Abstract
Background
Restless legs syndrome (RLS), a disorder characterized by an urge to move one’s legs during sleep or rest, leads to impaired sleep quality. Many patients with RLS report increased daytime sleepiness, but this has seldom been the focus of clinical research. The current study empirically investigated the prevalence and severity of daytime sleepiness in RLS.
Methods
This prospective study included 29 newly diagnosed treatment-naïve patients with RLS and 31 healthy controls and assessed standardized subjective (tiredness symptom scale [TSS], Stanford Sleepiness Scale [SSS], Epworth Sleepiness Scale [ESS]), cognitive (psychomotor vigilance task [PVT], Mackworth Clock Test [MCT]), and physiological measures (pupillary unrest index [PUI]). RLS symptom severity was assessed, and the effects of RLS on general health aspects and subjective sleep quality (Pittsburgh Sleep Quality Index) were compared to control data.
Results
Patients had moderate to severe RLS with significant negative effects on general health, quality of life, and sleep quality. Patients with RLS showed more subjective daytime sleepiness (ESS) and current sleepiness (TSS, SSS) than controls. The objective performance of patients in sustained attention tasks (P VT, MCT) was significantly worse than that of controls. Additionally, patients showed higher PUI scores.
Conclusion
In the present study, RLS was associated with markedly impaired subjective sleep quality and with subjectively and objectively increased daytime sleepiness. Since daytime sleepiness can be a major factor leading to compromised quality of life, assessing and treating sleepiness should be incorporated into standard diagnostics and treatment.
Zusammenfassung
Hintergrund
Das Restless-Legs-Syndrom (RLS) ist eine Erkrankung, die durch einen Bewegungsdrang in den Beinen während Schlaf- und Ruhephasen charakterisiert ist und zu einer Verminderung der Schlafqualität führt. Obwohl über Tagesschläfrigkeit von vielen Patienten berichtet wird, wurde dieses Symptom bisher selten in klinischen Studien untersucht. Die vorliegende Studie untersuchte die Prävalenz und Schwere von Tagesschläfrigkeit bei RLS mit empirischen Methoden.
Methode
Die prospektive Studie schloss 29 Patienten, bei welchen ein idiopathisches RLS kürzlich festgestellt und noch nicht therapiert wurde, und 31 gesunde Kontrollprobanden ein. Es wurden standardisierte subjektive (Tiredness Symptom Scale [TSS], Stanford Sleepiness Scale [SSS], Epworth Sleepiness Scale [ESS]), kognitive (Psychomotor Vigilance Task [PVT], Mackworth Clock Test [MCT]) und physiologische Messmethoden (Pupillenunruheindex [PUI]) verwendet. Zusätzlich wurde die Schwere der RLS-Symptome erfasst. Ebenso wurden die Auswirkungen von RLS auf den allgemeinen Gesundheitszustand sowie die Daten zur subjektiven Schlafqualität (Pittsburgh Sleep Quality Index) mit der Kontrollgruppe verglichen.
Ergebnisse
Die Patienten litten an moderatem bis schwerem RLS mit signifikanten negativen Auswirkungen auf den allgemeinen Gesundheitszustand, die Lebens- und Schlafqualität. Im Vergleich zu den Kontrollprobanden wiesen die RLS-Patienten eine höhere subjektive Einschlafneigung in Alltagssituationen (ESS) und aktuelle Müdigkeit (TSS, SSS) auf. Die objektive Leistung der Patienten war bei den Aufgaben zur Daueraufmerksamkeit (PVT, MCT) signifikant schlechter als die der Kontrollgruppe. Des Weiteren wiesen die Patienten einen höheren PUI auf.
Schlussfolgerung
In der vorliegenden Studie war RLS mit deutlich verringerter Schlafqualität sowie mit subjektiv und objektiv erhöhter Tagesschläfrigkeit assoziiert. Da Tagesschläfrigkeit ein wichtiger Faktor bezüglich verminderter Lebensqualität sein kann, sollte die Erfassung sowie die Behandlung von Schläfrigkeit in die Diagnose- und Therapiemaßnahmen bei RLS aufgenommen werden.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Restless legs syndrome (RLS), also known as Willis–Ekbom disease, is a severe neurological sensorimotor disease characterized by an urge to move one’s legs during sleep or rest. The estimated prevalence in the general adult population is 3%, and is higher in women (4.7%) than in men (2.8%). It is associated with iron deficiency and pregnancy, and the prevalence increases with age [2]. Five essential diagnostic criteria have been proposed by the International Restless Legs Syndrome Study Group (IRLSSG; [1], Table 1). The IRLSSG also highlighted disturbed sleep and lower quality of life as consequences of RLS. Sleep disruptions, including frequent awakenings during the night due to limb movements as well as difficulty falling asleep, are common concomitant features of RLS [1]. Additionally, cognitive deficits and general physical and mental health problems such as depression are associated with RLS [2]. In the upcoming International Classification of Diseases-11 (ICD-11, [25]), RLS is no longer classified within G25 (“Other extrapyramidal and movement disorders”). Instead, it is assigned to “sleep-related movement disorders,” and this change might reflect increasing awareness of the disease. Furthermore, its definition within ICD-11 corresponds to the essential diagnostic criteria of the IRLSSG; occasionally, co-occurring arm sensations are also considered. Moreover, ICD-11 states that symptoms are “sufficiently severe to result in significant distress or impairment in personal, family, social, educational, occupational, or other important areas of functioning (e.g., due to frequent disruptions in sleep)” [25].
RLS research often focuses on nocturnal sleep disturbances, which do affect daytime functioning and sleepiness [17], but little evidence exists regarding the prevalence of daytime sleepiness in patients with RLS. Moreover, the effects of daytime sleepiness on daily functioning have rarely been objectively assessed. Johns [8], the author of the widely used Epworth Sleepiness Scale (ESS), described excessive daytime sleepiness as a widespread symptom in patients with sleep disorders.
In a systematic review of daytime sleepiness measured using the ESS in treatment-naïve patients with idiopathic RLS, Fulda and Wetter [6] indicated increased daytime sleepiness (ESS score > 10) in 29.6% of patients. Song et al. [16] found excessive daytime sleepiness (ESS score > 10) in 22% of patients with RLS, and these patients reported more severe RLS symptoms than patients with RLS without excessive daytime sleepiness (ESS score ≤ 10). The authors suggested that improvement of RLS symptoms might simultaneously decrease daytime sleepiness [16]. This assumption is supported by research on RLS treatment using dopamine: Kallweit et al. [10] and Kallweit et al. [9] showed simultaneous improvement of RLS symptoms and symptoms of daytime sleepiness following administration of dopamine. One study focusing on the effects of RLS symptoms on quality of life reported that 31.1% of patients experienced excessive daytime sleepiness, noting that the most prevalent symptoms were insomnia, nocturia, sadness, and forgetfulness [13]. In a sample from the Sleep Heart Health Study (second examination), the mean ESS score was higher in participants presenting with RLS symptoms with at least moderate distress than in the total sample (8.5 ± 4.9 vs. 7.3 ± 4.2) [24]. Kallweit et al. [9] identified EDS (ESS > 10) in 32% of their sample of 37 patients with RLS, with 14% of the whole sample presenting with severe EDS (ESS > 14). In a 20-year follow-up study of a large cohort of 5102 middle-aged participants, the occurrence of RLS with daytime sleepiness in women was even associated with an increased mortality risk [20]. Further, in psychiatric patients with comorbid RLS, 46% of patients showed ESS scores above the cutoff of 10 [22].
In contrast to the evidence showing daytime sleepiness in patients with RLS, the IRLSSG listed a “lack of profound daytime sleepiness” as one of the clinical features supporting the diagnosis of RLS/Willis–Ekbom disease [1]. These seemingly contradictory positions may be influenced by differing concepts of daytime sleepiness or daytime fatigue.
These findings and recommendations stress the need for further empirical research to investigate daytime sleepiness in patients with RLS. Prior studies lacked control groups and only partially relied on standardized objective tests. Using a multidimensional assessment approach, the current study aimed to assess the prevalence and severity of daytime sleepiness as well as related performance deficits in patients with RLS compared to a control group.
Materials and methods
Measurements and procedure
Clinical evaluation of RLS symptoms and severity as well as general health, quality of life, and sleep quality assessments were implemented. Subjective and objective measurements were included to assess sleepiness. Current levels of subjective sleepiness were measured before assessing objective measures of sleepiness. The entire assessment took approximately 2 h and was usually performed in the mid-afternoon. Sleep–wake activity was monitored via actigraphy during the night prior to the assessment. All participants were instructed to take sufficient time for rest (usually equivalent to time in bed; minimum 6 h) to avoid any unusual sleep deprivation in the night prior to the assessment.
Essential criteria of RLS.
The presence of essential diagnostic criteria in accordance with the IRLSSG guidelines (Table 1) was assessed by clinical interview.
International Restless Legs Severity Scale.
The IRLSSG questionnaire (IRLSS) consists of ten questions regarding the subjective severity of RLS symptoms, ranging from 0 (none) to 4 (very severe). A score above 10 is considered moderate, a score above 20 points severe, and a score above 30 very severe [18].
Pittsburgh Sleep Quality Index.
The Pittsburgh Sleep Quality Index (PSQI) assesses the quality of sleep and awakening as well as somatic symptoms. A global score above 5 indicates poor sleep quality [4].
36-Item Short Form Health Survey.
The 36-Item Short Form Health Survey (SF-36) includes eight scales to assess physical and mental health. Scores range from 0 to 100, with higher scores representing a better health status [3].
Epworth Sleepiness Scale.
The ESS assesses the likelihood of dozing off or falling asleep in different everyday situations, in contrast to feelings of tiredness. The ESS [8] is a self-administered eight-item questionnaire (ratings of 0–3 for each item; total score of 24). Based on normative data from the German population, a score greater than 10 is considered “clinically suspicious,” and a score greater than 12 is considered “clinically relevant” [12].
Tiredness symptom scale.
In the tiredness symptom scale (TSS), patients identify 14 physiological and psychological symptoms with 0 (no) or 1 (yes). Higher scores indicate increased levels of subjective sleepiness [15].
Stanford Sleepiness Scale.
In the Stanford Sleepiness Scale (SSS), patients rate their current state of alertness on a seven-point Likert scale ranging from 1 (very alert) to 7 (unable to stay awake) [7].
Mackworth Clock Test.
The Mackworth Clock Test (MCT; VIGIL S1, Schuhfried GmbH, Mödling, Austria) objectively assesses sustained attention using a monotonous task. Participants are instructed to observe a monitor on which a dot follows an orbit of 32 small circles, jumping every 1.5 s, and to press a button every time the dot skips one of the circles. These critical stimuli appear in a randomized order with a total of 100 skips over the 25-min test duration. Several variables are measured, including mean reaction time (RT), number of lapses, and number of false alarms [11].
Psychomotor vigilance task.
The psychomotor vigilance task (PVT) objectively assesses attention using reactions to frequent simple visual stimuli presented on a screen during a 10-min test period. Participants are asked to press a button whenever time starts being counted on the screen [5]. The main outcome measure was the mean RT.
Pupillographic sleepiness test.
In the pupillographic sleepiness test (PST), the diameter of the pupil is measured during 11 min in total darkness. The mean variation of pupil size per unit time is expressed as the pupillary unrest index (PUI [mm/s]). A higher PUI indicates sleepiness [23].
Sleep–wake activity monitoring.
In addition to the above tests, actigraphy in ambulatory settings (miniaturized accelerometers; Motionlogger; Ambulatory Monitoring, Inc., Ardsley, NY, USA; recording length, 16 h, epoch length, 2 s, zero-crossing mode) were performed to record sleep–wake activity and motor activity, such as periodic limb movements, the night before the assessment. Actigraphy and sleep diary data were mainly used to check for adherence to the study protocol and sufficient rest. Sleep–wake parameters and periodic limb movements in sleep (PLMS) were approximately determined using the standard monitoring software used in the study hospital (Action4®; Ambulatory Monitoring, Inc., New York, NY, USA). Full attended cardiorespiratory polysomnography was performed (according to American Academy of Sleep Medicine guidelines) only when necessary in individual cases to rule out other sleep disorders, which was the case in 3 patients (data not reported here).
Participants
The patients in this study were recruited from a pool of potential candidates for intended pharmacological studies on RLS symptoms. A total of 29 patients with idiopathic RLS were included. They were newly diagnosed at the Center of Sleep Medicine, Department of Psychiatry and Psychotherapy, University of Regensburg, according to the IRLSSG diagnostic criteria [1] and were treatment naïve. Exclusion criteria were strict and included (i) history of, current use of, or dependence on substances according to the Diagnostic and Statistical Manual of Mental Disorders‑5 criteria for substance use disorders (alcohol, hypnotics, or other substances except nicotine); (ii) severe comorbid psychiatric, neurological, or medical disorders that could confound the study results; (iii) use of psychoactive substances or medications that could affect RLS symptoms, sleep quality, or wakefulness (e.g., amphetamines, methylxanthines, sedatives, hypnotics, antidepressants, antihistamines, neuroleptics, beta-blockers); and (iv) excessive tobacco smoking (more than 15 cigarettes/day). Due to the a priori inclusion and exclusion criteria of the subsequent pharmacological studies (e.g., need for effective contraception), recruitment was biased toward men. Eight participants were women and 21 were men, with a mean (±standard deviation) age of 52.9 ± 13.0 years. The age- and sex-matched control group consisted of 31 healthy adults with a mean age of 51.0 ± 13.3 years (11 women, 20 men). For the control group, participants were recruited among hospital staff and via flyers and newspaper ads. All participants provided written, informed consent.
Study design and data analyses
The study followed a between-subject, single-measures design, featuring a group of patients with RLS and a healthy control group. The study was approved by the local ethics committee of the University of Regensburg and fulfilled the principles of the World Medical Association Declaration of Helsinki.
Results from standardized test instruments were compared between patients and healthy controls. As the data were not normally distributed, Mann–Whitney U tests were conducted. Statistical analyses were performed using SPSS software (version 25.0; IBM Corp., Armonk, NY, USA). Results with p-values < 0.05 were considered statistically significant. The measurement of quantitative actigraphic data, including PLMS indices, sleep duration, and sleep latency, was limited due to methodological issues. Therefore, PLMS and sleep–wake activity data were evaluated qualitatively by visual inspection and were not used for statistical comparisons.
Results
RLS severity
In the patient group, the severity of RLS symptoms as measured by the IRLSS ranged from 11 to 36 (moderate to very severe). The average severity was 27.0 ± 5.9, with a median of 28. RLS symptoms were subjectively rated as very severe (IRLSS > 30) in 27.6% (8/29) of patients and severe (IRLSS > 20–30) in 55.2% (16/29).
RLS, sleep quality and duration, and health status
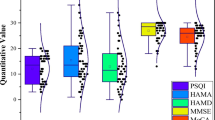
Patients had significantly higher global PSQI scores than healthy controls (score: 11.4 ± 3.9 vs. 3.6 ± 2.1; U [27.30] = 34.5; p < 0.001) and differed significantly in all seven subcomponent scores (all p < 0.029). The percentage of RLS patients with PSQI scores > 5, indicating “poor” sleep, was 89.3%, compared to 16.1% in the control sample. In patients with RLS, the usual sleep duration (PSQI sleep duration subscale) was less than 5 h in 42.9%, between 5 and 6 h in 17.9%, and 6–7 h in 42.9%. Among the healthy controls, the usual sleep duration was less than 6 h in 6.5%, 6–7 h in 41.9%, and more than 7 h in 51.6%. None of the controls reported sleeping less than 5 h. The difference between the two groups was highly significant (p < 0.001). In SF-36, patients had significantly worse scores than controls (all p ≤ 0.002) in all eight health status domains (i.e., physical functioning, physical role, bodily pain, general health perception, vitality, social functioning, emotional role, mental health; Fig. 1).
Effects of RLS on sleepiness
Global levels of subjective sleepiness.
ESS scores were significantly higher in patients than in controls (score: 10.4 vs. 6.7; p = 0.002). ESS scores > 10 were observed in 57% of patients and in 10% of controls.
Current levels of subjective sleepiness.
At baseline, patients with RLS had higher mean scores than controls on both the SSS (mean score: 3.3 vs. 1.8; p < 0.001) and the TSS (mean score: 5.0 vs. 1.3; p < 0.001).
Pupillographic sleepiness test.
PUI values were significantly higher in patients compared with controls (6.5 vs. 5.0 mm/min; p = 0.027), indicating a higher level of physiological sleepiness.
Psychomotor vigilance task.
On the PVT, the mean RT was significantly longer in patients than in controls (Table 2; p = 0.041).
Mackworth Clock Test.
RTs were significantly longer in patients than in controls (523 ms vs. 463 ms; p = 0.011). Lapses and false alarms were more frequent in patients than in controls, but the differences were not significant for either measure (lapses: 4.0 vs. 1.2; p = 0.068; false alarms: 3.0 vs. 1.9; p = 0.068).
The results of the subjective and objective measures of sleepiness are summarized in Table 2.
Discussion
RLS is commonly associated with poor sleep quality, manifesting in frequent awakenings, prolonged sleep latency, poor mental and physical health, and reduced quality of life; these aspects were confirmed in the current study. In addition, the increased prevalence of excessive daytime sleepiness in patients with RLS, which is a common but often neglected symptom, was supported by new empirical evidence. In contrast to previous studies, the current study relied on a between-subject design, including a healthy control group, as well as standardized subjective and objective measures to assess sleepiness.
In line with previous studies [2, 13], measures of global health were lower in patients with RLS compared to healthy controls. Moreover, sleep quality was impaired in a majority of patients. This corresponds to the description of RLS from the IRLSSG [1].
Regarding subjective sleepiness, patients with RLS scored higher than healthy controls on all assessments (ESS, SSS, TSS). Remarkably, 57% of patients had an ESS score > 10, which was distinctly higher than in prior studies [6, 9, 13, 16, 22]. Most likely, this is explained by the severity of RLS symptoms in the patient population in our study. A similar pattern emerged for objective measurements. Patient PUIs were significantly higher compared to those of controls, and patient RTs were also significantly longer on both the PVT and the MCT. These findings are typical for deficits in sustained attention related to sleepiness and can be considered cognitive deficits, as demonstrated in Broström et al. [2].
In their discussion of features supporting the diagnosis of RLS, Allen et al. [1] mention the “lack of expected daytime sleepiness” in patients with moderate to severe RLS, considering the degree of sleep loss in these patients. They refer to the point that the patients do not regularly nap, as is the case in central disorders of hypersomnolence. This seemingly discrepant finding is explained by the very nature of the RLS symptoms, which prevent patients from actually falling asleep specifically when they are tired and inclined to doze off due to the lack of sleep. However, this state should still be considered as sleepiness given the definition of excessive daytime sleepiness in the ICSD‑3 [21], which includes the “inability to stay (awake and) alert during the major waking episodes of the day.”
The findings of the current study underlined the importance of daytime sleepiness in the clinical presentation of RLS. Daytime sleepiness corresponds with reduced quality of life and health status as well as personal, social, and occupational functioning. It may also affect driving skills and road safety [19]. This is reflected by lower performance on the MCT, which is sensitive to a decreased fitness to drive according to Schwarz et al. [14].
There are several limitations to the present study. First, only 29 patients were included, which may not be sufficient to draw firm conclusions. However, diagnoses were carefully established via clinical interviews, and concomitant or confounding diagnoses were excluded. Therefore, it is unlikely that RLS mimics were included. In addition, the majority of patients had severe RLS symptoms, which adds weight to our findings.
Sex was not evenly distributed within the sample, with only eight women participating. Thus, the sample did not adequately reflect the distribution of RLS in the general population, showing a higher prevalence in women than in men [2]. Therefore, the results should be interpreted with caution and not be used to make definite recommendations for treatment and diagnosis. Nevertheless, this study highlights a neglected problem, and future studies with larger numbers of participants, including a higher proportion of women, may provide further crucial insight.
As all participants with RLS in the current study were newly diagnosed and treatment naïve, future research could additionally evaluate daytime sleepiness as a patient-reported outcome measure of RLS treatment. As suggested by Song et al. [16], Kallweit et al. [10], and Kallweit et al. [9], improvement in RLS symptoms should, in most cases, lead to a reduction in daytime sleepiness, except in cases where dopaminergic treatment causes sleepiness as a side effect [9]. The ESS is an easily and quickly administered, widely used, and accepted assessment tool for measuring daytime sleepiness, and it would be beneficial to incorporate it into diagnostic routines for RLS when objective testing of sustained attention is not feasible in a clinical setting.
Conclusion
RLS is commonly associated with low sleep quality, compromised mental and physical health, and decreased quality of life; these effects were confirmed in the current study. Additionally, the increased prevalence of excessive daytime sleepiness in patients with RLS was supported by new empirical evidence. Patients with RLS reported increased general daytime sleepiness as well as higher current sleepiness during the assessment in comparison to controls. This was confirmed on a physiological level by an increase in pupil size variation in the pupillographic sleepiness test. Moreover, patients performed worse on sustained attention tasks sensitive to increased sleepiness.
These findings demonstrate the potential benefits of incorporating daytime sleepiness as a potential symptom of RLS into diagnostic processes and treatment. Daytime sleepiness is closely associated with lower quality of life and impaired daily functioning. Further research is necessary to determine the generalizability of the findings to the wider patient population and to establish whether treating RLS also improves daytime sleepiness.
Practical conclusion
-
Excessive daytime sleepiness is a common symptom of restless legs syndrome (RLS) and associated with impaired quality of life and daily functioning.
-
Patients diagnosed with RLS should always be evaluated for excessive daytime sleepiness.
-
Managing RLS requires addressing sleep disturbances to alleviate daytime sleepiness and improve quality of life.
-
Treatment for RLS should target excessive daytime sleepiness.
Data availability statement
Study data are available from the corresponding author upon reasonable request.
References
Allen RP, Picchietti DL, Garcia-Borreguero D, Ondo WG, Walters AS, Winkelman JW, … & International Restless Legs Syndrome Study Group. (2014) Restless legs syndrome/Willis—Ekbom disease diagnostic criteria: updated International Restless Legs Syndrome Study Group (IRLSSG) consensus criteria-history, rationale, description, and significance. Sleep Med 15(8):860–873
Broström A, Alimoradi Z, Lind J, Ulander M, Lundin F, Pakpour A (2023) Worldwide estimation of restless legs syndrome: a systematic review and meta-analysis of prevalence in the general adult population. J Sleep Res 32(3):e13783. https://doi.org/10.1111/jsr.13783
Bullinger M (2000) Erfassung der gesundheitsbezogenen Lebensqualität mit dem SF-36-Health Survey. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 43(3):190–197. https://doi.org/10.1007/s001030050034
Buysse DJ, Reynolds CF III, Monk TH, Berman SR, Kupfer DJ (1989) The pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res 28:193–213
Dinges DF (1995) An overview of sleepiness and accidents. J Sleep Res 4(S2):4–14. https://doi.org/10.1111/j.1365-2869.1995.tb00220.x
Fulda S, Wetter TC (2007) Is daytime sleepiness a neglected problem in patients with restless legs syndrome? Mov Disord 22(Suppl 18):S409–S413. https://doi.org/10.1002/mds.21511
Hoddes E, Dement WC, Zarcone V (1972) The development and use of the Stanford Sleepiness Scale (SSS). Psychophysiology 10:431–436
Johns MW (1991) A new method for measuring daytime sleepiness: the epworth sleepiness scale. Sleep 14(6):540–545. https://doi.org/10.1093/sleep/14.6.540
Kallweit U, Khatami R, Pizza F, Mathis J, Bassetti CL (2010) Dopaminergic treatment in idiopathic restless legs syndrome: effects on subjective sleepiness. Clin Neuropharmacol 33:276–278
Kallweit U, Siccoli MM, Poryazova R, Werth E, Bassetti CL (2009) Excessive daytime sleepiness in idiopathic restless legs syndrome: characteristics and evolution under dopaminergic treatment. Eur Neurol 62(3):176–179
Kipman U, Fritz A (2014) VIGIL. Vigilanz. Psychologische Diagnostik von Aufmerksamkeits- und Konzentrationsfähigkeit im Kindergarten- und Schulalter. Eigenverlag: Österreichisches Zentrum für Begabtenförderung und Begabungsforschung
Sander C, Hegerl U, Wirkner K, Walter N, Kocalevent RD, Petrowski K, Glaesmer H, Hinz A (2016) Normative values of the Epworth Sleepiness Scale (ESS), derived from a large German sample. Sleep Breath 20(4):1337–1345
Sauerbier A, Sivakumar C, Klingelhoefer L, Martinez-Martin P, Perkins L, Inniss R, Rizos A, Trivedi D, Leta V, Wan YM, Parry M, van Wamelen D, Reichmann H, Chaudhuri KR (2019) Restless legs syndrome—the under-recognised non-motor burden: a questionnaire-based cohort study. Postgrad Med 131(7):473–478. https://doi.org/10.1080/00325481.2019.1658506
Schwarz JFA, Geisler P, Hajak G, Zulley J, Rupprecht R, Wetter TC, Popp RFJ (2016) The effect of partial sleep deprivation on computer-based measures of fitness to drive. Sleep Breath = Schlaf Atm 20(1):285–292. https://doi.org/10.1007/s11325-015-1220-0
Schulz H, Volk S, Yassouridis A (1991) Measuring tiredness by symptoms. Sleep Med Res 20:515
Song P, Park HS, Joo EY, Hong SB (2011) How do restless legs syndrome patients recognize daytime sleepiness?—The multiple sleep latency test. Sleep Med Res 2(3):102–106. https://doi.org/10.17241/smr.2011.2.3.102
Stepanski E, Lamphere J, Badia P, Zorick F, Roth T (1984) Sleep fragmentation and daytime sleepiness. Sleep 7(1):18–26
Walters AS, LeBrocq C, Dhar A, Hening W, Rosen R, Allen RP, Trenkwalder C (2003) Validation of the International Restless Legs Syndrome Study Group rating scale for restless legs syndrome. Sleep Med 4(2):121–132. https://doi.org/10.1016/S1389-9457(02)00258-7
Ward KL, Hillman DR, James A, Bremner AP, Simpson L, Cooper MN, Palmer LJ, Fedson AC, Mukherjee S (2013) Excessive daytime sleepiness increases the risk of motor vehicle crash in obstructive sleep apnea. J Clin Sleep Med 9(10):1013–1021. https://doi.org/10.5664/jcsm.3072
Mallon, L., Broman, J. E., & Hetta, J. (2008). Restless legs symptoms with sleepiness in relation to mortality: 20‐year follow‐up study of a middle‐aged Swedish population. Psychiatry and clinical neurosciences, 62(4), 457-463.
American Academy of Sleep Medicine (2023) International classification of sleep disorders, 3rd edn. American Academy of Sleep Medicine, Darien, IL
Weber FC, Danker-Hopfe H, Dogan-Sander E, Frase L, Hansel A, Mauche N, Mikutta C, Nemeth D, Richter K, Schilling C, Sebestova M, Spath MM, Nissen C, Wetter TC (2022) Restless legs syndrome prevalence and clinical correlates among psychiatric inpatients: a multicenter study. Front Psychiatry 13:846165. https://doi.org/10.3389/fpsyt.2022.846165
Wilhelm B, Korner A, Heldmaier K, Moll K, Wilhelm H, Lüdtke H (2001) Normwerte des pupillographischen Schläfrigkeitstests für Frauen und Männer zwischen 20 und 60 Jahren. Normal Values of the Pupillographic Sleepiness Test in Male and Female Subjects Aged 20 to 60 Years. Somnologie 5(3):115–120
Winkelman JW, Redline S, Baldwin CM, Resnick HE, Newman AB, Gottlieb DJ (2009) Polysomnographic and health-related quality of life correlates of restless legs syndrome in the sleep heart health study. Sleep 32:772–778
World Health Organization (2022) International Classification of Diseases Eleventh Revision (ICD-11). Geneva
Acknowledgements
We are grateful to Alexander S. Chockley for English language editing. We also thank Christiane Hirn for clinical assessment of the patients and Michael Bauer for data management and statistical support.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
Conception and design of the study: RFJP, GH, PG; data acquisition: TCW, GH, PG, RFJP; analysis of data: AE, JO, RFJP; first manuscript draft: AE, RFJP. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
A.-L. Eich, J. Ottersbach, P. Geisler, G. Hajak, T.C. Wetter and R. F. Popp declare that they have no competing interests.
For this article no studies with human participants or animals were performed by any of the authors. All studies mentioned were in accordance with the ethical standards indicated in each case.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.

Scan QR code & read article online
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Eich, AL., Ottersbach, J., Geisler, P. et al. Daytime sleepiness in patients with untreated restless legs syndrome. Somnologie 28, 149–155 (2024). https://doi.org/10.1007/s11818-024-00455-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11818-024-00455-6