae) on Cistanche armena (Orobanchaceae) and from Armenia. Fla Entomol 101(3):519–521. https://doi.org/10.1653/024.101.0314
Piwowarczyk R, Carlón L, Kasińska J, Tofil S, Furmańczyk P (2016) Micromorphological intraspecific differentiation of nectar guides and landing platform for pollinators in the Iberian parasitic plant Cistanche phelypæa (Orobanchaceae). Bot Lett 163(1):41–55. https://doi.org/10.1080/12538078.2015.1124287
Piwowarczyk R, Pedraja ÓS, Moral GM, Fayvush G, Zakaryan N, Kartashyan N, Aleksanyan A (2019) Holoparasitic Orobanchaceae (Cistanche, Diphelypaea, Orobanche, Phelipanche) in Armenia: distribution, habitats, host range and taxonomic problems. Phytotaxa 386(1):1–106. https://doi.org/10.11646/phytotaxa.386.1.1
Ricarte A, Marcos-García MA, Rotheray GE (2008) The early stages and life histories of three Eumerus and two Merodon species (Diptera: Syrphidae) from the Mediterranean region. Entomol Fenn 19:129–141. https://doi.org/10.33338/ef.84424
Ricarte A, Souba-Dols GJ, Hauser M, Marcos-García MA (2017) A review of the early stages and host plants of the genera Eumerus and Merodon (Diptera: Syrphidae), with new data on four species. PLoS ONE 12(12):1–22. https://doi.org/10.1371/journal.pone.0189852
Ricarte A, Nencioni A, Kočiš Tubić N, Grković A, Vujić A, Marcos-García MA (2018) The hoverflies of an oak Dehesa from Spain, with a new species and other insights into the taxonomy of the Eumerus tricolor group (Diptera: Syrphidae). Ann Zool 68:259–280. https://doi.org/10.3161/00034541ANZ2018.68.2.005
Rotheray GE (1991) Larval stages of 17 rare and poorly known British hoverflies (Diptera: Syrphidae). J Nat Hist 25(4):945–969. https://doi.org/10.1080/00222939100770621
Rotheray GE (1993) Colour guide to hoverfly larvae (Diptera, Syrphidae) in Britain and Europe. Dipterist Digest 9:1–156
Rotheray GE (2019) Ecomorphology of Cyclorrhaphan larvae (Diptera). Springer, Cham
Rotheray GE, Gilbert F (1999) Phylogeny of Palaearctic Syrphidae (Diptera): evidence from larval stages. Zool J Linn Soc. https://doi.org/10.1111/j.1096-3642.1999.tb01305.x
Rotheray GE, Gilbert F (2008) Phylogenetic relationships and the larval head of the lower Cyclorrhapha (Diptera). Zool J Linn Soc 153(2):287–323. https://doi.org/10.1111/j.1096-3642.2008.00395.x
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9(7):671–675. https://doi.org/10.1038/nmeth.2089
Souba-Dols GJ, Ricarte A, Hauser M, Speight M, Ángeles-Marcos M (2020) What do Eumerus Meigen larvae feed on? New immature stages of three species (Diptera: Syrphidae) breeding in different plants. Org Divers Evol 20:267–284. https://doi.org/10.1007/s13127-020-00437-0
Speight MCD, Fisler L, Pétremand G, Hauser M (2021) A key to the males of the Eumerus species known from Switzerland & surrounding parts of central Europe (Diptera: Syrphidae). Syrph the Net, the database of European Syrphidae, vol 112, 36 pp, Syrph the Net publications, Dublin
Ståhls G, Hippa H, Rotheray GE, Muona J, Gilbert FS (2003) Phylogeny of Syrphidae (Diptera) inferred from combined analysis of molecular and morphological characters. Syst Entomol 28(4):433–450. https://doi.org/10.1046/j.1365-3113.2003.00225.x
Stuke J-H (2000) Phylogentic relationships within the genus CheilosiaMeigen, 1822, as evidenced by the larval stages (Diptera, Syrphi-dae). Studia Dipterologica, Suppl. 8, 1–118 (in German)
Thompson CF (1999) A key to the genera of the flower flies (Diptera: Syrphidae) of the Neotropical Region including descriptions of new genera and species and a glossary of taxonomic terms. Contrib Entomol 3:319–378
Thompson JD, Higgins DG, Gibson TJ (1994) Clustal W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucl Acids Res 22:4673–4680. https://doi.org/10.1093/nar/22.22.4673
Tóth P, Undas AK, Verstappen F, Bouwmeester H (2016) Floral volatiles in parasitic plants of the Orobanchaceae. Ecological and taxonomic implications. Front Plant Sci 7:312. https://doi.org/10.3389/fpls.2016.00312
Waitzbauer W (1976) Eumerus compertus Villeneuve (Dipt., Syrphidae); larve und puparium. Zool Anz 196:16–22
Young AD, Lemmon AR, Skevington JH, Mengual X, Ståhls G, Reemer M, Jordaens K, Kelso S, Lemmon EM, Hauser M, de Meyer M, Misof B, Wiegmann BM (2016) Anchored enrichment dataset for true flies (order Diptera) reveals insights into the phylogeny of flower flies (family Syrphidae). BMC Evol Biol 16(1):143. https://doi.org/10.1186/s12862-016-0714-0
Acknowledgements
We acknowledge Dr. Andrés Campoy, for his collaboration by illustrating the head skeleton of the species. The studies were conducted in the technical services of the Technical University of Valencia (UPV) and of the University of Alicante (UA). Partial financial support was received from the research department of the University of Alicante in the framework of a predoctoral grant (UAFPU2019-03). Also, it has been partially supported by the Ministry of Science, Technological Development and Innovation of the Republic of Serbia (Grant Nos. 451-03-47/2023-01/200125 and 451-03-47/2023-01/200358).
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature.
Author information
Authors and Affiliations
Contributions
Conceptualization: AV, CPB, SRa, SRo, methodology: AA, AG, AJ, CPB, NKT; data curation: AA; writing—original draft: AA, AG; writing—review & editing: AJ, AV, CPB, NKT, SRa, SRo; funding acquisition: AV, SRo. All authors revised the manuscript and approved its final version.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Handling Editor: Dagmar Voigt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This manuscript has been written as a part of a PhD project conducted by Andrea Aracil at the University of Alicante (Spain).
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aracil, A., Grković, A., Pérez-Bañón, C. et al. A new species of phytophagous flower fly (Diptera, Syrphidae), feeding on holoparasitic broomrape plants (Orobanchaceae) for the first time in Europe. Arthropod-Plant Interactions 17, 401–418 (2023). https://doi.org/10.1007/s11829-023-09962-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11829-023-09962-z