Abstract
Heat stress is a major environmental stress that affects the growth and development of plants. Korean fir (Abies koreana), a rare species endemic to South Korea, is sensitive to global climate change. The effect of exogenous methyl jasmonate (MeJA) on heat stress tolerance was, therefore, investigated in this species. During heat stress, the expression levels of eight genes (AkNAC19, AkMPK6, AkERF4, AkEFP, AkNAC2, AkbHLH, AkHSP17.6, and AkMYB123) were assessed in needles of A. koreana following treatment with 0, 0.1, 1.0, or 2.0 mM MeJA. Optimal upregulation of expression of most genes was observed 24 h post-treatment with 2.0 mM MeJA. Similar results were obtained when gene expression was analyzed 1, 2, 4, and 8 days post-treatment with 2.0 mM MeJA. Under heat stress conditions, plants treated with 2.0 mM MeJA initially showed a rapid decline in electrolyte leakage and higher chlorophyll content after 28 days of heat stress; however, opposite trends were observed in untreated plants, indicating that MeJA mediated tolerance to heat stress. Higher levels of expression of AkERF4, AkNAC2, and AkHSP17.6 were observed in MeJA-treated needles than in untreated needles, indicating these genes were strongly associated with MeJA-mediated heat tolerance. Therefore, these results suggest that the ability of Korean fir to tolerate abiotic stress is associated with endogenous MeJA synthesis or signaling, and identifies AkERF4, AkNAC2, and AkHSP17.6 as potential candidates for genes involved in the stress-tolerance mechanism.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As a result of climate change, observations indicate worldwide increases in temperature and more frequent extreme heat waves. These events pose a threat to plant growth and ecosystems as heat stress damages cell membranes, inactivates enzymes, inhibits photosynthesis, and enhances respiration (Janni et al. 2020; Li et al. 2021; Zhao et al. 2021). The phytohormones, jasmonic acid (JA), and methyl jasmonic acid (MeJA), collectively known as jasmonates, are induced in response to heat stress and act as signaling molecules in stress signaling pathways, enabling plants to adapt to stressful environments (Creelman and Mullet 1995; Clarke et al. 2009; Howe et al. 2018; Wang et al. 2018, 2020; Kanagendran et al. 2019; Su et al. 2021; Nie et al. 2022). The application of exogenous MeJA has demonstrated the role played by MeJA in alleviating heat stress injury in many plant species (Clarke et al. 2009; Pan et al. 2019; Su et al. 2021; Nie et al. 2022). Exogenous MeJA significantly improves heat tolerance in perennial ryegrass (Lolium perenne) by altering osmotic adjustment, antioxidant defenses, and JA-responsive gene expression (Su et al. 2021), and by mediating gene expression across various pathways (Nie et al. 2022). Similarly, application of exogenous JA rescues heat stress-induced stigma secretion (Pan et al. 2019). Clarke et al. (2009) observed that, in Arabidopsis thaliana, exogenous MeJA protects cell membranes from heat stress and improved heat resistance. Moreover, exogenous JA rapidly and dynamically regulates expression of genes involved in plant defense mechanisms under abiotic stress. For example, JA regulates expression of NAC family transcription factors (TFs), which play vital roles in promoting plant adaptations to abiotic stress (Olsen et al. 2005; Jeong et al. 2010; Sun et al. 2013; Zhou et al. 2013; Liang et al. 2014). In addition, upregulation of LpNAC037, LpNAC045, and LpNAC054 occurs in exogenous MeJA-treated leaves of perennial ryegrass under heat stress (Su et al. 2021), upregulation of ethylene response factor (ERF) has been observed in MeJA-treated kiwi fruit under heat stress, and expression of bHLH, MYB, and MPK increases following treatment with exogenous MeJA in various plant species (Yue et al. 2012; Zhao et al. 2013; Pan et al. 2019; Li et al. 2020; Wu et al. 2023). Heat shock proteins (HSPs), controlled by heat stress transcription factors, play a central role in the heat stress response (Kotak et al. 2007; Scharf et al. 2012). Overexpression of Hsp70 improves the tolerance to heat stress of Arabidopsis thaliana (Koizumi et al. 2014), Capsicum annuum (Guo et al. 2014; Usman et al. 2015), and Oryza sativa (Wang et al. 2014). Application of exogenous MeJA under heat stress conditions leads to upregulation of HSP70 and HSP90 in opium poppy (Gurkok et al. 2015) and heat shock factors (Wang et al. 2016) and LpHsp010 (Su et al. 2021) in perennial ryegrass.
Transcriptomic analysis has shown that genes including NAC (AkNAC19 and AkNAC2), MPK (AkMPK6), ERF (AkERF4), EFP (AkEFP), bHLH (AkbHLH), HSP (AkHSP17.6), and MYB (AkMYB123), are upregulated in Abies koreana in response to heat stress (Hwang et al. 2018). Upregulation of these genes in other plant species under heat stress or other abiotic stresses is associated with exogenous JA treatment or endogenous JA synthesis, as described above. It remains unknown, however, whether upregulation of these genes under heat stress is associated with endogenous JA synthesis and signaling in A. koreana, as previous studies have not addressed the effects of exogenous JA on transcriptional regulation during heat stress in this species. We therefore investigated whether MeJA treatment led to the upregulation of these genes in A. koreana. In addition, we evaluated gene expression, electrolyte leakage, and chlorophyll content to determine whether MeJA treatment alleviated the adverse effects of heat stress in Korean fir.
Materials and methods
Plant materials
Plants used in this study were 5-year-old Korean fir (Abies koreana) obtained from the Plant Conservation Center, National Park Institute for Wildlife Conservation, Korea National Park Service. The pots containing seedlings were moved to a growth room within the laboratory and plants were allowed to adapt to growth conditions of 200 µmol/m2 s light intensity, 20 °C temperature, 60% relative humidity, and 16-h photoperiod for 1 month. Plants were then transferred to a plant growth chamber and exposed to a light intensity of 120 µmol/m2 s with other environmental parameters remaining the same.
Treatment of exogenous MeJA
MeJA solutions of 0.1, 1.0, and 2.0 mM were prepared using 0.1% ethanol and 0.05% Tween 20 (Bioshop, Canada, USA). Needles (leaves) of A. koreana plants were dipped in MeJA solution for 1 min. The dipping was performed twice at 5 min intervals. Needles were dipped in distilled water containing 0.05% Tween 20 as a control treatment. During dipping, the pots, but not the plants, were covered with a net to prevent soil loss during inversion. This net was removed after dipping and each plant was covered with a plastic cup for 24 h to prevent evaporation of the MeJA solution. The plants were then returned to the growth chamber. Each treatment was replicated three times and each replicate contained five plants. Samples of needles were collected from MeJA-treated and control plants 24 and 48 h post-treatment and frozen in liquid nitrogen prior to RNA extraction.
RNA extraction and gene expression analysis
Following Avelar Carpinetti et al. (2021), total RNA was extracted from samples with slight modifications. The frozen samples were ground to powder using a bead beater (Retsch MM400, Germany) and 100 mg of the powder was added to 0.9 mL extraction buffer solution [100 mM Tris–HCl (pH 8.0), 10% CTAB, 25 mM EDTA (pH 8.0), 2 M NaCl, 0.02% spermidine trihydrochloride, and 2% β-mercaptoethanol, warmed to 60 ℃]. The samples were mixed well, incubated for 10 min at 60 °C, and 0.9 mL chloroform: isoamyl alcohol (24:1) was added. After centrifugation at 7000 × g for 20 min at 4 °C, 0.6 mL of the supernatant was transferred to a 1.5 mL tube containing 0.9 mL of chloroform: isoamyl alcohol (24:1), and the centrifugation was repeated. Next, the supernatant was transferred to a new tube containing a half volume of 5 M LiCl. The tubes were incubated for 4 h at −20 ℃ and centrifuged at 16,000 × g for 30 min at 4 °C. The RNA pellet was washed three times with 75% ethanol and then dried. The RNA was dissolved in nuclease-free water prior to complementary DNA (cDNA) synthesis.
cDNA was synthesized from 1.0 μg RNA using the ReverTra Ace kit (Toyobo, Japan) according to the manufacturer’s instructions. qRT-PCR analysis was performed as described in Hwang et al. (2018, 2019). The expression levels of AkNAC19, AkMPK6, AkERF4, AkEFP, AkNAC2, AkbHLH, AkHSP17.6, and AkMYB123 were measured in needles relative to the expression of the AkACTIN reference gene using the CFX 96 real-time PCR system (Bio-Rad, USA). Relative gene expression levels were calculated using the quantitative comparative CT (DDCT or ∆∆CT) method. The primers used to amplify the genes of interest (AkNAC19, AkMPK6, AkERF4, AkEFP, AkNAC2, AkbHLH, AkHSP17.6, and AkMYB123) and AkACTIN are listed in Table 1. The analyses were repeated three times for all samples.
Analysis of gene expression at different time points after treatment with exogenous MeJA
Needles of 5-year-old plants were dipped in 2.0 mM MeJA and distilled water containing 0.05% Tween 20 was used as a control treatment. Each treatment was replicated three times and each replicate contained five plants. Samples of MeJA-treated needles were collected 1, 2, 4, and 8 days post-treatment for RNA extraction. RNA was also extracted from control samples 1 day post-treatment. Total RNA extraction, cDNA synthesis, and gene expression analysis were performed as described previously (Hwang et al. 2018).
Treatments of exogenous MeJA and heat stress
Five-year-old plants were dipped in 2.0 mM MeJA solution, as described above and plants dipped in distilled water containing 0.05% Tween 20 were used as untreated controls (no treatment; NT). To determine whether MeJA played a role in alleviating heat stress, plants were placed in growth chambers at either 20 °C (normal condition) or 30 °C (heat stress condition) and 60% RH and 16 h photoperiod. Each treatment was replicated three times and each replicate contained five plants. The electrolyte leakage percentage and chlorophyll content (chlorophyll a and chlorophyll b) were assessed in needles after 28 days of heat stress. In addition, needle samples were collected for RNA extraction after 3 and 28 days of heat stress. Total RNA extraction, cDNA synthesis, and gene expression analysis were performed as described above.
Measurement of electrolyte leakage
To determine electrolyte leakage, needle samples (~3.0 g) were immersed in 30 mL of deionized sterile water in a 50 mL conical tube and maintained in the dark at 25 °C for 24 h before the initial electrical conductivities (EC1 and W1) were evaluated. The conical tube was then incubated at 121 °C for 5 min and cooled to 25 °C prior to measurement of the final electrical conductivities (EC2, W2).
Electrolyte leakages (EL) were calculated using the following equation (Binder and Fielder 1995):
Measurement of chlorophyll content
Fresh needles, frozen in liquid nitrogen, were ground into powder (100 mg each) for total chlorophyll extraction, 1.0 mL of 80% Aceton (aceton:DW 8:2, v/v) (Sigma-Aldrich, USA) was added, and the samples were mixed well prior to centrifugation at 13,000 rpm for 5 min at 4 ℃. The supernatants were transferred to 96-well plates and their optical density was measured at 663 and 646 nm. The measurement was repeated three times. Chlorophyll a and chlorophyll b contents were calculated using the following equations (Lichtenthaler and Wellburn 1983):
Results
Role of exogenous MeJA in regulation of stress-responsive genes
Transcriptional upregulation in response to heat stress of the stress-responsive genes (AkNAC19, AkMPK6, AkERF4, AkEFP, AkNAC2, AkbHLH, AkHSP17.6, and AkMYB123) has been observed in A. koreana (Hwang et al. 2018). To investigate whether these genes were also upregulated following application of exogenous MeJA, A. koreana needles were treated with different concentrations of MeJA. Needles were harvested 24 and 48 h after treatment for analysis of gene expression levels. Gene expression in plants treated with 0.1 mM MeJA did not differ significantly from that in the control group at either 24 h or 48 h post-treatment (Fig. 1). Gene expression was noticeably upregulated following treatment with higher concentrations of MeJA (1.0 and 2.0 mM). However, although the expression levels of AkNAC19, AkERF4, AkEFP, AkNAC2, AkbHLH and AkHSP17.6 were highest 24 h after treatment with 2.0 mM MeJA. AkMPK6 expression did not differ significantly between these treatments (Fig. 1). The expression levels of most genes, other than AkNAC2 and AkHSP17.6, had declined by 48 h post-treatment, particularly following treatment with 2.0 mM MeJA. Upregulation of genes other than AkHSP17.6 was not observed 48 h post-treatment with other MeJA concentrations. Overall, upregulation of gene expression was associated with MeJA application, although the expression level varied with concentration and time point. As six out of the eight genes were strongly upregulated 24 h after treatment with 2.0 mM MeJA, this concentration was selected for further experiments.
Expression levels of the investigated genes in A. koreana needles 24 and 48 h post-treatment with different concentrations of MeJA. Data represent means of three replicates. The error bars show standard error of the mean. Statistically significant differences relative to each control of 24 and 48 h post-treatment are indicated using asterisks (*p < 0.05, **p < 0.01, ***p < 0.001; Student’s t test). Con: distilled water-treated control
Next, needles were treated with either 2.0 mM MeJA or distilled water containing 0.05% Tween 20 as a control. Gene expression levels in MeJA-treated needles were assessed at 1, 2, 4, and 8 days post-treatment and in control needles after 1 day. Gene expression was higher in MeJA-treated needles than in the control group, although the level of expression varied across time points (Fig. 2). Expression of all genes other than AkHSP17.6 was highest at 1 day post-treatment, consistent with the results of the first experiment. A strong or slight decline in expression of AkNAC19, AkERF4, AkEFP, AkNAC2, and AkMYB123 was observed over time. By contrast, expression of AkMPK6, AkbHLH, and AkHSP17.6 did not depend on time point. AkbHLH expression in treated needles at 2, 4, and 8 days post-treatment was lower than in the control and, at 4 days post-treatment, AkHSP17.6 expression in treated needles was also lower than in the control, although expression of this gene peaked 8 days post-treatment. These results suggested that treatment with 2.0 mM MeJA was associated with strongly upregulated expression of the selected genes in A. koreana, particularly during the first day with expression levels declining thereafter.
Expression of the investigated genes in A. koreana needles 1, 2, 4, and 8 days post-treatment with 2.0 mM MeJA. Data represent means of three replicates. The error bars show standard error of the mean. Statistically significant differences relative to control are indicated using asterisks (*p < 0.05, **p < 0.01, ***p < 0.001; Student’s t test). Con: distilled water-treated control
Role of exogenous MeJA in alleviating heat stress
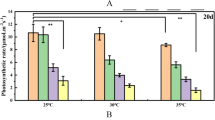
To determine whether treatment with exogenous MeJA alleviated the adverse effects of heat stress in A. koreana, plants treated with 2.0 mM MeJA (MJ) and untreated controls (NT) were grown for 28 days in growth chambers set either at 30 °C to induce heat stress or at 20 °C to provide a non-stressed environment (Fig. 3a). In addition, EL and chlorophyll content both play vital roles in the adaptation of plants to heat stress. EL in the needles was assessed after 28 days. Similar EL percentages were observed in the MeJA-treated and NT plants under non-stress conditions. Under heat stress conditions, however, although both treated and NT plants showed significantly elevated EL, the percentage of EL in NT plants was significantly higher than in MeJA-treated plants (Fig. 3b).
Comparison of changes in A. koreana needles treated with 2.0 mM MeJA (MJ) or distilled water (NT) after exposure to 28 days of non-stress (20 °C) or heat stress (30 °C) conditions. a Phenotypes of A. koreana treated with 2.0 mM MeJA (MJ) or distilled water (NT) after exposure to 28 days of non-stress (20 °C) or heat stress (30 °C) conditions. b Electrolyte leakage assay. c, d Analysis of chlorophyll contents. Data represent means of three replicates. The error bars show standard error of the mean. Statistically significant differences relative to controls (20 °C or 30 °C) are indicated using asterisks [*p < 0.05, **p < 0.01, ***p < 0.001 (20 °C) and ##p < 0.01, ###p < 0.001 (30 °C); Student’s t test]
In addition, the chlorophyll content [chlorophyll a (Chl a) and chlorophyll b (Chl b) was measured after 28 days. At 20 °C, the Chl a content in NT needles was slightly higher than that in MeJA-treated needles, whereas the opposite result was observed for Chl b. Although a significant decline in chlorophyll content was observed in both groups at 30 °C, levels of Chl a and Chl b in MeJA-treated needles were significantly or slightly higher than those in NT needles (Fig. 3c, d). These results suggested that MeJA treatment alleviated the adverse effects of heat stress in A. koreana by reducing the EL percentage and maintaining the chlorophyll content.
Expression of stress-responsive genes in MeJA-treated needles under heat stress
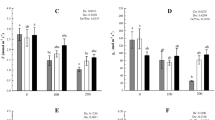
The previous experiment showed that MeJA treatment reduced the adverse effects of heat stress in A. koreana. We, therefore, evaluated levels of expression of stress-responsive genes in MeJA-treated and NT needles after 3 and 28 days of heat stress (30 °C), respectively (Figs. 4, 5). After 3 days in unstressed conditions (20 °C), the expression levels of all genes except AkNAC19 were similar in both MeJA-treated and NT needles; AkNAC19 expression was higher in MeJA-treated needles than in NT needles. At 30 °C, however, we observed that expression of all genes except AkMPK6 was upregulated after 3 days. Most genes were expressed at significantly higher levels in MeJA-treated needles than in NT needles after 3 days under heat stress conditions; the exception was AkEFP, which was expressed at a higher level in NT needles than in MeJA-treated needles (Fig. 4).
Expression levels of the investigated genes in A. koreana needles treated with 2.0 mM MeJA (MJ) or distilled water (NT) after exposure to 3 days of non-stress (20 °C) or heat stress (30 °C) conditions. Data represent means of three replicates. The error bars show standard error of the mean. Statistically significant differences relative to controls (20 °C or 30 °C) are indicated using asterisks [*p < 0.05, **p < 0.01 (20 °C) and #p < 0.05, ##p < 0.01, ###p < 0.001 (30 °C); Student’s t test]
Expression levels of the investigated genes in A. koreana needles treated with 2.0 mM MeJA (MJ) or distilled water (NT) after exposure to 28 days of non-stress (20 °C) or heat stress (30 °C) conditions. Data represent means of three replicates. The error bars show standard error of the mean. Statistically significant differences relative to controls (20 °C or 30 °C) are indicated using asterisks [*p < 0.05, **p < 0.01, ***p < 0.001 (20 °C) and #p < 0.05, ##p < 0.01, ###p < 0.001 (30 °C); Student’s t test]
After 28 days at 20 °C, the expression levels of all genes except AkNAC19 and AkbHLH were similar in MeJA-treated and NT needles. The result resembled that obtained after 3 days. After 28 days at 30 °C, however, AkERF4, AkNAC2, and AkHSP17.6 showed elevated levels of expression, and expression of these genes was significantly higher in MeJA-treated needles than in NT needles. By contrast, after 28 days of heat stress, expression of AkEFP and AkMYB123 was strongly elevated in NT needles to levels that were significantly higher than in MeJA-treated needles (Fig. 5). Overall, the genes investigated in this study were upregulated under heat stress conditions in MeJA-treated and NT plants. Upregulation of AkERF4, AkNAC2, and AkHSP17.6 was observed in MeJA-treated and NT needles soon after the onset of heat stress (day 3) as well as after an extended period of heat stress (day 28), although expression levels were higher at the earlier time point. This suggested that application of exogenous MeJA strongly upregulated expression of most genes throughout the duration of heat stress conditions.
Discussion
MeJA production is induced in plants exposed to heat stress as MeJA plays a role in enabling plants to adapt to heat stress conditions (Clarke et al. 2009; Howe et al. 2018; Wang et al. 2018, 2020; Kanagendran et al. 2019; Su et al. 2021; Nie et al. 2022). Hwang et al. (2018) reported that eight stress-induced genes (AkNAC19, AkMPK6, AkERF4, AkEFP, AkNAC2, AkbHLH, AkHSP17.6, and AkMYB123) were induced in A. koreana in response to heat stress and involved in the heat stress-tolerance mechanism. It remained unclear, however, whether these stress-induced genes were associated with MeJA production. We, therefore, designed the present study to investigate whether application of exogenous MeJA led to upregulation of these eight genes and to assess the role of MeJA in alleviating the deleterious effects of heat stress in A. koreana.
Here, treatment with MeJA led to upregulated expression of the stress-induced genes. Treatment with 0.1 mM MeJA was insufficient to upregulate gene expression, however, as no difference was observed between plants treated with this low concentration and untreated controls. Significant upregulation of gene expression was observed in plants treated with 1.0 or 2.0 mM MeJA with the higher concentration producing the greater effect (Fig. 1). Shahzad et al. (2015) reported that 0.1 mM or 0.2 mM MeJA enabled pea plants to adapt to heat stress and that high concentrations of MeJA promoted plant defense mechanisms, enabling adaptation to high temperature (40 °C) stress. Su et al. (2021) observed that treatment with 0.1 mM MeJA produced the most favorable effect on the heat tolerance of perennial ryegrass. This may be because MeJA causes strong upregulation of stress-responsive genes, thereby alleviating the deleterious effects of heat stress on plant growth. Rahman et al. (2022) observed that treatment of grapevine leaves with 100 µM MeJA led to significant upregulation of genes involved in metabolic pathways and stress-tolerance mechanisms. Treatment with 100 µM MeJA was associated with increased expression of MYB4 and MYB88 in Glycyrrhiza uralensis (Li et al. 2020), and ERF TFs in California poppy (Yamada et al. 2020) and tomato fruit (Yu et al. 2018). In addition, strong upregulation of the MPK was observed in Populus trichocarpa exposed to 0.5 mM MeJA (Wu et al. 2023).
The concentration of MeJA required to upregulate stress-induced gene expression in A. koreana differed from those used in the studies cited above, as did plant responses to stress. Although treatment with 0.1 mM MeJA led to upregulated gene expression in other plants, up to 2.0 mM MeJA was required in A. koreana. This may be because the physiological status of the plants, as well as the level of endogenous MeJA, differed between studies and species. In addition, the needles of A. koreana are thicker and more rigid in structure and texture than the leaves of the plant species studied previously, which may limit the effective absorption when needles are treated with low concentrations of MeJA. We found that the level of upregulation of gene expression following MeJA treatment depended on the time of testing, as the expression levels of most genes peaked at 24 h post-treatment and had declined by 48 h (Fig. 1). It is likely that MeJA upregulated most genes early in the post-treatment period. Rahman et al (2022) observed that the flavonoid genes F3H and PAL were strongly upregulated 24 h after MeJA treatment, but their expression levels declined by 48 h. Gurkok et al. (2015) also reported that HSP70 and HSP90 were upregulated in opium poppy 3 and 12 h after MeJA treatment. To validate our results, levels of gene expression were measured in A. koreana needles 0, 1, 2, 4, and 8 days after treatment with 2.0 mM MeJA (Fig. 2). Consistent with earlier studies, expression of most genes was highest 1 day (24 h) after treatment, confirming that gene expression in A. koreana was strongly upregulated by exogenous MeJA early in the post-treatment period.
EL and chlorophyll content indicate the level of injury caused to plants by abiotic stress. Higher EL percentages and decreased chlorophyll content are both negatively associated with plant growth. An elevated EL percentage and a decline in chlorophyll content were observed in both MeJA-treated and NT plants under heat stress in this study. Such heat stress-induced damage has been previously reported in many plant species (Zhou and Leul 1999; Hu et al. 2020; Liu and Huang 2000; Su et al. 2021). Treatment with 2.0 mM MeJA alleviated the deleterious effects of heat stress in A. koreana, as lower EL percentages were observed under heat stress conditions in MeJA-treated plants than in NT plants (Fig. 3b). Similarly, the chlorophyll content in MeJA-treated plants was higher than that in NT plants (Fig. 3c, d). Such MeJA-mediated alleviation of heat stress injuries has been reported previously (Hu et al. 2013; Shahzad et al. 2015; Bertini et al. 2019; Su et al. 2021; Nie et al. 2022). MeJA protects plants from photosynthetic damage by maintaining chlorophyll content (Bertini et al. 2019) and by increasing expression of chlorophyll biosynthesis genes under heat stress (Nie et al. 2022). Su et al (2021) reported that MeJA could maintain chlorophyll content and decrease EL in perennial ryegrass leaves under heat stress. In addition, MeJA-associated reduction in EL has been observed in Arabidopsis thaliana and pea plants under heat stress (Hu et al. 2013; Shahzad et al. 2015). An increase in chlorophyll and carotenoid contents, soluble proteins, flavonoids, lignin, and enzymatic antioxidants, which play a crucial role in stress tolerance, was also recently reported in MeJA-treated leaves of Isatis indigotica (Liu et al. 2022).
To clarify whether MeJA-mediated heat stress alleviation was associated with expression of AkNAC19, AkMPK6, AkERF4, AkEFP, AkNAC2, AkbHLH, AkHSP17.6, and AkMYB123, the expression levels of these genes were assessed in MeJA-treated and NT needles under heat stress (30 °C) and non-stress (20 °C) conditions (Figs. 4, 5). All genes except AkMPK6 showed upregulated expression after 3 days of heat stress. Moreover, gene expression levels in MeJA-treated needles were higher than those in NT leaves. These results indicated not only that these genes were involved in heat stress tolerance but also that their expression was upregulated by MeJA (Fig. 4). After exposure to heat stress for 28 days, we observed elevated expression of AkERF4, AkNAC2, and AkHSP17.6. Again, expression of these genes was significantly higher in the MeJA-treated needles than in NT needles. Expression levels of AkNAC19 and AkMPK6 decreased in both MeJA-treated and NT needles (Fig. 5). Therefore, the greater tolerance of heat stress shown by MeJA-treated plants may result from upregulated expression of AkERF4, AkNAC2, and AkHSP17.6, both early and late in the period of exposure to heat stress. These three genes are strong candidates for genes that act to alleviate the deleterious effects of heat stress. In addition, AkMPK6 may be involved in the stress-tolerance mechanism at the post-translational modification, as its expression was not upregulated under heat stress conditions in either MeJA-treated or NT needles.
Heat stress-induced upregulation of NAC TFs has been observed in A. koreana, Arabidopsis thaliana, and ryegrass (Morishita et al. 2009; Hwang et al. 2018; Su et al. 2021). Su et al (2021) reported that upregulation of three NAC TFs, LpNAC037, LpNAC045, and LpNAC054, by MeJA enabled ryegrass to tolerate heat stress. Similarly, upregulation of ERF genes during heat stress has been observed in A. koreana, Arabidopsis thaliana, and pak choi (Hsieh et al. 2013; Xu et al. 2016; Hwang et al. 2018). Increased expression of ERF genes in MeJA-treated leaves is consistent with other studies. For instance, Yamada et al. (2020) and Yu et al. (2018) observed that MeJA strongly induced expression of several ERF genes in California poppy and tomato fruit, respectively. More recently, Nie et al. (2022) and Su et al. (2021) have shown that improved tolerance of ryegrass to heat stress is associated with MeJA-induced upregulation of HSP genes. In the current study, 2.0 mM MeJA was required to upregulate genes involved in the stress-tolerance mechanisms of A. koreana. Application of MeJA at this concentration improved plants’ tolerance of heat stress by reducing EL and maintaining chlorophyll content. The beneficial effects of MeJA treatment were strongly associated with upregulation of gene expression, mostly notably of AkERF4, AkNAC2, and AkHSP17.6.
Conclusion
In this study, treatment with exogenous MeJA led to upregulated expression of eight genes (AkNAC19, AkMPK6, AkERF4, AkEFP, AkNAC2, AkbHLH, AkHSP17.6, and AkMYB123) involved in stress-tolerance mechanisms in Abies koreana. A high concentration (2.0 mM) of MeJA was required to upregulate these genes significantly. In addition, the effect of MeJA on gene expression varied over time post-treatment. Expression levels of most genes were highest 1 day post-treatment. Abiotic stresses such as heat shock damage plant cells through increasing EL percentage and reducing chlorophyll content. MeJA treatment alleviated the adverse effects of heat stress on A. koreana by reducing EL and maintaining chlorophyll content. These effects were strongly associated with higher levels of expression of AkERF4, AkNAC2, and AkHSP17.6 in MeJA-treated plants than in the NT plants. This suggested that the ability of A. koreana to tolerate heat stress may be associated with endogenous MeJA synthesis or signaling pathways. In addition, AkERF4, AkNAC2, and AkHSP17.6 are potential candidates for target genes for manipulation to improve the tolerance of A. koreana to heat stress, although further studies are required to validate the functions of these genes in abiotic stress tolerance in this species.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author (H.C. Park) upon reasonable request.
References
Bertini L, Palazzi L, Proietti S, Pollastri S, Arrigoni G, de Laureto PP, Caruso C (2019) Proteomic analysis of MeJa-induced defense responses in rice against wounding. Int J Mol Sci 20:2525. https://doi.org/10.3390/ijms20102525
Binder WD, Fielder P (1995) Seasonal changes in chlorophyll fluorescence of white spruce seedlings from different latitudes in relation to gas exchange and winter storability. New for 11:207–232
Clarke SM, Cristescu SM, Miersch O, Harren FJM, Wasternack C, Mur LAJ (2009) Jasmonates act with salicylic acid to confer basal thermotolerance in Arabidopsis thaliana. New Phytol 182:175–187. https://doi.org/10.1111/j.1469-8137.2008.02735.x
Creelman R, Mullet JE (1995) Jasmonic acid distribution and action in plants: regulation during development and response to biotic and abiotic stress. Proc Natl Acad Sci USA 92:4114–4119. https://doi.org/10.1073/pnas.92.10.4114
Guo M, Zhai Y-F, Lu J-P, Chai L, Chai W-G, Gong Z-H, Lu M-H (2014) Characterization of CaHsp70-1, a pepper heat-shock protein gene in response to heat stress and some regulation exogenous substances in Capsicum annuum L. Int J Mol Sci 15:19741–19759. https://doi.org/10.3390/ijms151119741
Gurkok T, Turktas M, Parmaksiz I, Unver T (2015) Transcriptome profiling of alkaloid biosynthesis in elicitor induced opium poppy. Plant Mol Biol Report 33:673–688. https://doi.org/10.1007/s11105-014-0772-7
Howe GA, Major IT, Koo AJ (2018) Modularity in jasmonate signaling for multistress resilience. Annu Rev Plant Biol 69:387–415. https://doi.org/10.1146/annurev-arplant-042817-040047
Hsieh E-J, Cheng M-C, Lin T-P (2013) Functional characterization of an abiotic stress-inducible transcription factor AtERF53 in Arabidopsis thaliana. Plant Mol Biol 82:223–237. https://doi.org/10.1007/s11103-013-0054-z
Hu Y, Jiang L, Wang F, Yu D (2013) Jasmonate regulates the INDUCER OF CBF expression-C-repeat binding factor/DRE binding factor1 cascade and freezing tolerance in Arabidopsis. Plant Cell 25:2907–2924. https://doi.org/10.1105/tpc.113.112631
Hu S, Ding Y, Zhu C (2020) Sensitivity and responses of chloroplasts to heat stress in plants. Front Plant Sci 11:375. https://doi.org/10.3389/fpls.2020.00375
Hwang JE, Kim YJ, Shin MH, Hyun HJ, Bohnert HJ, Park HC (2018) A comprehensive analysis of the Korean fir (Abies koreana) genes expressed under heat stress using transcriptome analysis. Sci Rep 8:10233. https://doi.org/10.1038/s41598-018-28552-1
Hwang JE, Kim YJ, Jeong DY, Park HC (2019) Transcriptome analysis of Korean fir (Abies koreana) in response to elevated carbon dioxide and high temperature. Plant Biotechnol Rep 13:603–612. https://doi.org/10.1007/s11816-019-00553-0
Janni M, Gullì M, Maestri E, Marmiroli M, Valliyodan B, Nguyen HT, Marmiroli N, Foyer C (2020) Molecular and genetic bases of heat stress responses in crop plants and breeding for increased resilience and productivity. J Exp Bot 71:3780–3802. https://doi.org/10.1093/jxb/eraa034
Jeong JS, Kim YS, Baek KH, Jung H, Ha S-H, Choi YD, Kim M, Reuzeau C, Kim J-K (2010) Root-specific expression of OsNAC10 improves drought tolerance and grain yield in rice under field drought conditions. Plant Physiol 153:185–197. https://doi.org/10.1104/pp.110.154773
Kanagendran A, Chatterjee P, Liu B, Sa T, Pazouki L, Niinemets Ü (2019) Foliage inoculation by Burkholderia vietnamiensis CBMB40 antagonizes methyl jasmonate-mediated stress in Eucalyptus grandis. J Plant Physiol 242:153032. https://doi.org/10.1016/j.jplph.2019.153032
Koizumi S, Ohama N, Mizoi J, Shinozaki K (2014) Functional analysis of the Hikeshi-like protein and its interaction with HSP70 in Arabidopsis. Biochem Biophys Res Commun 450:396–400. https://doi.org/10.1016/j.bbrc.2014.05.128
Kotak S, Larkindale J, Lee U, von Koskull-Döring P, Vierling E, Scharf K-D (2007) Complexity of the heat stress response in plants. Curr Opin Plant Biol 10:310–316. https://doi.org/10.1016/j.pbi.2007.04.011
Li Y, Chen X, Wang J, Zou G, Wang L, Li X (2020) Two responses to MeJA induction of R2R3-MYB transcription factors regulate flavonoid accumulation in Glycyrrhiza uralensis Fisch. PLoS ONE 15:e0236565. https://doi.org/10.1371/journal.pone.0236565
Li N, Euring D, Cha JY, Lin Z, Lu M, Huang L-J, Kim WY (2021) Plant hormone-mediated regulation of heat tolerance in response to global climate change. Front Plant Sci 11:627969. https://doi.org/10.3389/fpls.2020.627969
Liang C, Wang Y, Zhu Y, Tang J, Hu B, Liu L, Ou S, Wu H, Sun X, Chu J, Chu C (2014) OsNAP connects abscisic acid and leaf senescence by fine-tuning abscisic acid biosynthesis and directly targeting senescence-associated genes in rice. Proc Natl Acad Sci USA 111:10013–10018. https://doi.org/10.1073/pnas.1321568111
Lichtenthaler HK, Wellburn AR (1983) Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem Soc Trans 11:591–592. https://doi.org/10.1042/bst0110591
Liu X, Huang B (2000) Heat stress injury in relation to membrane lipid peroxidation in creeping bentgrass. Crop Sci 40:503–510. https://doi.org/10.2135/cropsci2000.402503x
Liu R, Wang Z, Zheng J, Xu Z, Tang X, Huang Z, Zhang N, Dong Y, Li T (2022) The effects of methyl jasmonate on growth, gene expression and metabolite accumulation in Isatis indigotica Fort. Industrial Crops Products 177:114482. https://doi.org/10.1016/j.indcrop.2021.114482
Morishita T, Kojima Y, Maruta T, Nishizawa-Yokoi A, Yabuta Y, Shigeoka S (2009) Arabidopsis NAC transcription factor, ANAC078, regulates flavonoid biosynthesis under high-light. Plant Cell Physiol 50:2210–2222. https://doi.org/10.1093/pcp/pcp159
Nie G, Zhou J, Jiang Y, He J, Wang Y, Liao Z, Appiah C, Li D, Feng G, Huang L, Wang X, Zhang X (2022) Transcriptome characterization of candidate genes for heat tolerance in perennial ryegrass after exogenous methyl jasmonate application. BMC Plant Biol 22:68. https://doi.org/10.1186/s12870-021-03412-9
Olsen AN, Ernst HA, Leggio LL, Skriver K (2005) NAC transcription factors: structurally distinct, functionally diverse. Trends Plant Sci 10:79–87. https://doi.org/10.1016/j.tplants.2004.12.010
Pan C, Yang D, Zhao X, Jiao C, Yan Y, Lamin-Samu AT, Wang Q, Xu X, Fei Z, Lu G (2019) Tomato stigma exsertion induced by high temperature is associated with the jasmonate signalling pathway. Plant Cell Environ 42:1205–1221. https://doi.org/10.1111/pce.13444
Rahman FU, Zhang Y, Khan IA, Liu R, Sun L, Wu Y, Jiang J, Fan X, Liu C (2022) The promoter analysis of VvPR1 gene: a candidate gene identified through transcriptional profiling of methyl jasmonate treated grapevine (Vitis vinifera L.). Plants 11:1540. https://doi.org/10.3390/plants11121540
Scharf K-D, Berberich T, Ebersberger I, Nover L (2012) The plant heat stress transcription factor (Hsf) family: structure, function and evolution. Biochim Biophys Acta 1819:104–119. https://doi.org/10.1016/j.bbagrm.2011.10.002
Shahzad R, Waqas M, Khan AL, Hamayun M, Kang S-M, Lee I-J (2015) Foliar application of methyl jasmonate induced physio-hormonal changes in Pisum sativum under diverse temperature regimes. Plant Physiol Biochem 96:406–416. https://doi.org/10.1016/j.plaphy.2015.08.020
Su Y, Huang Y, Dong X, Wang R, Tang M, Cai J, Chen J, Zhang X, Nie G (2021) Exogenous methyl jasmonate improves heat tolerance of perennial ryegrass through alteration of osmotic adjustment, antioxidant defense, and expression of jasmonic acid-responsive genes. Front Plant Sci 12:664519. https://doi.org/10.3389/fpls.2021.664519
Sun L, Zhang H, Li D, Huang L, Hong Y, Ding XS, Nelson RS, Zhou X, Song F (2013) Functions of rice NAC transcriptional factors, ONAC122 and ONAC131, in defense responses against Magnaporthe grisea. Plant Mol Biol 81:41–56. https://doi.org/10.1007/s11103-012-9981-3
Usman MG, Rafii MY, Ismail MR, Malek MA, Latif MA (2015) Expression of target gene Hsp70 and membrane stability determine heat tolerance in chili pepper. J Am Soc Hortic Sci 140:144–150. https://doi.org/10.21273/JASHS.140.2.144
Wang Y, Lin S, Song Q, Li K, Tao H, Huang J, Chen X, Que S, He H (2014) Genome-wide identification of heat shock proteins (Hsps) and Hsp interactors in rice: Hsp70s as a case study. BMC Genomics 15:344. https://doi.org/10.1186/1471-2164-15-344
Wang Y, Dai Y, Tao X, Wang J-Z, Cheng H-Y, Yang H, Ma X-R (2016) Heat shock factor genes of tall fescue and perennial ryegrass in response to temperature stress by RNA-seq analysis. Front Plant Sci 6:1226. https://doi.org/10.3389/fpls.2015.01226
Wang Y, Xu H, Liu W, Wang N, Qu C, Jiang S, Fang H, Zhang Z, Chen X (2018) Methyl jasmonate enhances apple’ cold tolerance through the JAZ-MYC2 pathway. Plant Cell Tissue Org Culture (PCTOC) 136:75–84. https://doi.org/10.1007/s11240-018-1493-7
Wang J, Song L, Gong X, Xu J, Li M (2020) Functions of jasmonic acid in plant regulation and response to abiotic stress. Int J Mol Sci 21:1446. https://doi.org/10.3390/ijms21041446
Wu J, Liang X, Lin M, Lan Y, Xiang Y, Yan H (2023) Comprehensive analysis of MAPK gene family in Populus trichocarpa and physiological characterization of PtMAPK3-1 in response to MeJA induction. Physiol Plant 175:e13869. https://doi.org/10.1111/ppl.13869
Xu H, Chen L, Song B, Fan X, Yuan X, Chen J (2016) De novo transcriptome sequencing of pakchoi (Brassica rapa L. chinensis) reveals the key genes related to the response of heat stress. Acta Physiol Plant 38:252. https://doi.org/10.1007/s11738-016-2269-5
Yamada Y, Nishida S, Shitan N, Sato F (2020) Genome-wide identification of AP2/ERF transcription factor-encoding genes in California poppy (Eschscholzia californica) and their expression profiles in response to methyl jasmonate. Sci Rep 10:18066. https://doi.org/10.1038/s41598-020-75069-7
Yu W, Zhao R, Sheng J, Shen L (2018) SIERF2 is associated with methyl jasmonate-mediated defense response against Botrytis cinerea in tomato fruit. J Agri Food Chem 66:9923–9932. https://doi.org/10.1021/acs.jafc.8b03971
Yue H, Nie S, Xing D (2012) Over-expression of Arabidopsis Bax inhibitor-1 delays methyl jasmonate-induced leaf senescence by suppressing the activation of MAP kinase 6. J Exp Bot 63:4463–4474. https://doi.org/10.1093/jxb/ers122
Zhao M-L, Wang J-N, Shan W, Fan J-G, Kuang J-F, Wu K-Q, Li X-P, Chen W-X, He F-Y, Chen J-Y, Lu W-J (2013) Induction of jasmonate signaling regulators MaMYC2s and their physical interactions with MaICE1 in methyl jasmonate-induced chilling tolerance in banana fruit. Plant Cell Environ 36:30–51. https://doi.org/10.1111/j.1365-3040.2012.02551.x
Zhao J, Lu Z, Wang L, Jin B (2021) Plant responses to heat stress: physiology, transcription, noncoding RNAs, and epigenetics. Int J Mol Sci 22:117. https://doi.org/10.3390/ijms22010117
Zhou W, Leul M (1999) Uniconazole-induced tolerance of rape plants to heat stress in relation to changes in hormonal levels, enzyme activities and lipid peroxidation. Plant Growth Regul 27:99–104. https://doi.org/10.1023/A:1006165603300
Zhou Y, Huang W, Liu L, Chen T, Zhou F, Lin Y (2013) Identification and functional characterization of a rice NAC gene involved in the regulation of leaf senescence. BMC Plant Biol 13:132. https://doi.org/10.1186/1471-2229-13-132
Acknowledgements
We thank the Plant Conservation Center, National Park Institute for Wildlife Conservation, Korea National Park Service, for providing the five-year-old Korean fir (Abies koreana) used in this study. This work was supported by a grant from the National Institute of Ecology (NIE), funded by the Ministry of Environment (MOE) of the Republic of Korea (NIE-B-2024-15).
Funding
This work was supported by a grant from the National Institute of Ecology (NIE), funded by the Ministry of Environment (MOE) of the Republic of Korea, NIE-B-2024-15, Hyeong Cheol Park.
Author information
Authors and Affiliations
Contributions
DAL and HCP designed, planned, and organized the experiments. DAL and DAP performed the experiments. DAL and HCP analyzed the data. DAL and HCP wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lee, D.Y., Park, D.Y. & Park, H.C. Exogenous methyl jasmonate mediates tolerance of heat stress in Korean fir (Abies koreana). Plant Biotechnol Rep (2024). https://doi.org/10.1007/s11816-024-00912-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11816-024-00912-6