Abstract
The mirid bug Pameridea roridulae lives mutalistically on the protocarnivorous plant Roridula gorgonias. The latter resembles an effective, three-dimensional flypaper trap which captures numerous flying insects. We have recently shown that P. roridulae bugs are not trapped by the plant, because they are covered with a layer of epicuticular grease, which is considerably thicker than in other insects. The present study demonstrates that the bugs’ morphology and locomotory characteristics also contribute to their specialisation for life on the adhesive plant surface. A structural analysis of the mirid bug’s attachment system, and an experimental study on its attachment ability were carried out. In traction force tests, maximum forces of 8.8 mN were measured on adaxial R. gorgonias leaves, corresponding to 126 times the bug’s body weight. On smooth surfaces, generated forces were only 47 times the bug’s body weight. Compared to closely related mirid bug species avoiding contact with plant adhesive secretion, P. roridulae is distinctly stronger and heavier, and holds its body close to the plant substrate. Two locomotion strategies on the glandular hairy plant surfaces are suggested for mirid bug species from the tribus Dicyphini: (1) avoidance strategy, characterised by the slim body held at a large distance from the plant surface by using long, slender legs, and (2) defense strategy, where trapping of the heavy bugs, situated close to the plant surface, is overcome by generating strong forces during locomotion and by having a thick anti-adhesive epicuticular greasy layer on the bugs’ cuticle.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
South African bugs Pameridea roridulae Reuter (Heteroptera, Miridae, Bryocorinae, Dicyphini) are obligately associated with protocarnivorous plants Roridula gorgonias Planch. (Roridulaceae; Reuter 1907; Dolling and Palmer 1991; Ellis and Midgley 1996; Anderson and Midgley 2002; Anderson 2006). The host plant is densely covered with glandular trichomes of three different types (short, middle-sized, and long tentacle-shaped ones; Fenner 1904; Bruce 1907; Voigt et al. 2009). It is a three-dimensional, meshed, sticky, flypaper trap that has been observed to capture numerous insects of considerable body mass (Marloth 1903, 1910). Nevertheless, P. roridulae live and walk confidently on the sticky plant surface. The surface of these mirid bugs is covered with an anti-adhesive, greasy, epicuticular layer that prevents the bugs’ adherence to the plant secretion (Voigt and Gorb 2008) and enables them to live in a digestive mutualism with R. gorgonias (Ellis and Midgley 1996). Mirid bugs feed on the insects captured by plant trichomes and defecate on their leaves (Marloth 1903; Loyd 1934). The nitrogen in the bugs’ faeces is absorbed, for nutrition, through the thin leaf cuticle of the plant, since the plants themselves produce no enzymes to digest captured insects (Marloth 1910; Lloyd 1934; Ellis and Midgley 1996; Anderson and Midgley 2002, 2003). Furthermore, juveniles of Pameridea are primary pollinators of Roridula’s flowers (Anderson et al. 2003).
Recent coevolutionary studies on mutualistic interactions resulted in phylogenetic and geographical associations, suggesting cospeciation, coadaptation, and long-term coexistence in the Roridula-Pameridea complex (Anderson et al. 2004; Anderson 2006). In host choice experiments with Roridula dentata L. (Roridulaceae) and Pameridea marlothi Poppius (Heteroptera, Miridae), bugs preferred host plants over unrelated host plant species and closely related sister species (Anderson et al. 2004). The presence of an intermediate number of P. marlothi caused positive growth rates in R. dentata (Anderson and Midgley 2007).
Only a few data about mutualistic effects on bug’s fitness and specific adaptations are currently available. Since relationships between insects and plants are highly diverse associations, they need broad studies of various factors and features at different conditions for a better understanding and evaluation (Thompson 1988). Besides genetic, fossil, taxonomic, geographical, ecological, and biochemical evidence, insights into the functional morphology and ethology may indicate associations and coadaptations (Futuyma and Slatkin 1983).
Similar to R. gorgonias and P. roridulae, relationships between mirid bugs and carnivorous plants have been reported from representatives of the plant genera Byblis and Drosera in Australia (e.g. Schuh 1995). These bug species are representatives of the mirid bug subfamilies Orthotylinae and Bryocorinae, which are known to be specialised in living on glandular hairy plants (Reuter 1913; Kullenberg 1946; Falkingham 1995; Dolling and Palmer 1991; Wheeler 2001; Sugiura and Yamazaki 2006; Voigt et al. 2007). Such mirid bugs have been reported to bear elongated, curved, sharp claws and free, rather large pseudopulvilli (Reuter 1913; Wagner 1955). Besides specialised claws, they have slim bodies as well as long, slender legs (Roberts 1930; Southwood 1986; Voigt et al. 2007). In contrast to P. roridulae, other related species have been observed avoiding contact with sticky glandular plant secretions by means of morphological adaptations.
Additionally, mirid bugs may show a particular behaviour. For example, Dicyphus errans Wolff (Heteroptera, Miridae, Bryocorinae, Dicyphini) stalks along the plant surface touching only trichome tips and stops frequently to groom itself (Southwood 1986; Voigt et al. 2004). For D. errans, non-glandular and glandular hairy plant substrates have been experimentally demonstrated to be important attachment substrates (Voigt et al. 2007), whereas geometrical variables of trichomes (length and diameter) significantly influenced the bug’s attachment ability. Trichomes have been found to provide suitable interlocking sites for attachment and locomotion of this tiny mirid bug. It clings to single trichomes using one part of the paired claw.
Preliminary observations on P. roridulae let us suggest a different behavior strategy. This insect keeps its body close to the plant substrate and frequently contacts adhesive trichome secretion that covers almost the entire surface of R. gorgonias and adheres occasionally to bristles on the mirid bug′s body surface (Voigt and Gorb 2008). It runs quickly between glandular trichomes, moves sometimes with jump-like movements or short flights. However, it grooms itself infrequently. Compared to D. errans, morphological differences are also ascertainable in P. roridulae; the latter appears burlier. Although the length of the body and legs is similar to that in D. errans, the width differs between the species and is distinctly larger in P. roridulae (Table 1).
Possibly, not only the anti-adhesive, greasy, epicuticular layer enables these bugs to live on the sticky plant surface, but also other morphological and behavioral characteristics contribute to it′s ability. The stronger build of P. roridulae may result in a larger muscle mass, due to which mirid bugs could generate stronger forces during locomotion on the sticky substrate, where they have possibly to free themselves from trichomes’ viscous secretion. As a specialist species, it is strongly associated with its single host plant (Anderson et al. 2004; Anderson 2006). Similar to related species of Miridae, they have to attach properly to the surface, not only during locomotion and feeding, but also during copulation, molting and oviposition of eggs into the plant tissue (Wheeler 2001).
Thus, the question arises, how do P. roridulae bugs attach and walk on their host plant? Do their attachment devices and attachment ability differ from those of related mirid bugs? Are they specialised for the surface of R. gorgonias? Previously, pretarsal structures in the genus Pameridea have been described to be similar to those of the genus Dicyphus, bearing short, curved claws, triangular pseudopulvilli and a straight, bristle-like parempodia (Dolling and Palmer 1991). However, up to now, no illustrations of these structures are available in the literature.
The present study should contribute to the better understanding of the complex mutualistic relationship between the protocarnivorous plant and the associated mirid bugs, in particular at the interface between the plant surface and insect attachment system. Using light and cryo-scanning electron microscopy (cryo-SEM), we analyzed the pretarsal structures of P. roridulae. Its forces, generated in a traction experiment, were measured on various substrates: glandular hairy adaxial and abaxial leaf surfaces of the bug’s host plant, smooth adaxial and sparsely hairy abaxial leaves of Rumex obtusifolius L. (Polygonaceae) and glass.
Materials and methods
Insects and plants
Shrubs of R. gorgonias (seeded, potted, 1–3 year old) with P. roridulae, were obtained from a private glasshouse culture (Klaus Keller, Augsburg, Germany). They were kept under laboratory conditions during experiments (23.7 ± 1.7°C, 47.3 ± 10.0% RH, 16 h photoperiod), and fed with wingless, adult Drosophila melongaster Meigen (Diptera, Drosophilidae; Zoo-Schöniger, Stuttgart, Germany).
Leaves from plants of Rumex obtusifolius L. (Polygonaceae) at eight-leaf stage were collected from the wayside in a humid mixed forest in Stuttgart-Büsnau (Baden-Württemberg, Germany). Its adaxial leaf surface is uneven because of the prominent venation and irregularly shaped convex epidermal cells and has a maximum height of about 9 μm (Holloway 1967; Gorb and Gorb 2009). The cells are relatively large (50.0 ± 9.04 μm long, 30.7 ± 7.45 μm wide), covered with a cuticle and a smooth, amorphous, epicuticular wax layer (0.1 ± 0.05 μm thick). Sporadically, very sparse small wax crystals occur in some places on the surface. The crystals have irregular scale-like or granular shapes and vary in size (0.5 ± 0.16 μm long, 0.3 ± 0.07 μm wide, and 0.1 ± 0.0 μm thick) (Gorb and Gorb 2009). The abaxial leaf side is roughly similar to the adaxial, however, convex cells are striated and tiny (Holloway 1967).
Observations and structural studies
The attachment positions of five male and five female freely walking P. roridulae to the plant surface were studied (1) visually without optical instruments, (2) visually with a stereomicroscope Olympus SZX 12 with a DF PLAPO 1xPF objective (Olympus Corp., Tokyo, Japan), and (3) using digital recordings. Images were taken using (1) a digital single lens reflex camera Canon EOS 20D combined with the Canon macro lens EF 100 mm, 1:2.8, USM and the Canon macro twin lite MT-24EX (Canon Inc., Japan), and (2) a Nikon Coolpix E995 digital camera adapted to the stereomicroscope with a C-Mount adapter and MDC 2 relay lens MXA 29005 (Nikon Corp., Tokyo, Japan).
To analyze the morphology of the mirid bug′s attachment system and the interaction with the plant surface, a cryo-SEM Hitachi S-4800 (Hitachi High-Technologies Corp., Tokyo, Japan) equipped with a Gatan ALTO 2500 cryo-preparation system (Gatan Inc., Abingdon, UK) was used. Fresh samples of mirid bug legs and plant leaves were cut out using a razor blade, mounted on metal holders by Tissue-Tek® O.C.T.TM Compound (Sakura Finetek Europe B. V., Zoeterwoude, Netherlands), frozen in the preparation chamber at −140°C, sputter-coated with gold–palladium (6 nm thickness) and examined in a frozen state in the cryo-SEM at 3 kV and −120°C.
To visualize in detail how mirid bugs’ claws interlock with trichomes on the host plant surface, leaf samples of R. gorgonias with attached P. roridulae were prepared, clamped perpendicularly on holders, and examined in the cryo-SEM as described above. Additionally, from SEM micrographs of 10 randomly selected pretarsi of adult specimens (males and females pooled together), the inner length of the claw, diameter of a circle fitting the inner curvature of the claw, and length of the setiform parempodia were estimated, using Sigma Scan Pro 5 (SPSS, Inc., Chicago, IL, USA) software.
Traction force experiment
Traction force measurements were carried out according to Gorb et al. (2004) and Voigt et al. (2007). Insects were anaesthetised with CO2 and attached to a 10 cm long human hair with a molten wax droplet. Their forewings were glued together. Bugs were weighed using an analytical balance AG 204 Delta Range (Mettler Toledo GmbH, Greifensee, Switzerland). Following anaesthesia, insects were allowed to recover for 1 h. Then, the free end of the hair was connected to the force sensor.
Fresh leaves were removed from the middle part of R. gorgonias plants and attached to a horizontal glass plate using double-sided tape. Pieces (5 × 5 cm2) were cut out with a razor blade from the intercostal leaf areas of the non-host plant R. obtusifolius. Using a FORT-10 force transducer (10 g capacity, Biopac Systems Ltd., Santa Barbara, CA, USA), the traction force was measured in males and females of P. roridulae. Each specimen was tested (1) walking distally on the adaxial and abaxial leaf surface of R. gorgonias (contact angle of water could not be determined), (2) walking proximally on the adaxial and abaxial leaf surface of R. gorgonias, (3) walking distally on the non-host plant R. obtusifolius (contact angle of water 40–70°, surface energy 35.43 mN*m−1; Gorb and Gorb 2009), and (4) walking on a glass surface (contact angle of water 55°, surface energy 42.9 mN*m−1). The succession of different substrates (1–4) was randomly organised during the experiment. Before the exchange of substrates, bugs were allowed to groom and to walk over KIMTECHScienceTM precision wipes (Kimberly-Clark Europe Ltd, Surrez, UK).
Using AcqKnowledge 3.7.0 software (Biopac Systems Ltd, Goleta, CA, USA), force–time curves were recorded to estimate the maximal traction force produced by a single bug during five consecutive runs on a test substrate. On each plant surface, five males and five females were tested individually. In total, 70 individual tests with 10 different specimens were carried out (N = 7 substrates, n = 10 individual tests). Kruskal–Wallis one-way ANOVA on ranks followed by all pair-wise multiple comparision procedures (Dunn’s test) was applied to determine differences in forces between test surfaces (software SigmaStat 3.1.1®, Systat Software, Inc., Richmond, California, USA). Force measurements in various walking directions on the same leaf surface of R. gorgonias were included because trichomes are oriented distally and thus some anisotropical effects could occur. In contrast to R. gorgonias, the dock plant R. obtusifolius represents a non-host plant having a totally different surface. Since no structural anisotropy was found on dock plant leaf surfaces, traction was tested only for mirid bugs walking distally on the leaf.
Results
The attachment system of Pameridea roridulae
Mirid bugs were able to move quickly and confidently, and to attach properly to the whole plant surface (stems, both leaf sides and inflorescence; Fig. 1a–h). Frequently they were observed resting in axillae (Fig. 1b, d, e). Predominant interlocking sites were trichome stems, namely their bases, up to the lower one-third of the trichome (Fig. 1f–n, p, q–s, u, w, y, z). Infrequently, claws clung more distally to the trichomes (Fig. 1v). The leg span (distance between left and right leg pretarsi) fits approximately to the width of the leaf lamina. Thus, bugs grip trichomes at both leaf margins at once, and hold their body above the leaf lamina. However, bugs also attached to the smooth plant epidermis between trichomes, positioning their tarsus almost parallel and very close to the surface (Fig. 1f). Claw interlocking, where claw curvature fits the trichome stem, occurred on filiform thread-shaped (Fig. 1o), small (Fig. 1v) and middle-sized trichomes (Fig. 1t). On thicker tentacle-shaped ones, the position of claws was similar to that on smooth surfaces (Fig. 1x). Claws moved apart under load, applied on the flat surface, allowing pseudopulvilli to get a grip on the plane surface.
Images of various positions of Pameridea roridulae bugs and their tarsi on the surface of the host plant Roridula gorgonias. a–c A female bug, adhering to trichomes, close to a captured blow fly (a); at the plant stem in an axilla (b); and close to the leaf tip (c). d, e The male bug in diverse similar attachment positions. f A female, adhering to the smooth plant surface between trichomes; pretarsi almost orientated parallel to the plant surface. g A female and 5th instar nymph clinging to the plant surface at opposite leaf sides. h–j 5th instar nymph demonstrating similar behavior as in f (h), and pretarsi interlocked with trichomes (i, j). k–z Details of pretarsi adhering to the plant surface at various positions. Note: claw interlocking close to the base of a single trichome (k, l, q, p, r, u, x, y, z), at higher sites of the trichome stem (t, v), and attachment to the smooth plant surface between trichomes (m, n, s). The body length of the bugs is about 5 mm
Cryo-SEM studies of the pretarsus clarified its position on the plant surface (Fig. 2a) and the structure of attachment devices (Fig. 2b–f). Paired curved claws are 23.1 ± 6.77 μm (mean ± SD) long and connected to the unguitractor plate at their bases (Fig. 2c). They have slightly tapered tips (claw teeth, 2.6 ± 0.51 μm long) and widened basal lobe-like structures. The latter ones bear paired, trapezium-shaped pseudopulvilli and between them, two setiform parempodia (13.8 ± 1.0 μm long). Dorsally, a stronger (guard) seta is visible (Fig. 2f). The claw surface bears longitudinal striae. The inner side of claws appears sharp-edged. Adhering to a single short trichome, its stem is clamped beween the inner side of one claw part and a single pulvillus (Fig. 2d, e). The average diameter of an ideal circle fitting the concavity of claws is 16.1 ± 4.14 μm.
Cryo-SEM micrographs of the attachment devices in Pameridea roridulae bugs. a Female’s abdominal tip and hind legs, adhering to the glandular hairy abaxial plant surface of Roridula gorgonias. b Female tarsus. c Female pretarsus (unguitractor plate hidden by tarsomere 3 and other pretarsal structures). d, e Male pretarsus interlocked with a short glandular trichome. The middle of the trichome stem is clamped between a single claw, its basal lobe-like structure and the pseudopulvillus. f A male pretarsus attached to the smooth metallic surface of a SEM sample holder. Pseudopulvilli are pressed against the substrate beneath the outspread claws. The guard seta is clearly visible. (bs) basal lobe-like structure; (cl) claw; (co) grooming comb; (ct) claw tooth; (fe) femur; (gs) guard seta; (mt) middle-sized trichome; (ps) pseudopulvillus; (pt) pretarsus; (st) short trichome; (ta) tarsus; (t1) 1st tarsomere; (t2) 2nd tarsomere; (t3) 3rd tarsomere; (th) trichome glandular head; (ts) trichome stem; (tt) tentacle-shaped trichome; (ti) tibia; (tj) tibia-tarsus joint. Scale bars: a = 1,000 μm; b, d = 50 μm; c, e, f = 20 μm
Traction force of Pameridea roridulae
Plant substrates used in the traction force experiment varied in the structure of their surfaces (Fig. 3). The adaxial leaf side of R. gorgonias is densely covered with filiform, thread-shaped, distally pointing trichomes and interdispersed short glandular trichomes (Fig. 3a). The abaxial leaf side bears short and medium-sized glandular trichomes, and the spaces between them are rather smooth, having slight cell irregularities (Fig. 3b). Leaf margins are densely covered with long, medium-sized and short trichomes.
Cryo-SEM micrographs of plant substrates used in traction force experiment with Pameridea roridulae bugs. a, b The leaf surface of Roridula gorgonias bears various types of trichomes on the adaxial (a), and on the abaxial leaf side (b). c–e The uneven adaxial leaf surface of Rumex obtusifolius shows irregular convex cells and stomata (c, e), sparse cuticular foldings (arrow) and wax granules (d). f, g The abaxial leaf surface is similar to the adaxial, however, prominent veins are covered with small papilla-shaped trichomes (b). (ft) filiform, thread-shaped trichomes; (mt) middle-sized trichomes; (st) short trichomes; (tt), tentacle-shaped trichomes. Scale bars: a, b, c, f, g = 50 μm; d = 1 μm; e = 20 μm
Leaves of R. obtusifolius appear adaxially predominantly smooth, slightly textured by convex cells having sparse cuticular foldings (Fig. 3c), and a few discrete patches of epicuticular crystal wax granules and stomata (Fig. 3d–e). On the abaxial leaf side, convex cells and venation are more prominent. Larger veins are covered with papilla-shaped trichomes (Fig. 3f). Numerous stomata are found (Fig. 3g).
The body mass of P. roridulae ranged from 6.7 ± 0.35 mg (mean ± SD) in males to 7.0 ± 0.63 mg in females without a statistical difference between the sexes (t-test, t = −0.864, P = 0.413, n = 10). Traction forces differed significantly depending on the surface (Fig. 4). The highest forces, up to 8.8 mN, were generated on the adaxial leaf surface of R. gorgonias, corresponding to 126 times the bug’s body mass. The lowest forces were measured on the adaxial leaf side of R. obtusifolius and on a glass surface (47 times the bug’s body weight). Leaf side and pulling direction did not significantly influence the bug’s performance on R. gorgonias. On R. obtusifolius, higher forces were generated on the rougher abaxial surface. Exept for the distal direction on the abaxial leaf side of R. gorgonias, the adaxial leaf side of R. obtusifolius and the glass surface, no statistical differences were found between sexes on the same surface (Table 2).
Forces generated by Pameridea roridulae in traction experiments on leaf surfaces of Roridula gorgonias (traction in distal and proximal direction) and Rumex obtusifolius (traction in distal direction), and on cleaned glass (control). a Box-and-whisker diagram showing the traction force of single mirid bugs measured on various surfaces. The ends of the boxes define the 25 and 75th percentiles, with a line at the median and error bars defining the 10 and 19th percentiles. Asterisks show statistical differences between males and females (for statistical differences see Table 2). b Statistical differences between surfaces (Kruskal–Wallis one-way ANOVA on ranks, for males: H6,173 = 101.5, P ≤ 0.001; for females: H6,173 = 111.7, P ≤ 0.001; all pairwise multiple comparison procedures, Tukey test, P < 0.05 for both, separately males and females)
Discussion
Mirid bug behaviour
The locomotion mode of P. roridulae and the manner in which it attaches to plants affirm previous reports that this bug does not avoid contact with the sticky sites of the host plant surface (Voigt and Gorb 2008). Rather it frequently touches adhesive plant secretion droplets since it adheres, predominantly, to the trichome base at the lower third of the trichome stem. Thus, the body is held very close to the plant surface, unlike related species. For example, Dicyphus errans adheres to the glandular surface of Ononis sp., positioning the long tibiae and the especially long hind femuri almost vertically to the plant surface (Southwood 1986). Only the tarsal apexes come in contact with the plant surface and the body is held far away from it.
Pretarsal adaptations to the trichome-covered terrain
Although the pretarsus of P. roridulae bears similar structures found in related bryocorine bug species (Schuh 1976; Cobben 1978; Wheeler 2001), claws in P. roridulae are distinctly shorter (mean length 23.1 μm) and thicker, e.g., compared to those of the mirid bug D. errans (mean length 74.0 μm, Voigt et al. 2007). The body length is similar in both mirid bug species (Table 1), but the body of P. roridulae appears stronger and thicker. The mean female body mass in P. roridulae is 7.0 mg corresponding to 1.8 times that of the female D. errans. The body mass, in combination with trichome stem flexibility, may explain why the bugs P. roridulae predominantly cling to the basal part of trichomes. Knowing trichome spring constants and bending behaviour (tentacle-shaped: 0.02 Nm−1, bending at the tip; medium-sized: 0.08 Nm−1, bending at the tip; short: 0.95 Nm−1, bending at the base Voigt et al. 2009), the trichome’s deflection, caused by the load of a mirid bug, can be estimated. Since the geometry of trichomes is similar, the deflection of short trichomes may also be considered as for the base of tentacle-shaped ones. Assuming a resting female (7 mg) having all six legs clinging to trichomes, a tentacle-shaped trichome would bend 1 mm at the tip but only about 20 μm at the base. For comparison, a female D. errans (3.8 mg) would cause a trichome deflection of 250 μm at the trichome’s tip and about 7 μm at the base. Less trichome bending allows more effective bug attachment, as shown in previous studies on D. errans, where long and thin trichomes with a high aspect ratio resulted in lower traction forces (Voigt et al. 2007).
The heavier body of P. roridulae led us to assume a generally larger portion of muscles enabling this bug species to generate stronger forces compared to lighter related species. The assumption is confirmed by traction forces measured with P. roridulae on various surfaces. Traction force corresponds to the friction between insect pretarsi and the substrate. The proper adherence to trichomes and applying a pulling force to them while remaining in a certain position, is important in behavioural situations where mirid bugs’ bodies move back and forth or up and down the substrate (Kullenberg 1946; Voigt et al. 2004; Voigt 2005). (1) During sucking the head and thorax are repeatedly moved up and down, supporting the rostrum bending inside the prey. (2) During the final phase of copulation the male and female couple their abdominal tips, heads facing in opposite directions, and move their bodies back and forth, as in a “tug of war”. (3) The oviposition requires strong up and down movements of the female abdomen and therefore strong attachment to the plant substrate in order to insert the saw-shaped ovipositor into the plant tissue. (4) Molting nymphs and emerging imagines free themselves from old cuticle by pulling their body distally. For P. roridulae, the challenge is to perform these actions even upon its adhesive host plant terrain. Possibly, for this reason P. roridulae may generate higher traction forces (126 times higher than the body mass) than the related D. errans (34.2 times higher than the body mass) on both a glandular hairy plant surface as well as on glass (P. roridulae: 47, and D. errans: 6.1 times higher than the body mass). The latter values indicate that mirid bugs may also adhere sufficiently to microstructured and smooth surfaces such as those of R. obtusifolius and glass. Thus, smooth sites on R. gorgonias (trichome-free spaces on the leaf and wide, non claw-fitting trichome stems) provide suitable attachment substrates for P. roridulae bugs. Considering the lowest generated force on a smooth glass surface (females: 0.7 mN, median), a bug may still free itself from 10 tentacle-shaped trichomes adhering coincidentally to the bug’s epicuticle (median adhesion force of a single trichome: 0.07 mN, Voigt and Gorb 2008).
Pameridea roridulae bugs were observed predominantly interlocking to trichomes using claws. Although their claws are much shorter (average length 23 μm) than those of D.errans (74 μm), the average diameter of an ideal circle fitting the concavity of claws in both species is about 16 μm. Since mean stem diameters of trichomes on the surface of R. gorgonias are from 75 μm in median-sized to 120 μm in tentacle-shaped ones (Voigt et al. 2009), claws of P. roridulae may only interlock with some sites of these trichome stems. Nevertheless, on rough surfaces, higher traction forces were measured compared to smooth ones. For example, on the abaxial leaf side of R.obtusifolius, small papilla-shaped trichomes provided interlocking sites for claws and therefore, a stronger traction force of mirid bugs was recorded than on the smooth adaxial leaf side. P.roridulae bugs performed best on their host plant. Since forces did not differ significantly between leaf sides and pull direction, leaves of R.gorgonias, in general, can be assumed to be a suitable attachment substrate for male and female bugs.
Sexual dimorphism in traction force generation
Compared to females, males generated significantly higher forces on the abaxial leaf side of R. gorgonias, on the adaxial leaf side of R. obtusifolius, and on glass. Differences between sexes have been previously found in traction force experiments with leaf beetles Chrysolina polita L. (Coleoptera, Chrysomelidae; Stork 1980) and ladybird beetles Coccinella septempunctata L. (Coleoptera, Coccinellidae; Gorb et al. 2008). Males of both species adhere stronger to smooth surfaces, whereas females performed better on rough ones. However, attachment systems of beetles (hairy) and mirid bugs (smooth) differ completely and cannot be compared outright in the context of our results (Beutel and Gorb 2001). Since significances between male and female force values occur on predominantly smooth substrates, one may conjecture that differences in material properties between male’s and female’s pseudopulvilli exist. These pad-like attachment structures in mirid bugs, adhering to smooth surfaces, need further micromechanical analyses for better understanding of their functionality depending on sex and species.
Two locomotion strategies of mirid bugs
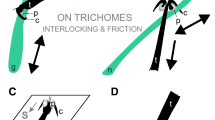
Generally, in species of the order Heteroptera, a notable diversity of pretarsal structures has been reported and discussed in the evolutionary context (Schuh 1976; Cobben 1978). Some peculiar host plant relations have been previously assumed, but the adaptive value of these structures is difficult to interpret in direct functional connection with the substrate due to the absence of detailed analyses. Considering published data on both D. errans (Southwood 1986; Voigt et al. 2007) and P. roridulae (Voigt and Gorb 2008) in combination with results obtained in the present study, we may hypothesize two different strategies of locomotion and attachment on glandular hairy plant surfaces in species within one and the same mirid tribe Dicyphini of the subfamily Bryocorinae (Fig. 5). (1) The “avoidance strategy”, as used by D. errans, consists of keeping the body at a large distance from the plant surface and glandular secretions of trichomes (Fig. 5a). This strategy relies on a light and slim body, long and slender, carefully-moving legs, whose tibiae are oriented almost vertically to the substrate. Pretarsi are equipped with long, narrow claws. Moreover, frequent resting and grooming are observed in these bugs, indicating that they do not have a protective layer on their epicuticle maintaining the body clean and free from adhesive plant secretion. (2) P. roridulae has a “defense strategy” allowing close contact with the plant surface and the trichomes’ adhesive secretion (Fig. 5b). The body is heavy, with relatively thick legs that are predominantly used for attachment to the trichome bases by short claws. Mirid bugs with “defense strategy” generate stronger forces during locomotion, enabling them to walk, unhinderd, on a plant surface covered with sticky trichomes and to free themselves from adhesive secretions. Additionally, the greasy secretion, found in the P. roridulae epicuticle, contributes to reducing the adhesion of the plant adhesive to the bug. One may conjecture that the defense strategy is a specific adaptation of bugs P. roridulae for a life on the glandular plant surface of R. gorgonias. This would support recently published coevolutionary considerations on cospeciation, coadaptation, and long-term coexistence in the Roridula-Pameridea complex (Anderson et al. 2004; Anderson 2006). On the other hand, the avoidance strategy could be a typical feature of generalists, like D. errans, having a broad range of host plants.
Two assumed strategies of mirid bugs of the tribus Dicyphini (Heteroptera, Miridae, Bryocorinae) walking on plant surfaces, which are densely covered with glandular trichomes. a “Avoidance strategy”: the light, slim body is held at a large distance from the plant surface and adhesive secretion of trichomes by using long, slender legs. Additionally, bugs frequently rest and groom to keep their body clean. b “Defense strategy”: the heavy, tough body is situated close to the plant surface, frequently contacting trichomes and their viscous secretion. Bugs overcome the adhesion of the plant surface by generating strong forces during locomotion and having a thick anti-adhesive epicuticular greasy layer on their cuticle
To what extent the hypothesized strategies may be the case for other mirid bug species, is difficult to appraise, because neither detailed comparative nor experimental studies have been previously carried out. Representatives of the mirid bug subfamilies Orthotylinae and Bryocorinae are generally known to be specialised in living on glandular hairy plants (Reuter 1913; Kullenberg 1946; Cassis 1984; Falkingham 1995; Dolling and Palmer 1991; Wheeler 2001; Sugiura and Yamazaki 2006; Voigt et al. 2007). Several of them even live on insectivorous plants of the genera Drosera and Byblis (e.g. China and Carvalho 1951; China 1953; Southwood 1986; Falkingham 1995). These bugs also feed on trapped insects, but unlike P. roridulae, they avoid the contact with the sticky surface of their host plants (Russell 1953).
Only one previous report on different modes of locomotion on glandular plant surfaces is found in literature, however, for other true bug families than Miridae (Schwoerbel 1956). Stilt bugs Gampsocoris punctipes Germ. (Heteroptera, Berytidae) avoid the contact with glandular plant secretion of the spiny restharrow Ononis spinosa L. (Fabaceae) by means of their slim, small body and long, slender legs, whereas juveniles Corizus hyoscami L. (Heteroptera, Rhopalidae) appear stronger and walk unhamperedly between trichomes like a “snowplow” on the same plant surface (Schwoerbel 1956). Thus, for non-mirid species, the avoidance strategy is not only characteristic of generalists, because the specialist G. punctipes is obligatory associated with O. spinosa.
Concluding remarks
Pameridea roridulae lives in a complex, three-dimensional, sticky terrain where it moves without hindrance. In contrast to related species within the tribe Dicyphini of the family Bryocorinae, P. roridulae do not avoid adhesive secretions but rather walk between glandular trichomes and touch them very frequently. Previously, an anti-adhesive epicuticular layer protecting mirid bugs from being captured by the adhesive plant secretion has been described. The present results show that the mode of attachment and locomotion on the plant surface also contributes to the specialisation of P.roridulae to a life on the protocarnivorous flypaper trap R.gorgonias. There are direct, biomechanical interactions at the interface between the plant surface and insect attachment system. Dicyphine bugs use claws to cling to trichomes and use pad-like pseudopulvilli for adhesion to smooth hairless patches or to thick trichome stems. This strategy allows them to generate distinctly higher traction forces if compared to other mirid bug species. Thus, P.roridulae may even overcome contact with the adhesive secretion of numerous trichomes. Based on available data, we have hypothesized, here, two strategies of locomotion and attachment on glandular hairy plant surfaces within the mirid bug tribus Dicyphini: (1) avoidance strategy, characterised by the slim body held at a large distance from the plant surface by using long, slender legs, and (2) defense strategy, where trapping of the heavy bugs, situated close to the plant surface, is overcome by generating strong forces during locomotion and by having a thick anti-adhesive epicuticular greasy layer on the bugs’ cuticle. The proper attachment and locomotion on the host plant surface may result in the better fitness of insects. Additionally, living on an adhesive plant trap has several long-term adventages for P. roridulae (e.g., protection, less competition, permanent food supply). The defense strategy could be specific to Pameridea species indicating specialized adaptations of bugs to the plant surface, which would support previously suggested coevolutionary processes in the Roridula-Pameridea complex. Further comparative broad screenings of pretarsal structures combined with attachment experiments on different substrates and analyses of epicuticular grease, including various species of mirid bugs and glandular hairy plants, will shed light on the evolutionary tendencies in this insect-plant mutualism.
References
Anderson B (2006) Inferring evolutionary patterns from the biogeographical distributions of mutualists and exploiters. Biol J Linn Soc 89:541–549
Anderson B, Midgley JJ (2002) It takes two to tango but three is a tangle: mutualists and cheaters on the carnivorous plant Roridula. Oecologia 132:369–373
Anderson B, Midgley JJ (2003) Digestive mutualism, an alternate pathway in plant carnivory. Oikos 102:221–224
Anderson B, Midgley JJ (2007) Density-dependent outcomes in a digestive mutualism between carnivorous Roridula plants and their associated hemipterans. Oecologia 152:115–120
Anderson B, Midgley JJ, Stewart BA (2003) Facilitated selfing offers reproductive assurance: a constrained mutualism between a hemipteran and carnivorous plant. Am J Bot 90:1009–1015
Anderson B, Olivieri I, Lourmas M, Stewart BA (2004) Comparative population structures and local adaptation of two mutualists. Evolution 58:1730–1747
Beutel RG, Gorb SN (2001) Ultrastructure of attachment specializations of hexapods (Arthropoda): evolutionary patterns inferred from a revised ordinal phylogeny. J Zool Syst Evol Research 39:177–207
Bruce AN (1907) On the distribution, structure, and function of the tentacles of Roridula. Notes RBG Edin 17:83–98
Cassis G (1984) A systematic study of the subfamily Dicyphinae (Heteroptera: Miridae). Ph.D. dissertation, Oregon State University, Corvallis, USA. 389 pp
China WE (1953) Two new species of the genus Cyrtopeltis (Hemiptera) associated with sundews in Western Australia. West Aust Nat 4:1–8
China WE, Carvalho JCM (1951) A new ant-like mirid from Western Australia (Hemiptera, Miridae). Ann Mag Nat Hist 4:221–225
Cobben RH (1978) Evolutionary trends in Heteroptera. Part II. Mouthpart-structures and feeding strategies. Meded. Landbouwhogeschool Wageningen, 78-5, H. Veenman & Zonen, B. V., Wageningen. 407 pp
Dolling WR, Palmer JM (1991) Pameridea (Hemiptera: Miridae): predaceous bugs specific to the highly viscid plant genus Roridula. Syst Ent 16:319–328
Ellis AG, Midgley JJ (1996) A new plant-animal mutualism involving a plant with sticky leaves and a resisdent hemipteran insect. Oecologia 106:478–481
Falkingham C (1995) Carnivorous plants-carnivorous bugs. Is there a symbiotic relationship? Victiorian Naturalist 112:222–223
Fenner CA (1904) Beiträge zur Kenntnis der Anatomie, Entwicklungsgeschichte und Biologie der Laubblätter und Drüsen einiger Insektivoren. Flora Allg Bot Z 93:335–433
Futuyma DJ, Slatkin M (1983) Epilogue: the study of coevolution. In: Futuyma DJ, Slatkin M (eds) Coevolution. Sinauer Associates, Inc, Sunderland, pp 459–464
Gorb E, Gorb S (2009) Effects of surface topography and chemistry of Rumex obtusifolius leaves on the attachment of the beetle Gastrophysa viridula. Entomol Exp Appl 130:220–222
Gorb E, Kaster V, Peressadko A, Arzt E, Gaume L, Rowe N, Gorb S (2004) Structure and properties of the glandular surface in the digestive zone of the pitcher in the carnivorous plant Nepenthes ventrata and its role in insect trapping and retention. J Exp Biol 207:2947–2963
Gorb E, Hosoda N, Gorb S (2008) Nano-porous substrates reduce beetle attachment force. Proccedings of the 9th biennial conference on engineering systems design and analysis ESDA08, Haifa, Israel. pp 1–6
Holloway (1967) Wettability of plant surfaces. Ph.D. dissertation, University of London, UK
Kullenberg B (1946) Studien über die Biologie der Capsiden. Zool Bidrag Uppsala 23:1–522
Lloyd FE (1934) Is Roridula a carnivorous plant? Can J Res 10:780–786
Marloth R (1903) Some recent observations on the biology of Roridula. Ann Bot 17:151–158
Marloth R (1910) Further observations on the biology of Roridula. Trans Roy Soc South Afr 2:59–62
Reuter OM (1907) Ad cognitionem Capsidarum aethiopcarum scripsit. Öfv F Vet Soc Förh 49, 27 pp
Reuter OM (1913) Lebensgewohnheiten und Instinkte der Insekten bis zum Erwachen der sozialen Instinkte. Friedländer & Sohn, Berlin, 448 pp
Roberts JI (1930) The tobacco capsid (Engytatus volucer Kirk.) in Rhodesia. Bull Entomol Res 21:169–183
Russell MC (1953) Notes on insects associated with sundews (Drosera) at Lesmurdie. West Aust Nat 4:9–12
Schuh RT (1976) Pretarsal structures in the Miridae (Hemiptera) with cladistic analysis of relationships within the family. Am Mus Novit 2601, 39 pp
Schuh RT (1995) Plant bugs of the world (Insecta: Heteroptera: Miridae): Systematic catalog, distributions, host list, and bibliography. The New York Entomological Society, 1329 pp
Schwoerbel W (1956) Beobachtungen und Untersuchungen zur Biologie einiger einheimischer Wanzen. Zool Jb Syst 84:329–354
Southwood R (1986) Plant surfaces and insects-an overview. In: Juniper B, Southwood R (eds) Insects and the plant surface. Edward Arnold Publishers, London, pp 1–22
Stork NE (1980) Experimental analysis of adhesion of Chrysolina polita (Chrysomelidae: Coleoptera) on a variety of surfaces. J Exp Biol 88:91–107
Sugiura S, Yamazaki K (2006) Consequences of scavenging behaviour in a plant bug associated with a glandular plant. Biol J Linn Soc 88:593–602
Thompson JN (1988) Coevolution and alternative hypotheses on insect/plant interactions. Ecology 69:893–895
Voigt D (2005) Untersuchungen zur Morphologie, Biologie und Ökologie der räuberischen Weichwanze Dicyphus errans Wolff (Heteroptera, Miridae, Bryocorinae). Dissertation, TU Dresden, Germany, http://nbn-resolving.de/urn:nbn:de:swb:14-1138036391273-82564
Voigt D, Gorb S (2008) Insect trap as habitat: cohesion failure mechanism prevents adhesion of bugs Pameridea roridulae (Heteroptera, Miridae, Bryocorinae) to the sticky surface of the plant Roridula gorgonias (Roridulaceae). J Exp Biol 211:2647–2657
Voigt D, Wyss U, Mölck G (2004) Videodokumentation über die Biologie und das Verhalten der räuberisch lebenden Weichwanze Dicyphus errans Wolff (Heteroptera, Miridae, Dicyphinae). Mitt DPG 34:44–45
Voigt D, Gorb E, Gorb S (2007) Plant surface–bug interactions: Dicyphus errans stalking along trichomes. Arthropod-Plant Interactions 1:221–243
Voigt D, Gorb E, Gorb S (2009) Hierarchical organisation of the trap in the protocarnivorous plant Roridula gorgonias (Roridulaceae). J Exp Biol 212:3184–3191
Wagner E (1955) Bemerkungen zum System der Miridae (Hemiptera, Heteroptera). Dtsch Entomol Z 2:230–242
Wagner (1970/71) Die Miridae Hahn, 1831, des Mittelmeerraumes und der Makronesischen Inseln (Hemiptera, Heteroptera), Teil 1. Entomol Abh Mus Tierk Dresden 37, 484 pp
Wheeler AG (2001) Biology of the plant bugs (Hemiptera: Miridae): pests, predators, opportunists. Cornell University Press, London, 507 pp
Acknowledgments
Klaus Keller (Augsburg, Germany) is kindly acknowledged for provision of plants and bugs. The bug species was determined by Kurt Arnold (Geyer, Erzgebirge, Germany). Victoria Kastner (Max-Planck Institute for Metals Research, Stuttgart, Germany) provided linguistic corrections of the manuscript. This study was supported by the Federal Ministry of Education and Research, Germany (BMBF project Inspirat 01RI0633D).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Heikki Hokkanen.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Voigt, D., Gorb, S. Locomotion in a sticky terrain. Arthropod-Plant Interactions 4, 69–79 (2010). https://doi.org/10.1007/s11829-010-9088-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11829-010-9088-1