Abstract
The volatile organic compounds (VOCs) 1-octen-3-ol and 3-octanone produced by the entomopathogenic fungus Metarhizium brunneum are known to have pesticidal properties at high doses against a range of invertebrate pests. Very little is known about their behavior-modifying (semiochemical) properties. This study focused on investigating the behavioral responses of three subterranean crop pests, wireworm (Agriotes lineatus), western corn rootworm (Diabrotica virgifera virgifera), and garden chafer (Phyllopertha horticola), to relatively low doses of 1-octen-3-ol and 3-octanone. The behavior of wireworms and corn rootworms were slightly influenced by the VOCs, yet not significantly. Western corn rootworms appeared to be slightly attracted by 100 µl and 200 µl 1-octen-3-ol and 100 µl dose of 3-octanone, respectively but slightly repelled by the higher dose of 3-octanone. Wireworms appeared to be slightly repelled by 1-octen-3-ol and high dose 3-octanone, but slightly attracted by the 100 µl dose of 3-octanone. The VOCs had no significant impact on garden chafer. In silico studies showed that corn rootworm odorant binding proteins (OBPs) had a strong binding affinity of 1-octen-3-ol and high dose 3-octanone, indicating that these VOCs can be detected and recognized by corn rootworm. OBPs are well conserved between species; thus, wireworm and garden chafer OBPs should also be able to bind with the VOCs. Further trials will be done to confirm that VOCs could be used as semiochemicals. Appropriate formulation of the VOCs should increase their efficacy and prevent rapid dissipation of the VOCs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Soil microbes produce a wide array of volatile organic compounds (VOCs), which have disparate ecological roles such as stimulating plant growth, inhibiting competitors, and influencing the behavior (e.g., attract/repel) of insects and other invertebrates (Davis et al. 2013; Kanchiswamy et al. 2015; Werner et al. 2016; Fincheira and Quiroz 2018). The potential exists for these VOCs to be developed into biopesticides for improved monitoring and control of soil dwelling invertebrate pests impacting plant health, thus improving food security. Entomopathogenic fungi (EPF) such as Beauveria bassiana, Metarhizium anisopliae, and Isaria fumosorosea are constitutively producing an array of VOCs whose ecological role is poorly understood (Bojke et al. 2018). EPF VOCs are responsible for the attractant and repellent behavior exhibited by some insects such as collembola, ants, termites, or mole crickets (Butt et al. 2016; Hamzah et al. 2020; Weithmann et al. 2020; Hummadi et al. 2021). VOCs of Metarhizium brunneum were recently shown to have antimicrobial properties in vitro (Hummadi et al. 2021) with two of the volatiles, 1-octen-3-ol and 3-octanone, attracting nematodes and mollusks at low doses but repelling them at higher concentrations (Khoja et al. 2019, 2021; Hummadi et al. 2021). At even higher doses, these compounds proved lethal to insects, nematodes, and mollusks (Hummadi et al. 2021; Khoja et al. 2019, 2021). The alcohol 1-octen-3-ol is an attractant for several mosquito species, grain beetles, collembola, and tsetse flies (Hall et al. 1984; Kline et al. 2007; Pierce et al. 1991). Similarly, the ketone 3-octanone is also known to influence insect behavior. For example, it is the active component of the alarm pheromone of the leaf cutting ant Acromyrmex (Blum and Brand 1972; Iwabuchi et al. 1987). Determining the semiochemical properties of microbial VOCs could help in the identification of attractant compounds which could be used to improve pest monitoring, mass trapping or attract-and-kill pest control strategies. In the attract-and-kill strategy, attractants are used to lure the pest to a control agent and thereby significantly reducing pesticide inputs. Repellents are equally important as they could protect plants without killing the pest and non-target organism. Both attractants and repellents could be used in push–pull pest control strategies, where repellents drive pests out of the main crop to a trap crop or an attractant (Cook et al. 2007; Khan et al. 2016).
Many major pests of economic significance have a subterranean phase in their life cycle, such as larval stages of click beetles, chafers, rootworms, cutworms, vine weevil, and leatherjackets (Bažok et al. 2021; Hann et al. 2015; Parker 2005). The majority of these pests are difficult to control with conventional chemical pesticides, partly due to the buffering capacity of the soil and the pests cryptic nature. The problem is compounded by the fact that many pesticides have been withdrawn or restricted in use, clearly creating a demand for new products and strategies to monitor and control these pests. Many research projects looked at the development of bioinsecticides using entomopathogenic fungi (EPF) such as Metarhizium; yet, the efficacy of EPF can be limited if the edaphic conditions are limiting their growth (Rath et al. 1995; Kabaluk and Ericsson 2007; Kabaluk et al. 2007; Larroudé and Thibord 2017). On the contrary, VOCs and semiochemicals could be a sustainable active ingredient and used immediately for pest monitoring and control. Carbon dioxide is an attractant for many soil insects (Bernklau and Bjostad 2008; Erb et al. 2013; Barsics et al. 2014; Ambele et al. 2019). Many other attractants have been identified, mostly plant or microbe-derived volatiles (Barsics et al. 2017; Hammack 2001; Hiltpold and Hibbard 2016). Odorant binding proteins (OBPs) located in the maxillary and labial palps play a pivotal role in the detection and subsequent behavioral response to these odors (Zhou et al. 2008; Pelosi et al. 2014; Brito et al. 2016). Insects possess a variable number of OBPs (e.g., 17 for Ceratitis capitata or Locusta migratora to 111 for Aedes aegypti). Each OBP has affinity for several odorant molecules. For example Carpomya vesuviana OBP5 and OBP6 which have the ability to bind a broad spectrum of low molecular weight compounds (Li et al. 2017) or Bactrocera dorsalis OBP56f-2 which can bind methyl eugenol, trans-2-hexenal and 4-carvomenthenol (Chen et al. 2021). Moreover, multiple OBPs can bind to the same compounds with different affinity. In fact, Chen et al. (2021) showed that the B. dorsalis mutant with knock down OBP56f-2 still retained some attraction to methyl eugenol, suggesting other OBPs may also have some affinity for this compound.
The aim of this study was to analyze the influence of 1-octen-3-ol and 3-octanone on the behavior of three major crop pests larvae, namely wireworm (larvae of click beetles), western corn rootworm and garden chafer (Ruther and Mayer 2005; Benjamin et al. 2018). These Coleoptera were chosen as they have different life cycles and behaviors. Indeed, wireworms are a generalist pest that spend 2 to 5 years in the soil before pupating, while corn rootworm, a specialist pest, and garden chafer, a generalist pest, complete their life cycle in one year. In vivo, trials were performed on the three species to test the attractant and repellent properties of 1-octen-3-ol and 3-octanone; moreover, as corn rootworm is fully sequenced, we choose this species as a model to perform in silico studies to identify corn rootworm OBPs and establish if they had affinity for the VOCs 1-octen-3-ol and 3-octanone.
Materials and methods
Insect source and maintenance
Late instar wireworm (Agriotes lineatus) collected in Britany (France) in September 2020, were kept in 1L pots filled with medium loam soil and fed with potato slices. Western corn rootworm (Diabrotica v. virgifera) eggs were collected by the Austrian Agency for Health and Food Safety Ltd. in Spring 2020. The eggs were incubated at 25 °C in a soil-sand mixture until hatching. Emergent larvae were provided corn seedlings as a food source. Only first and second instar larvae were used. These were gently transferred to the experimental containers using a fine paint brush. Garden chafer (Phyllopertha horticola) larvae, collected in August–September 2020 from pesticide free sites in Tyrol (Austria), were quarantined for a week and healthy third instar individuals were used in the trials.
Behavioral response choice trial
Preliminary lab studies showed that direct exposure to liquid 1-octen-3-ol and 3-octanone were toxic to wireworm, western corn rootworm, and garden chafer at doses of 0.5 µl per gram of soil or less (Bourdon et al. 2022). High mortality (> 90%) was observed in less than 4 days.
The behavioral responses of test insects to the VOCs to these same compounds was investigated in choice studies conducted in 5 l plastic (HDPE) buckets (30 × 13 × 13 cm) filled with 4 l medium loam mixed with 10% silver sand (jardineries Truffaut). The test arena was evenly allocated into three zones: treated, central, and untreated (Fig. 1). The treated and untreated zone were either placed on the right or left side of the buckets to account for external attraction cues. Three five-day old corn seedlings were planted at opposite ends of the test area, approximately 5 cm from the edge of the container and 3 cm from each other. Two sublethal doses (100 µl and 2 × 100 µl, which correspond to 0.05 µl/g of soil and 0.1 µl/g of soil) were tested for 1-octen-3-ol and for 3-octanone (Sigma-Aldrich, France). Each VOC was injected (100 µl) into 7 mm dia Sharrow cellulose filter tips (Wilsons & Co Ltd) which were placed either directly behind the central 5-day old maize seedlings or between the seedlings for the 2 × 100 µl treatment (Fig. 1). Filters were covered with 1 cm of soil immediately after they had been loaded with the VOC. Four larvae were released in the center of each bucket on the soil surface. There were ten replicates for each treatment and dose. Untreated buckets were prepared by injecting water in sharrow filters placed in the “treated” side. Five days post treatment, each zone was collected individually and checked manually to recover live and dead larvae. Due to the very delicate nature of corn rootworm, the recovery method had to be modified to avoid as much handling as possible. Briefly, the soil was separated in two parts (treated and untreated, see Fig. 1). Each half of the soil was mixed with water, the living larvae could be recovered floating on the water, while the dead ones could not be found. Larvae from all treatments were incubated in clean, fresh soil for 5 days to assess the final mortality.
Behavioral response choice trial design. In the treated area, either one filter was placed in position A, or two filters were placed in position B1 and B2. In each filter 100 µl of 1-octen-3-ol or 3-octanone was injected. The two black arrows indicate how the soil was split for corn rootworm position assessment. The assessment was made by carefully washing the soil to recover the floating living larvae
The response index (RI) was calculated according to Usseglio et al. (2017), using the equation:
where T and C represent the number of insects found in treated and untreated control zones, respectively. Total is the number of insects which responded and excludes the unresponsive larvae in the central zone and those never found. Positive RI values indicate attraction while negative values indicate repellency. The trials were performed in greenhouses (temperature ranging between 20 and 25 °C, RH = 75 ± 16% for A. lineatus trial, RH = 46 ± 4% for D. v. virgifera and P. horticola).
Identification of corn rootworm OBPs
In silico analyses were performed on western corn rootworm. This species was used as a model, as it is fully sequenced with its genome obtained from NCBI (National Center of Biotechnology Information) (GCF_003013835.1). Previously annotated OBPs from mosquitoes (Aedes aegypti and Aedes albopictus) were obtained from the paper by Zhou et al. (2008). Additional classic, Plus-C, Minus-C and atypical OBPs were obtained from A. aldopictus (FJ040863.1, FJ040862.1, FJ040861.1) (Armbruster et al. 2009), Locusta migratora (FJ959365, JN247410, JN129989) (Yu et al. 2009), Tribolum castaneum (KQ971354.1, KQ971352.1) (Richards et al. 2008). All these sequences were blasted using local BLASTp module in BioEdit Tool, using a blast cutoff of 10 to identify all corn rootworm OBPs (Hall et al. 2011). All identified OBPs were tested on motif search website (https://www.genome.jp/tools/motif/) to verify that the identified sequences were OBPs. Corn rootworm OBPs were classified according to their number of conserved cysteine residues. Phylogenetic analysis were performed on MEGA11 using the maximum likelihood method and the Jones–Taylor–Thornton method as an amino acid-based substitution model (Fatma et al. 2021). The phylogenetic tree was generated with bootstrap values of 1000 replicates using OBPs from D. v. virgifera, D. melanogaster, and Cylas formicarius (https://www.ncbi.nlm.nih.gov/nuccore/?term=cylas+formicarius+odorant).
Binding affinity of the VOCs to OBPs
To predict the binding affinities of the VOCs with the OBPs, we followed the protocol described by Fatma et al. (2021). Briefly, the thread-based structure model of the multiple classic, plus-C and minus-C OBPs was predicted using RoseTTAFold module of Robetta (https://robetta.bakerlab.org/submit.php). Selected models were verified by ERRAT and procheck (https://saves.mbi.ucla.edu/) to analyze the patterns of nonbonded atomic interaction and obtain information on the overall structural geometry of the proteins (Colovos and Yeates 1993; Laskowski et al. 1996).
Binding site prediction was then performed using CASTp webserver (http://sts.bioe.uic.edu/castp/calculation.html). The 3D structure of 1-octen-3-ol, 3-octanone, as well as the corn rootworm attractants: (E)-β-caryophyllene, carbon dioxide, indole, (+)-linalool and 4-methoxycinnamaldehyde (Hammack 2001; Hiltpold and Hibbard 2016; Walsh et al. 2020) were obtained from PubChem. Finally, the molecular docking was performed using Autodock Vina (Trott and Olson 2010). The protein–ligand interactions were determined using Protein–Ligand Interaction Profiler (https://plip-tool.biotec.tu-dresden.de/plip-web/plip/index) (Adasme et al. 2021).
Statistical analysis
For the attractant/repellent trial, the difference among treatment RIs was determined using an ANOVA followed by a Tukey post hoc analysis pairwise T test, to determine the difference between treatment pairs. Moreover, the number of larvae not found (considered as dead) was compared using a Kruskal Wallis test. All statistical analysis were performed using R version 4.1.2 (R Core Team 2021).
Results
Behavioral response choice trial
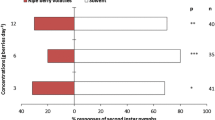
The VOCs elicited different responses in the test insects. They had no effect on garden chafer which were spread evenly between the treated and untreated area (Fig. 2, Table 1). On the contrary, corn rootworms were slightly attracted toward 1-octen-3-ol, while wireworms were slightly repelled by this VOC as well as with the high dose of 3-octanone (Fig. 2); yet, no significant differences were found between the treatment and control. The absence of significant differences can be explained by the high dispersion of the insects in the untreated buckets (Fig. 2). Moreover, many wireworms did not appear to have moved since 46% to 84% were found in the central zone (Table 1). The presence of wireworms in the water-treated side of the untreated bucket should not be due to external cues as the water-treated side was placed either right or left of the buckets. Their presence on the water-treated side appears to be due to random movement toward the plants.
Response index (RI) of the three insect species. Positive RI values indicate attractancy, while negative values indicate repellency. Corn rootworms were slightly attracted by 1-octen-3-ol but were repelled by the high dose of 3-octanone. Garden chafers were spread nearly evenly between the treatment and control. Wireworms were repelled by 1-octen-3-ol and the high dose of 3-octanone
During the assessment, 7.5% to 17.5% wireworm could not be located and were assumed dead (Table 2) as these quickly turn black and therefore are difficult to find in the soil. The number of dead larvae was not significantly different between the treatments (p = 0.8932, df = 4), indicating that the mortality was not induced by the treatment. Corn rootworm larvae mortality ranged between 45 and 77.5% (Table 2); however, no significant differences were found between the treatments and control (p = 0.08965, df = 4), confirming deaths were not induced by the VOCs. Most garden chafer were recovered, except in the 1-octen-3-ol treatment where 2.5% of the larvae could not be found (Table 2).
Identification of corn rootworm OBPs
86 OBPs were identified in the genome of corn rootworm. 19 OBPs were classic OBPs with six conserved cystine residues, 48 were minus-C OBPs with 4 or 5 cystine residues, 17 were Plus-C OBPs with 7 or 8 cystine residues and an additional conserved proline residue, and 2 were atypical OBPs with 9 or 10 conserved cystine residues.
The phylogenetic tree (Fig. 3) showed that the majority of D. v. virgifera OBPs were not closely related and belong to different subgroups. OBPs from D. v. virgifera and C. formicarius were closely related as compared to D. melanogaster OBPs.
Phylogenetic tree of Diabrotica v. virgifera (in yellow), Drosophila melanogaster (in blue) and Cylas formicarius (in red) OBPs. The red box highlights the sequences used for molecular docking. The phylogenetic tree shows that OBPs are well conserved between even between different order, some D. v. virgifera OBPs are closely related to D. melanogaster OBPs
Binding affinity of the VOCs to OBPs
Four classic OBPs, one Plus-C and one Minus-C OBP were selected for evaluation of the ERRAT score, Ramachandran plots of the predicted models and Q-mean. The best models score for each selected OBP is presented in Table 3. The binding affinity of 1-octen-3-ol and 3-octanone was compared with known attractants which included linalool and 4-methoxycinnamaldehyde (Hammack 2001; Hiltpold and Hibbard 2016; Walsh et al. 2020).
Carbon dioxide had the least binding affinity with the OBPs (Fig. 4, Table 4), while (E)-β-caryophyllene and 3-octanone had the strongest binding affinity followed by indole and 1-octen-3-ol (Fig. 4, Table 4).
Binding affinity of four classic corn rootworm OBPs (DvirOBP10, DvirOBP21, DvirOBP22, DvirOBP80), one Minus-C (DvriOBP28) OBP, and one Plus-C OBP (DvirOBP12) to seven potential attractants. Lowest score indicate better binding affinity. (E)-β-caryophyllene and 3-octanone had the best binding affinity with the OBPs, while carbon dioxide had the least binding affinity
Carbon dioxide only had hydrogen bonds with the OBPs, while the other ligands had a majority of hydrophobic bonds (Fig. 5). Hydrogen bonds are usually responsible for the binding of small proteins such as carbon dioxide, while larger proteins recognition is more dependent on hydrophobic bonds (Hubbard and Haider 2010). The ligands with more hydrophobic bonds were (+)-linalool, 1-octen-3-ol, 3-octanone and (E)-β-caryophyllene, while carbon dioxide, 4-methoxycinnamaldehyde and indole had the lowest number of binding interactions (Fig. 5).
Number of hydrogen and hydrophobic bond between seven different ligands ((+)-linalool, (E)-beta-caryophyllene, 1-octen-3-ol, 3-octanone, 4-methoxycinnamaldehyde, carbon dioxide and indole) to four classic corn rootworm OBPs (DvirOBP10, DvirOBP21, DvirOBP22, DvirOBP80), one Minus-C (DvriOBP28) OBP, and one Plus-C OBP (DvirOBP12)
Discussion
This study shows that the Metarhizium-derived VOCs 1-octen-3-ol and 3-octanone had no significant effect on soil insects; yet, more wireworms were found in the control zone while corn rootworm were mostly found in the treated zone. In silico experiments presented herein validated that corn rootworm had odor binding proteins with strong affinity for these compounds. The relatively conserved OBPs (Fig. 3) suggest that wireworms and garden chafer should be able to bind the VOCs similarly. In silico studies are a good way to screen potential new attractants or repellents; yet they are limited to insect species which have been fully sequenced and cannot be used on their own to determine if a compound has attractant or repellent properties. The different behavioral responses observed in this study indicate diverse preferentiality for specific rhizosphere VOCs in isolation, supporting prior works (Fäldt et al. 1999; Sawahata et al. 2008; Badri and Vivanco 2009; Erb et al. 2013; Holighaus et al. 2014; Bont et al. 2017). It is probable that in nature, plants and fungi emit a bouquet of VOCs, and the overall blend might have a greater influence on insect’s behavior than single compounds (Hammack 2001; Renou and Anton 2020).
The behavioral response of invertebrate to volatiles cues is also known to be dose dependent. Cook et al. (2007) showed that the attraction levels of 1-octen-3-ol and R-1-octen-3-ol differed between the mosquitoes Aedes aegypti and Culex quinquefasciatus. Moreover, it was shown that low doses of VOCs could attract mosquitoes, mollusks, and insects, while higher doses were repellent (Xu et al. 2015; Khoja et al. 2019, 2021; Hummadi et al. 2021).
The absence of response of garden chafer to 1-octen-3-ol and 3-octanone could indicate that this species does not have specific receptors for these compounds. Alternatively, it could be that garden chafers do not centrally process these in terms of chemotaxis, and use other orientation cues. In fact, these two VOCs are commonly emitted by numerous fungi (Insam and Seewald 2010) as well as trees such as Quercus robur or Q. petraea (Weissteiner et al. 2012), indicating that they could be commonly abundant in the soil and therefore of little utility to the insect. Measuring the release rates of the VOCs, their absorption, and their diffusion in the soil was out of the scope of this study, but we can expect the VOCs to have dissipated quickly as has been seen for similar compounds (Cui et al. 2021). Rapid dispersal would lead to a short window for potential action, should one insect be less mobile or have a less rapid response to any given stimuli then the behavioral effect would be likely to be much less evident. Moreover, the life cycle of wireworms and corn rootworms could also explain the limited efficacy of the VOCs to attract or repel these species. Wireworms only feed actively during 29% of their life cycle (Furlan 2004), and only on a limited number of seeds and tubers (Chaton et al. 2008). Therefore, wireworms which are not in their feeding phase may avoid unnecessary movement to conserve energy (Charnov 1974). This would explain the high number of wireworms found in the central area (Table 1). Due to the limited number of wireworm available for our study, we could not pre-select larvae in feeding stage for the trials. First and second instar corn rootworm were used for these trials because they are the most damaging stages; however, the natural mortality of first instar can reach 95%, while second instar mortality can reach 10% (Toepfer and Kuhlmann 2006), which explains the high number of mortality found in our study.
Many of these issues have the potential for mitigation through the development of appropriate formulation technologies. Further trials will be done to encapsulate the VOCs into absorbent materials to significantly reduce the volatility of the compounds and extend their period of activity. The VOCs will be tested for attract-and-kill or push–pull strategies to increase their efficacy and drag the pests out of the crops. Push–pull strategies using the VOCs and a trap crop such as wheat or peas could be effective against wireworm (Adhikari 2017). The repellent effect of the VOCs could also explain why Kabaluk and Ericsson (2007) observed wireworms avoiding soil treated with Metarhizium spores, moreover, they found that the avoidance increased with the increase of concentration of conidia even from distance, suggesting that VOCs may play a role in insect avoidance of entomopathogens. Push–pull strategies for western corn rootworm could be effectively achieved through the combined deployment of attractive VOCs in a trap crop and a simultaneous repellent within the main crop. Such repellents could include methyl anthranilate (Bernklau et al. 2019).
The VOCs used in this paper had no significant influence the behavior of soil insects. However, the high mortality and absence of movement of the larvae impacted negatively the results from this study. The slight behavioral change observed were encouraging, thus, we will do further trials to confirm the effect of encapsulated VOCs on soil insects.
Data Availability
All the data belong to Certis Belchim, and they would prefer not to share the full data set.
References
Adasme MF, Linnemann KL, Bolz SN et al (2021) PLIP 2021: expanding the scope of the protein–ligand interaction profiler to DNA and RNA. Nucleic Acids Res 49:W530–W534. https://doi.org/10.1093/nar/gkab294
Adhikari A (2017) Evaluation of trap crops for the management of wireworms in spring wheat in Montana. Arthropod-Plant Interact. 11:755
Ambele CF, Bisseleua HDB, Akutse KS et al (2019) Testing a co-formulation of CO2-releasing material with an entomopathogenic fungus for the management of subterranean termite pests. Mycol Prog 18:1201–1211
Armbruster P, White S, Dzundza J et al (2009) Identification of genes encoding atypical odorant-binding proteins in Aedes albopictus (Diptera: Culicidae). J Med Entomol 46:271–280. https://doi.org/10.1603/033.046.0211
Badri DV, Vivanco JM (2009) Regulation and function of root exudates. Plant Cell Environ 32:666–681. https://doi.org/10.1111/j.1365-3040.2009.01926.x
Barsics F, Haubruge É, Francis F, Verheggen FJ (2014) The role of olfaction in wireworms: a review on their foraging behavior and sensory apparatus. Biotechnol Agron Soc Environ 18:524–535
Barsics F, Delory BM, Delaplace P et al (2017) Foraging wireworms are attracted to root-produced volatile aldehydes. J Pest Sci 90:69–76. https://doi.org/10.1007/s10340-016-0734-y
Bažok R, Lemić D, Chiarini F, Furlan L (2021) Western corn rootworm (Diabrotica virgifera virgifera LeConte) in Europe: current status and sustainable pest management. Insects 12:1–26. https://doi.org/10.3390/insects12030195
Benjamin EO, Grabenweger G, Strasser H et al (2018) The environmental and economic benefits of biological control of western corn rootworm Diabrotica virgifera virgifera and wireworms Agriotes spp. in maize and potatoes for selected European countries. J Plant Dis Prot 6:1–13. https://doi.org/10.1007/s41348-018-0156-6
Bernklau EJ, Bjostad LB (2008) Identification of feeding stimulants in corn roots for western corn rootworm (Coleoptera: Chrysomelidae) larvae. J Econ Entomol 101:341–351. https://doi.org/10.1603/0022-0493(2008)101[341:IOFSIC]2.0.CO;2
Bernklau EJ, Hibbard BE, Bjostad LB (2019) Repellent effects of methyl anthranilate on western corn rootworm larvae (Coleoptera: Chrysomelidae) in soil bioassays. J Econ Entomol 112:683–690. https://doi.org/10.1093/jee/toy346
Blum MS, Brand JM (1972) Social insect pheromones: their chemistry and function. Integr Comp Biol 12:553–576. https://doi.org/10.1093/icb/12.3.553
Bojke A, Tkaczuk C, Stepnowski P, Gołębiowski M (2018) Comparison of volatile compounds released by entomopathogenic fungi. Microbiol Res 214:129–136. https://doi.org/10.1016/j.micres.2018.06.011
Bont Z, Arce C, Huber M et al (2017) A herbivore tag-and-trace system reveals contact- and density-dependent repellence of a root toxin. J Chem Ecol 43:295–306. https://doi.org/10.1007/s10886-017-0830-3
Bourdon P-A, Zottele M, Baxter I et al (2022) Fumigation of three major soil pests (Agriotes lineatus, Diabrotica virgifera virgifera, Phyllopertha horticola) with 3-octanone and 1-octen-3-ol enantiomers. Biocontrol Sci Technol 32:863–876. https://doi.org/10.1080/09583157.2022.2057436
Brito NF, Moreira MF, Melo ACA (2016) A look inside odorant-binding proteins in insect chemoreception. J Insect Physiol 95:51–65. https://doi.org/10.1016/j.jinsphys.2016.09.008
Butt TM, Coates CJ, Dubovskiy IM, Ratcliffe NA (2016) Entomopathogenic fungi: new insights into host–pathogen interactions. Elsevier, Hoboken
Charnov EL (1974) Optimal foraging, the marginal value theorem. Theor Popul Biol 9:129–136
Chaton PF, Lempérière G, Tissut M, Ravanel P (2008) Biological traits and feeding capacity of Agriotes larvae (Coleoptera: Elateridae): a trial of seed coating to control larval populations with the insecticide fipronil. Pestic Biochem Physiol 90:97–105. https://doi.org/10.1016/j.pestbp.2007.09.001
Chen X, Lei Y, Li H et al (2021) CRISPR/Cas9 mutagenesis abolishes odorant-binding protein BdorOBP56f-2 and impairs the perception of methyl eugenol in Bactrocera dorsalis (Hendel). Insect Biochem Mol Biol 139:1–7. https://doi.org/10.1016/j.ibmb.2021.103656
Colovos C, Yeates TO (1993) Verification of protein structures: patterns of nonbonded atomic interactions. Protein Sci 2:1511–1519. https://doi.org/10.1002/pro.5560020916
Cook SM, Khan ZR, Pickett JA (2007) The use of push-pull strategies in integrated pest management. Annu Rev Entomol 52:375–400. https://doi.org/10.1146/annurev.ento.52.110405.091407
Cui K, Yang S, Zou N et al (2021) Residual behavior of the potential grain fumigant 1-octen-3-ol in wheat during fumigation and ventilation processes. Pest Manag Sci 77:2933–2938. https://doi.org/10.1002/ps.6329
Davis TS, Crippen TL, Hofstetter RW, Tomberlin JK (2013) Microbial volatile emissions as insect semiochemicals. J Chem Ecol 39:840–859. https://doi.org/10.1007/s10886-013-0306-z
Erb M, Huber M, Christelle AMR et al (2013) The role of plant primary and secondary metabolites in root-herbivore behaviour, nutrition and physiology. Adv Insect Phys 45:53–95
Fäldt J, Jonsell M, Nordlander G, Borg-Karlson AK (1999) Volatiles of bracket fungi Fomitopsis pinicola and Fomes fomentarius and their functions as insect attractants. J Chem Ecol 25:567–590. https://doi.org/10.1023/A:1020958005023
Fatma T, Zafar Z, Fatima S et al (2021) Computational assessment of Botrytis cinerea lipase for biofuel production. Catalysts 11:1–19. https://doi.org/10.3390/catal11111319
Fincheira P, Quiroz A (2018) Microbial volatiles as plant growth inducers. Microbiol Res 208:63–75. https://doi.org/10.1016/j.micres.2018.01.002
Furlan L (2004) The biology of Agriotes sordidus Illiger (Col., Elateridae). J Appl Entomol 128:696–706
Hall D et al (1984) 1-Octen-3-ol. A potent olfactory stimulant and attractant for tsetse isolated from cattle odours. Int J Tropic Insect Sci 5:335–339
Hall T, Biosciences I, Carlsbad C (2011) BioEdit: an important software for molecular biology. GERF Bull Biosci 2:60–61
Hammack L (2001) Single and blended maize volatiles as attractants for diabroticite corn rootworm beetles. J Chem Ecol 27:1373–1390. https://doi.org/10.1023/A:1010365225957
Hamzah HA, Rixson D, Paul-Taylor J et al (2020) Inclusion and release of ant alarm pheromones from metal–organic frameworks. Dalt Trans 49:10334–10338. https://doi.org/10.1039/d0dt02047h
Hann P, Trska C, Wechselberger KF et al (2015) Phyllopertha horticola (Coleoptera: Scarabaeidae) larvae in eastern Austrian mountainous grasslands and the associated damage risk related to soil, topography and management. SpringerPlus 4:1–15. https://doi.org/10.1186/s40064-015-0918-6
Hiltpold I, Hibbard BE (2016) Neonate larvae of the specialist herbivore Diabrotica virgifera virgifera do not exploit the defensive volatile (E)-β-caryophyllene in locating maize roots. J Pest Sci 89:853–858. https://doi.org/10.1007/s10340-015-0714-7
Holighaus G, Weißbecker B, von Fragstein M, Schütz S (2014) Ubiquitous eight-carbon volatiles of fungi are infochemicals for a specialist fungivore. Chemoecology 24:57–66. https://doi.org/10.1007/s00049-014-0151-8
Hubbard, R., & Haider, M. (2010). Hydrogen bonds in proteins: Role and strength. eLS:1–7
Hummadi EH, Dearden A, Generalovic T et al (2021) Volatile organic compounds of Metarhizium brunneum influence the efficacy of entomopathogenic nematodes in insect control. Biol Control 155:1–12. https://doi.org/10.1016/j.biocontrol.2020.104527
Insam H, Seewald MSA (2010) Volatile organic compounds (VOCs) in soils. Biol Fertil Soils 46:199–213. https://doi.org/10.1007/s00374-010-0442-3
Iwabuchi K, Takahashi J, Sakai T (1987) Occurrence of 2, 3-octanediol and 2-hydroxy-3-octanone, possible male sex pheromone in Xylotrechus chinensis chevrolat (Coleoptera: Cerambycidae). Appl Entomol Zool 22:110–111
Kabaluk JT, Ericsson JD (2007) Environmental and behavioral constraints on the infection of wireworms by Metarhizium anisopliae. Environ Entomol 36:1415–1420. https://doi.org/10.1093/ee/36.6.1415
Kabaluk JT, Goettel M, Ericsson JD et al (2007) Promise versus performance: working toward the use of Metarhizium anisopliae as a biological control for wireworms. IOBC/WPRS Bull 30:69–77
Kanchiswamy CN, Malnoy M, Maffei ME (2015) Chemical diversity of microbial volatiles and their potential for plant growth and productivity. Front Plant Sci 6:151. https://doi.org/10.3389/fpls.2015.00151
Khan Z, Midega CAO, Hooper A, Pickett J (2016) Push-pull: chemical ecology-based integrated pest management technology. J Chem Ecol 42:689–697. https://doi.org/10.1007/s10886-016-0730-y
Khoja S, Eltayef KM, Baxter I et al (2019) Fungal volatile organic compounds show promise as potent molluscicides. Pest Manag Sci 75:3392–3404. https://doi.org/10.1002/ps.5578
Khoja S, Eltayef KM, Baxter I et al (2021) Volatiles of the entomopathogenic fungus, Metarhizium brunneum, attract and kill plant parasitic nematodes. Biol Control 152:1–11. https://doi.org/10.1016/j.biocontrol.2020.104472
Kline DL, Allan SA, Bernier UR, Welch CH (2007) Evaluation of the enantiomers of 1-octen-3-ol and 1-octyn-3-ol as attractants for mosquitoes associated with a freshwater swamp in Florida, USA. Med Vet Entomol 21(4):323–331. https://doi.org/10.1111/j.1365-2915.2007.00697.x
Larroudé P, Thibord J-B (2017) Interet de l’utilisation de Metarhizium brunneum pour lutter contre les taupins en grandes cultures. In: AFPP-11e Conférence internationale sur les ravageurs et auxiliaires en agriculture
Laskowski RA, Rullmannn JA, MacArthur MW et al (1996) AQUA and PROCHECK-NMR: programs for checking the quality of protein structures solved by NMR. J Biomol NMR 8:477–486. https://doi.org/10.1007/BF00228148
Li Y, Zhou P, Zhang J et al (2017) Identification of odorant binding proteins in Carpomya vesuviana and their binding affinity to the male-borne semiochemicals and host plant volatiles. J Insect Physiol 100:100–107. https://doi.org/10.1016/j.jinsphys.2017.05.013
Parker W (2005) Practical implementation of a wireworm management strategy – lessons from the UK potato industry. Insect Pathogens Insect Paras Nematodes: IOBC wprs Bulletin 28(2):87–90
Pelosi P, Mastrogiacomo R, Iovinella I et al (2014) Structure and biotechnological applications of odorant-binding proteins. Appl Microbiol Biotechnol 98:61–70. https://doi.org/10.1007/s00253-013-5383-y
Pierce, A. M., Pierce Jr, H. D., Oehlschlager, A. C., & Borden, J. H. (1991). 1-Octen-3-ol, attractive semiochemical for foreign grain beetle, Ahasverus advena (Waltl) (Coleoptera: Cucujidae). Journal of Chemical Ecology (3), 567–580.
R Core Team (2021) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Rath AC, Anderson GC, Worledge D, Koen TB (1995) The effect of low temperatures on the virulence of Metarhizium anisopliae (DAT F-001) to the subterranean scarab, Adoryphorus couloni. J Invertebr Pathol 65:186–192
Renou M, Anton S (2020) Insect olfactory communication in a complex and changing world. Curr Opin Insect Sci 42:1–7. https://doi.org/10.1016/j.cois.2020.04.004
Richards S, Gibbs RA, Weinstock GM et al (2008) The genome of the model beetle and pest Tribolium castaneum. Nature 452:949–955. https://doi.org/10.1038/nature06784
Ruther J, Mayer CJ (2005) Response of garden chafer, Phyllopertha horticola, to plant volatiles: from screening to application. Entomol Exp Appl 115:51–59. https://doi.org/10.1111/j.1570-7458.2005.00264.x
Sawahata T, Shimano S, Suzuki M (2008) Tricholoma matsutake 1-ocen-3-ol and methyl cinnamate repel mycophagous Proisotoma minuta (Collembola: Insecta). Mycorrhiza 18:111–114. https://doi.org/10.1007/s00572-007-0158-x
Toepfer S, Kuhlmann U (2006) Constructing life-tables for the invasive maize pest Diabrotica virgifera virgifera (Col.; Chrysomelidae) in Europe. J Appl Entomol 130:193–205. https://doi.org/10.1111/j.1439-0418.2006.01060.x
Trott O, Olson AJ (2010) AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 31:455–461. https://doi.org/10.1002/jcc.21334
Usseglio VL, Pizzolitto RP, Rodriguez C et al (2017) Volatile organic compounds from the interaction between Fusarium verticillioides and maize kernels as a natural repellents of Sitophilus zeamais. J Stored Prod Res 73:109–114. https://doi.org/10.1016/j.jspr.2017.08.001
Walsh GC, Ávila CJ, Cabrera N et al (2020) Biology and management of pest diabrotica species in South America. Insects 11:1–18. https://doi.org/10.3390/insects11070421
Weissteiner S, Huetteroth W, Kollmann M et al (2012) Cockchafer larvae smell host root scents in soil. PLoS ONE 7:0045827. https://doi.org/10.1371/journal.pone.0045827
Weithmann S, von Hoermann C, Schmitt T et al (2020) The attraction of the dung beetle Anoplotrupes stercorosus (Coleoptera: Geotrupidae) to volatiles from vertebrate cadavers. Insects 11:1–16. https://doi.org/10.3390/insects11080476
Werner S, Polle A, Brinkmann N (2016) Belowground communication: impacts of volatile organic compounds (VOCs) from soil fungi on other soil-inhabiting organisms. Appl Microbiol Biotechnol 100:8651–8665. https://doi.org/10.1007/s00253-016-7792-1
Xu P, Zhu F, Buss GK, Leal WS (2015) 1-Octen-3-ol—the attractant that repels. F1000Research 4:1–10. https://doi.org/10.12688/f1000research.6646.1
Yu F, Zhang S, Zhang L, Pelosi P (2009) Intriguing similarities between two novel odorant-binding proteins of locusts. Biochem Biophys Res Commun 385:369–374. https://doi.org/10.1016/j.bbrc.2009.05.074
Zhou JJ, He XL, Pickett JA, Field LM (2008) Identification of odorant-binding proteins of the yellow fever mosquito Aedes aegypti: genome annotation and comparative analyses. Insect Mol Biol 17:147–163. https://doi.org/10.1111/j.1365-2583.2007.007
Acknowledgements
The authors thank Certis-Belchim and Swansea University for funding this work.
Funding
The study was funded by Certis-Belchim and Swansea University.
Author information
Authors and Affiliations
Contributions
PAB. IB. AM and TMB conceived and designed research. PAB and MZ conducted experiments. PAB and ZZ performed the bioinformatics analysis, PAB analyzed data. PAB, KW, MZ and HS provided the insects. PAB and TB wrote the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Additional information
Handling Editor: Todd Kabaluk.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Pierre-Antoine Bourdon worked with Swansea and Certis at the time of study, but now recently changed to Agriodor. Ian Baxter worked with Certis at the time of the study.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bourdon, PA., Zottele, M., Zafar, Z. et al. Behavioral response of three subterranean pests (Agriotes lineatus, Diabrotica virgifera virgifera, Phyllopertha horticola) to the fungal volatile organic compounds 1-octen-3-ol and 3-octanone. Arthropod-Plant Interactions 17, 473–483 (2023). https://doi.org/10.1007/s11829-023-09959-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11829-023-09959-8