Abstract
The influence of TDZ on adventitious shoot regeneration from leaves of the pear cultivars Conference and Abate Fétel, and the rootstock Farold®87 was investigated. Our main aim was to set up efficient in vitro regeneration protocols for all these pear genotypes by using expanding leaves from elongated shoot cultures as starting plant material. Our best results in terms of percentage of regeneration were achieved by using half-strength Murashige and Skoog basal medium supplemented with 1 μM NAA, combined with 13.5 μM TDZ for Conference (87.3%) and Farold®87 (53.3%), and 9 μM TDZ for Abate Fétel (68%). The impact on leaf organogenesis of the antibiotics timentin, cefotaxime, and carbenicillin, alone or in combination, usually used for the control of Agrobacterium overgrowth, and of kanamycin, commonly used for the selection of putatively transformed plants, were also evaluated to be exploited in future transformation trials. In general, the use of carbenicillin (475 mM), cefotaxime/carbenicillin (210/238 mM) and cefotaxime (630 mM) did not negatively affect the regeneration efficiency of Conference, Abate Fétel and Farold®87, respectively. The use of 4 μM kanamycin should be suitable to select transformed shoots from Abate Fétel and Farold®87 leaves, while a lower concentration or a different selection strategy should be applied for Conference. We report new regeneration and selection protocols usable for the application of new biotechnologies in the genetic improvement of pear cultivars and rootstocks.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Italy is the second-biggest producer of pears in the world, reaching the production of 619.470 tons with 26.600 cultivated hectares in 2020, and standing just behind China (FAOSTAT 2020). In particular, the Emilia Romagna region is the main area of pear cultivation in our country, spreading across different provinces as Reggio Emilia, Ferrara, Modena, Bologna, and Ravenna. Although the improvement of pomological and agronomic traits has been the main aim of European pear breeding programs since several decades, the main focus is currently on increasing adaptability to environmental stresses and resistance against pathogens (Bellini and Nin 2002; Dondini and Sansavini 2012). Generally, standard breeding programs still offer the possibility to release new improved pear cultivars and rootstocks but always with long production times and with the difficulty of solving specific problems of a cultivar without losing its main peculiarities. In fact, conventional breeding is difficult and demands several years because of pear, as perennial fruit species, is characterized by a high level of heterozygosity and a long juvenile period (4–6 years) (Caboni et al. 1999; Rivalta et al. 2002; Prohens et al. 2022). Thus, New Genomic Techniques (NGTs) based on the use of different biotechnological tools offer the possibility to improve, in shorter time, the existing and well-known pear commercial rootstocks and cultivars by modifying specific traits to obtain plants with higher production capacity and fruits nutritionally improved (Sabbadini et al. 2021). However, the application of new biotechnological tools requires the optimization of protocols specifically conceived to modify the genome of the cultivar or rootstock of interest. In particular, a successful in vitro genome modification strategy, as genetic transformation using Agrobacterium, requires firstly the optimization of suitable and reproducible procedures for in vitro regeneration of unipolar adventitious shoots or bipolar somatic embryos, consisting in the dedifferentiation of the starting tissue and its reorganization by meristem formation (with or without callus as intermediary) (Ricci et al. 2020a, b). In general, genotype, type of starting plant material and composition of in vitro shoot induction medium have a great impact on adventitious shoot organogenesis, and on the recovery of putatively transformed plants. Juvenile (seed-derived) and adult (somatic) tissues are the two main categories of plant material which may be considered as starting explants for regeneration attempts of transformed lines. For instance, leaf tissue from elongated shoot cultures, represents an optimal source of somatic cells for adventitious cell regeneration and Agrobacterium-mediated genetic transformation procedures. A leaf represents also a true-to-type starting material, differently from those obtained from seedling sources, which is considered of great importance for vegetatively propagated crops. Then, it is well known that many other factors influence the adventitious regeneration of putatively transformed plants, such as antibiotic compounds used for Agrobacterium decontamination in the medium after infection, and selective agents used to distinguish putatively transformed lines and limit the regeneration of non-transformed shoots (escapes) (Sabbadini et al. 2019). There are different selectable markers available but, among these, the antibiotic kanamycin is the most typically used in fruit tree species genetic transformation experiments using Agrobacterium. Potential susceptibility of plant tissues to both decontamination and selective compounds must be taken into consideration in setting up a suitable and efficient procedure to genetically modify a plant of interest.
Although fruit tree species are universally recognized for their recalcitrance in regenerating adventitious shoots (Bell et al. 2012; Ricci et al. 2020a, b), various attempts in terms of pear shoot organogenesis have been successfully performed by using different starting explants such as roots (Viseur 1990), embryonic axes (Browning et al. 1987), cotyledons (Browning et al. 1987), protoplasts (Ochatt and Caso 1986; Ochatt et al. 1988), shoot apices (Lane 1979; Caboni et al. 2000, 2002), anther-derived embryos (Kadota et al. 2002), and leaves (Abu-Qaoud et al. 1991; Bell et al. 2012; Caboni et al. 2000; Chevreau and Leblay 1992; Chevreau et al. 1989; Martinelli et al. 2014; Javadi et al. 2013; Leblay et al. 1991; Poudyal et al. 2008; Predieri et al. 1989; Yousefiara et al. 2014a, b; Zhu 2000; Tang et al. 2008). Referring to the above-mentioned list, many studies described how different factors can have an effect on pear leaf organogenesis, including growth stage of the mother plant (Chevreau and Leblay 1992; Yousefiara et al. 2014a), choice and orientation of the starting explant (Chevreau and Leblay 1992; Yousefiara et al. 2014a), plant growth regulators (PGRs) (especially cytokinins) and basal salt composition of the shoot induction medium (Abdollahi et al. 2006; Caboni et al. 1999; Tang et al. 2008; Yousefiara et al. 2014a), darkness and/or light demand for the induction phase (Leblay et al. 1991), gelling agents (Chevreau et al. 1997), carbohydrates (Chevreau et al. 1989; Leblay et al. 1991), and genotype (Abdollahi et al. 2006; Chevreau et al. 1997; Leblay et al. 1991; Predieri et al. 1989; Tang et al. 2008; Yousefiara et al. 2014a; Zhu 2000). Despite high values of regeneration frequency have been reported for many genotypes (both rootstocks and cultivars), these results were not always reproducible, as shown by the significant variability among replicates of the same trial, and among trials repeated at different time frames (Bell et al. 2012; Chevreau and Leblay 1992; Leblay et al. 1991). This fact may depend on the heterogeneity among starting explants in a specific trial and/or on the alteration of the physiological condition of the mother plant during periodical subcultures (Chevreau and Leblay 1992). However, although the huge amount of work existing on pear organogenesis, only few authors describe the sensitivity of pear tissue to decontamination and selective antibiotics (Abdollahi et al. 2006; Chevreau et al. 1997; Predieri et al. 1989198919891989). These studies mainly evaluated the effect of cefotaxime and kanamycin, as the most widely used antibiotics in Agrobacterium-mediated transformation experiments, on shoot organogenesis starting from leaf explants of the cultivar Conference.
The aim of the present work was to develop new and efficient adventitious shoot regeneration protocols from leaves of three P. communis L. genotypes, two pear cultivars Conference and Abate Fétel, plus the rootstock Farold®87, because of their commercial importance in Europe, especially in Italy, and because they can be easily propagated in vitro (Predieri et al. 1989). As already discussed, many factors influence leaf in vitro organogenesis (Ricci et al. 2020a, b), thus in the current study, we investigated the effects of both thidiazuron (TDZ), at different concentrations in the culture medium, and of different decontamination antibiotics, such as cefotaxime, timentin and carbenicillin, on adventitious shoot organogenesis from leaves of Conference, Abate Fétel, and Farold®87. In addition, the sensitivity of leaves for each of these genotypes to different concentrations of kanamycin was studied in order to identify the most appropriate concentration to be used as selective treatment for identifying new putative transgenic lines after Agrobacterium-mediated transformation.
Materials and methods
In vitro establishment of shoot cultures
Three P. communis L. genotypes have been considered for this study: two important commercial cultivars, Conference, Abate Fétel, and the Old Home x Farmingdale (OH x F) clonal rootstock Farold®87. Pear shoot cultures were in vitro established using 0.5-cm-long shoot tips cut from shoots (approximately 10 cm long) of 10-year-old greenhouse-grown trees of Conference, Abate Fétel and Farold®87 at Vitroplant Italia, Cesena, Italy. Pear shoot tips were surface sterilized by washing them in 1% (V/V) commercial bleach solution for 15 min, followed by three washes with sterile distilled water. In vitro shoots were proliferated by sub-culturing at 4-week intervals onto a shoot multiplication medium consisting of Quoirin and Lepoivre (QL) (Duchefa Biochemie, Haarlem, The Netherlands) basal salt and vitamins (Quoirin and Lepoivre 1977), 30 g L−1 sucrose and 6.5 g L−1 plant agar (Duchefa Biochemie, Haarlem, The Netherlands), at 24 ± 1 °C under a photoperiod of 16-h light (70 µmol/m2/s) provided by white fluorescent tubes. The shoot multiplication medium for Conference and Farold®87 was supplemented with 6.6 µM N6-benzylaminopurine (BAP) (Duchefa Biochemie, Haarlem, The Netherlands) and 1.5 µM indole-3-butyric acid (IBA) (Duchefa Biochemie, Haarlem, The Netherlands). For Abate Fétel, 8.9 µM BAP and 0.1 µM α-naphthalene acetic acid (NAA) (Duchefa Biochemie, Haarlem, The Netherlands) were added to the shoot multiplication medium. The pH was always adjusted to 5.8 with 1 M KOH before autoclaving at 121 °C for 20 min. Pear proliferating shoots were then moved to the elongation medium to expand leaf tissue, as requested for this study. Composition and preparation of the elongation medium were the same as for the shoot multiplication medium, except for PGRs concentrations, and the addition of 0.5 g L−1 activated charcoal (Duchefa Biochemie, Haarlem, The Netherlands). The elongation medium for Abate Fétel and Farold®87 was supplemented with 2.5 µM isopentenyl adenine (2ip) (Duchefa Biochemie, Haarlem, The Netherlands) and 0.5 µM IBA, while for Conference, 0.2 µM BAP and 0.5 µM IBA were used. In vitro shoots were kept on the elongation medium for three weeks at the same temperature and light conditions as mentioned above, and then used for in vitro regeneration experiments.
In vitro adventitious shoot regeneration of the three pear genotypes
The first five-six apical expanded leaves excised from 3-week-old elongated shoot cultures of Conference, Abate Fétel, and Farold®87 were used as starting explants for in vitro shoot induction experiments (Fig. 1 a). The abaxial surface of each explant (petiole not included as reported by Leblay et al. 1991) was wounded three-four times at each side perpendicular to the midrib, and then randomly plated, abaxial side down, on 25 ml of regeneration medium (poured into sterile plastic Petri dishes 9 cm × 1.5 cm) (Fig. 1 b, c). This regeneration medium consisted of half-strength Murashige and Skoog as basal salt (Murashige and Skoog 1962), 30 g L−1 sucrose, 6.5 g L−1 plant agar, thidiazuron (TDZ) at different concentrations (2.25, 4.5, 9 or 13.5 µM), combined with 1 µM NAA, for a total of six different media combinations (including negative controls where TDZ was not used) (Table 1). The pH value was adjusted to 5.8 with 1 M KOH before autoclaving at 121 °C for 20 min. After 3 weeks in darkness, leaves were moved to an auxin-free identical medium and kept at 24 ± 1 °C in light conditions (16-h photoperiod at a light intensity of 40 µmol/m2/s) for 4 weeks.
Starting explant used in this study. a 3-week-old elongated shoot of Conference (bar = 1 cm). The red arrow indicates the type of leaf used; b apical expanding leaf of Conference wounded three times perpendicular to the midrib (bar = 2 mm), and c leaves of Conference positioned abaxial side down on regeneration medium (bar = 1 cm)
Effect of decontamination antibiotics on adventitious shoot regeneration
Different antibiotics, usually used for the control of Agrobacterium overgrowth during transformation trials (Bell et al. 1999; Chevreau et al. 1997; Mourgues et al. 1996; Sun et al. 2011), were supplemented to each optimized regeneration medium (Table 2), and their effect on the regeneration of Conference, Abate Fétel, and Farold®87 was tested following the same regeneration protocol previously described.
Antibiotics stock solutions were filter-sterilised and added to the regeneration medium after autoclaving and cooling down to 50 °C.
Kanamycin sensitivity tests on pear leaves regeneration
The toxicity threshold of kanamycin was determined by placing expanding leaves of Conference, Abate Fétel, and Farold®87 in sterile plastic Petri dishes (9× 1.5 cm) containing 25 ml of the optimized regeneration medium supplemented with the suitable decontamination antibiotics, and increasing concentrations of kanamycin (0, 4, 10, 15, 31, 63 or 93 μM). Media preparation and leaves growing conditions were the same as previously reported. Kanamycin stock solution was filter-sterilised and added to the medium after autoclaving and cooling down.
Elongation, rooting and acclimatization of adventitious shoots
1-cm-long shoots regenerated from in vitro leaves for each of the three pear genotypes were isolated and moved to elongation medium (as described above) for 3 weeks. Single elongated shoots were then rooted for 3 weeks onto a medium consisting of QL basal salt and vitamins (Quoirin and Lepoivre 1977), 30 g L−1 sucrose and 6.5 g L−1 plant agar, at 24 ± 1 °C under a photoperiod of 16-h light (70 µmol/m2/s) provided by white fluorescent tubes. The rooting medium for Abate Fétel and Farold®87 was supplemented with 2.5 µM 2ip and 2 µM IBA, while for Conference, 0.2 µM BAP and 2 µM IBA were used (Chevreau et al. 1992). Rooted shoots were finally acclimatized in pots (7 × 7 cm) containing commercial peat and grown in the greenhouse.
Statistical analysis
For each treatment included in this study, three independent experiments consisting of five petri dishes each, with ten leaves per dish, were carried out. Data on leaf regeneration frequency expressed as (number of leaves regenerating at least one shoot/total leaves treated) × 100 were transformed by the arcsine square root transformation, ARSIN (SQRT (X)), before analysis. Data on the number of regenerating shoots are presented as the mean ± SE of the total number of shoots regenerating from starting explants. The results recorded 7 weeks after the beginning of the culture were analysed by one-way ANOVA using Statistica7 software (Statsoft Tulsa, CA, USA), and means were separated using the Newman–Keuls test (p < 0.05).
Results
Effect of TDZ concentrations on pear leaf adventitious regeneration
Increasing concentrations of TDZ (from 0 up to 13.5 µM) used in combination with 1 μM NAA were supplemented to the basal regeneration medium to stimulate leaf organogenesis and then evaluate the adventitious shoot regeneration frequency from leaves of Conference, Abate Fétel, and Farold®87 (Table 1 and Fig. 2). For almost all regeneration media (except for the medium where no PGRs were used) and with all genotypes, a 100% of callogenesis was observed 7 weeks after the beginning of the culture both at the proximal part of the leaf, where the petiole was removed, and along the wounds made perpendicular to the midrib (Fig. 2); slight differences were detected for callus size, especially for leaves of Farold®87, which in all the media supplemented with TDZ showed smaller calli, in particular at the proximal part of the leaf, compared to Conference and Abate Fétel (Fig. 2 and Supplementary Fig. 1). Data on leaf regeneration frequency and on the number of regenerating shoots were recorded 7 weeks after the beginning of the induction culture (Table 1). Most of the adventitious shoots of Conference, Abate Fétel, and Farold®87 arose after 3 weeks in dark conditions and continued to appear up to the end of the seventh week (data not shown). Differently, most of the shoots of Abate Fétel and Farold®87 developed in the proximal area of the leaf when explants of both genotypes were placed on regeneration media supplemented with TDZ (regardless of the concentration used) (Fig. 2i–l and 2 o—r, respectively), while buds of Conference also appeared in the wounded part, especially when the explants were cultured on medium containing 2.25 µM TDZ (Fig. 2 c and Supplementary Fig. 1 a). Furthermore, regeneration frequency of Conference and Abate Fétel was not influenced by the concentration of TDZ supplemented to the regeneration medium, since no statistical differences were observed at 7 weeks of culture (Table 1). However, except for the induction medium containing 9 µM TDZ, all TDZ concentrations used with Conference leaves reported higher efficiency (more than two-fold) compared to Abate Fétel leaves cultured on the same media (Table 1). The frequency of regeneration differed according to the cultivar: Conference and Abate Fétel showed higher percentages (87.3% and 68%, respectively) when the leaves were placed on basal medium supplemented with 13.5 or 9 μM TDZ in combination with 1 μM NAA, respectively (Table 1 and Supplementary Fig. 2). In contrast, regeneration percentage of Farold®87 was highly affected by the amount of TDZ, since the medium containing 13.5 µM TDZ was more efficient (more than double) in inducing adventitious regeneration compared to the other TDZ concentrations tested. In particular, the use of TDZ at 13.5 µM induced the highest regeneration frequency (53.3%) for Farold®87 leaves followed by media containing 9, 4.5 and 2.25 µM TDZ with 25.3%, 30.7% and 18.7% of adventitious shoot regeneration, respectively (Table 1 and Fig. 2). Lastly, despite TDZ concentrations tested seemed to have no influence on the regeneration frequency of Conference leaves, the average number of regenerating shoots per leaf of this genotype was significantly lower when starting explants were cultured on regeneration media supplemented with 9 or 13.5 µM TDZ (a mean of 1.5 ± 0.1, or a mean of 2.2 ± 0.1 shoots per explant, respectively) (Table 1). On the contrary, the effect of TDZ on the average number of regenerating shoots per leaf of both Abate Fétel and Farold®87 was not dose dependent, since no significant differences were observed 7 weeks after the beginning of the culture. However, all regeneration media tested with Farold®87 leaves showed lower efficiency in terms of the average number of regenerating shoots per starting explant compared to the leaves from the cultivars cultured on the same media (Table 1).
Effect of different decontamination antibiotics on regeneration efficiency of pear leaves
Different antibiotics, normally used to control Agrobacterium overgrowth during transformation trials, were supplemented to the optimized regeneration media, to evaluate their effect on shoot organogenesis from Conference, Abate Fétel, and Farold®87 leaves, after 7 weeks of culture (Table 2). The addition of timentin (347 mM), or carbenicillin (475 mM), in the regeneration medium containing 13.5 µM TDZ and 1 µM NAA did not affect adventitious regeneration from Conference leaves, since no significant differences were observed compared to the control, in terms of both frequency of regeneration and mean number of regenerating shoots per starting explant at 7 weeks after the beginning of the culture. In contrast, when cefotaxime was used alone or in combination with timentin or carbenicillin, shoot organogenesis from Conference leaves decreased in comparison with the control (Table 2). In particular, when cefotaxime was applied alone at 630 mM, both frequency of regeneration and average number of regenerating shoots per leaf reduced more than two-fold (46% and a mean of 0.8 ± 0.1 shoots per explant) compared to the control (87.3% and a mean of 2.2 ± 0.1 shoots per explant). Furthermore, the use of cefotaxime, timentin, and carbenicillin (alone or in combination) in the regeneration media had a negative influence on adventitious shoots organogenesis from both Abate Fétel and Farold®87 leaves compared to the controls (Table 2). In particular, the addition of cefotaxime (630 mM) or carbenicillin (475 mM) in the optimized medium containing 9 µM TDZ and 1 µM NAA reduced more than two-fold both the frequency of regeneration and average number of regenerating shoots per leaf of Abate Fétel compared to their combined use. In contrast, when cefotaxime was used alone at 630 mM in the medium supplemented with 13.5 µM TDZ and 1 μM NAA, both values of frequency of regeneration and average number of regenerating shoots per leaf of Farold®87 were significantly higher (24.7% and a mean of 0.5 ± 0.09 shoots per explant) compared to its use combined with timentin or carbenicillin 7 weeks after the beginning of the culture (an average of 12% with a mean of 0.2 ± 0.04 shoots per explant) (Table 2).
Kanamycin sensitivity tests on pear leaves
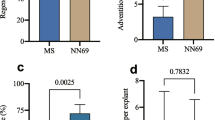
To establish the concentration of kanamycin to be used for the selection of putative transformed events in future infections with Agrobacterium, leaves of Abate Fétel, Farold®87, and Conference were cultured in each optimized regeneration medium (Table 1) supplemented with the suitable concentration of decontamination antibiotics (Table 2), and increasing concentration of kanamycin (0, 4, 10, 15, 31, 63 or 93 μM). As supposed, an increase in the concentration of kanamycin in the shoot induction medium led to a dramatic decrease in the leaf regeneration efficiency in both Abate Fétel (Fig. 3 a–b) and Farold®87 (Fig. 4 a–b) after 7 weeks of culture. In particular, even when kanamycin was used at 4 μM, (the lowest concentration tested in our study) the shoot regeneration frequency of both genotypes decreased drastically to 8.1% for Abate Fétel and 7.3% for Farold®87, as well as the average number of shoots per explant of both genotypes (a mean of 0.14 ± 0.05 shoots per explant of Abate Fétel, and a mean of 0.07 ± 0.02 shoots per explant of Farold®87) compared to the respective controls (53% and a mean of 1.2 ± 0.15 shoots per explant of Abate Fétel, and 30% and a mean of 0.5 ± 0.07 shoots per explant of Farold®87) (Fig. 3 a–b and Fig. 4 a–b). In addition, the use of kanamycin concentrations equal or higher than 10 μM arrested completely the adventitious regeneration starting from leaves of both Abate Fétel and Farold®87 (Fig. 3 a–b and Fig. 4 a–b). In the above-mentioned culture conditions, we also observed a lower frequency of callogenesis (data not shown) until to a complete lack of it when pear leaves were cultured on kanamycin equal or higher than 15 μM for 7 weeks (Fig. 3 c–i and Fig. 4 c–i). No adventitious shoot regeneration has been observed when leaves of Conference were cultured on the previously optimized shoot induction medium with any of the kanamycin concentrations tested (data not shown).
Kanamycin sensitivity test on Abate Fétel leaves. a Frequency of regeneration, expressed as (number of leaves regenerating at least one shoot/total explants) × 100, and b the average number of regenerating shoots per leaf (LF) recorded after 7 weeks from the beginning of the culture. Data were analysed by one-way ANOVA, and Newman–Keuls test (p < 0.05) ± SE (n = 150) was used to identify significant differences. Results showed in the picture represent the mean ± SE of three independent experiments. Leaf explants of Abate Fétel (c–i) cultured on the optimized regeneration medium supplemented with increasing concentrations of kanamycin (0, 4, 10, 15, 31, 63 or 93 μM) after 7 weeks from the beginning of the culture (bars = 1 cm)
Kanamycin sensitivity test on Farold®87 leaves. a Frequency of regeneration, expressed as (number of leaves regenerating at least one shoot/total explants) × 100, and b the average number of regenerating shoots per leaf (LF) acquired after seven weeks from the beginning of the culture. Data were analysed by one-way ANOVA, and Newman–Keuls test (p < 0.05) ± SE (n = 150) was used to identify significant differences. Results showed in the picture represent the mean ± SE of three independent trials. Leaves of Farold®87 (c–i) placed on the optimized regeneration medium supplemented with increasing concentrations of kanamycin (0, 4, 10, 15, 31, 63 or 93 μM) after 7 weeks from the beginning of the culture (bars = 1 cm)
In vitro rooting and acclimatization of regenerating shoots
Regenerating buds of Conference, Abate Fétel, and Farold®87 grown in the above-mentioned experiments were in vitro elongated, rooted, and acclimatized in the greenhouse (Fig. 5 a, b, c). About 80% of them developed 2-cm-long in vitro roots after 3 weeks in the genotype-dependent rooting induction medium (Fig. 5 b), and about 75% of them (for each pear genotypes) were successfully acclimatized in the greenhouse, where they showed a homogeneous development and a true-to-type phenotype (Fig. 5 c).
Discussion
Our study showed that adventitious shoot regeneration can be induced with high efficiency starting from leaves of the pear cultivars Conference, Abate Fétel, and the rootstock Farold®87. The individuation of the proper type and concentrations of PGRs supplemented to the basal regeneration medium is one of the essential steps in clarifying suitable in vitro stimulus capable of positively affecting leaf organogenesis (Ricci et al. 2020a, b). Our best results in terms of frequency of regeneration achieved on Conference, Abate Fétel, and Farold®87 genotypes were 87.3% (with mean number of shoots per explant of 2.2 ± 0.1), 68% (with mean number of shoots per explant of 1.9 ± 0.1), and 53.3% (with mean number of shoots per explant of 0.8 ± 0.1), respectively. These results were achieved by using 1 μM NAA in combination with 13.5 μM TDZ for Conference and Farold®87, and 9 μM TDZ for Abate Fétel. However, leaf tissue of the two cultivars showed an efficient shoot organogenesis even with different concentrations of TDZ, with regeneration percentages up to 85.3% (with mean number of shoots per explant up to 2.9 ± 0.2), and 59.3% (with mean number of shoots per explant up to 1.9 ± 0.2) for Conference and Abate Fétel leaves cultured in presence of 4.5 μM TDZ and 13.5 μM, respectively. On the contrary, Farold®87 presented lower regeneration percentages (from 18.7% up to 25.3%) when leaves were cultured on basal media supplemented with TDZ equal or lower than 9 μM. Similarly, Chevreau et al., (1989) described an adventitious regeneration protocol for two cultivars of P. communis L., reporting that 3 μM TDZ (used in combination with NAA) seemed to be optimal for inducing adventitious shoot regeneration from leaf tissue of the pear cultivars Seckel and Louise Bonne, reaching a regeneration percentage approximately of 80% and 40%, respectively. Furthermore, four pear cultivars tested by Leblay et al., (1991) behaved in the same way: when Comice, Passe-Crassane, Williams and Conference leaves were cultured in basal regeneration media supplemented with TDZ concentrations from 2.5 up to 5 μM shoot regeneration frequencies ranging from 60% up to 100%, with mean number of adventitious shoots per explant ranging from 3.2 ± 0.2 to 6.6 ± 0.1 were obtained. Moreover, Javadi et al. (2013) have optimized systems for in vitro leaf organogenesis of three different pear cultivars, which allowed to obtain regeneration rates of 64% and 40% (with mean number of shoots per explant of 10.6 ± 0.2 and 3.3 ± 0.1) when Dargazi and Harrow Delight, respectively, were cultured on basal media supplemented with 16 μM TDZ. The same authors were also able to induce adventitious regeneration (even if with lower efficiency) from leaves of pear cultivar Bartlett using TDZ at 4 μM and then reaching a regeneration rate of 24% with mean number of shoots per explant of 1.8 ± 0.1. Concerning P. communis L. rootstocks, good results in terms of regeneration percentages were obtained on OHF333 (Old Home x Farmingdale) (66% regeneration, with mean number of shoots per explant of 1.2 ± 0.1), BP10030 (98% regeneration, with mean number of shoots per explant of 1.8 ± 0.1), and Pyrodwarf®(S) (Bonne Louise d’Avranches x Old Home) (65% regeneration, with mean number of shoots per explant of 2.1 ± 0.2), through the use of 1 μM TDZ in combination with 0.1 μM NAA for OHF333 leaves and 15 μM TDZ in combination with 1 μM NAA for both leaf explants of BP10030 and Pyrodwarf®(S) (Martinelli et al., 2014; Zhu and Welander 2000). Although all these studies showed the strong impact of TDZ in stimulating adventitious regeneration starting from leaf explants of different pear genotypes (including cultivars and rootstocks), other authors were able to induce shoot organogenesis starting from leaf tissue of P. communis L. (including Conference and Abate Fétel) using BAP as cytokinin (Poudyal et al. 2008; Predieri et al. 1989). As demonstrated by Poudyal et al., (2008), a regeneration percentage of 60% with a mean number of regenerating shoots of 4.6 ± 0.2 was recorded when leaves of the pear cultivar Abate Fétel were placed in a medium containing 22 μM BAP and 5 μM NAA. In contrast, when Predieri and collaborators (1989) cultured the same explants in presence of the same PGRs treatment, shoot organogenesis from leaf tissue of Abate Fétel was very low; the few regenerated shoots were fragile and they could not be proliferated. While, the highest regeneration percentage (up to 40%) was recorded by the same authors when they placed Conference leaves on the same basal medium enriched with 22 μM BAP combined with 5 μM NAA (Predieri et al. 1989). Differently, during our preliminary experiments on the adventitious shoot regeneration from Conference, Abate Fétel, and Farold®87 leaves, we observed lower regeneration efficiencies (close to zero most of the time) using BAP as cytokinin (data not shown).
To optimize the different steps of a protocol to be used in future Agrobacterium-mediated transformation trials for these pear cultivars and the rootstock, leaves of each genotype were cultured on each optimized regeneration medium supplemented with different antibiotics (alone or in combination), which are usually efficacious for Agrobacterium decontamination after co-culture and during regeneration and selection phases of putatively transformed plants. Looking at our results, different aspects must be highlighted. The use of timentin (347 mM) or carbenicillin (475 mM) did not have a negative impact on adventitious shoot regeneration from Conference leaves. Whereas, carbenicillin (238 mM) used in combination with cefotaxime (210 mM) seemed to be less lethal than its use alone on adventitious shoot organogenesis from Abate Fétel leaves (Table 2). Furthermore, the addition of cefotaxime at 630 mM in the suitable regeneration medium had a negative effect on adventitious regeneration from leaf tissues of both Conference and Abate Fétel. In fact, both frequency of regeneration and average number of regenerating shoots per leaf of these two cultivars were reduced more than two-fold in comparison with their respective controls. In contrast, when cefotaxime was used alone at the concentration tested, seemed to be less toxic than its use combined with carbenicillin or timentin on the adventitious shoot regeneration from Farold®87 leaves (in terms of both percentage of regeneration and average number of regenerating shoots per leaf) (Table 2). Despite neutral or beneficial effects of cefotaxime concerning shoot regeneration have been shown in many plant species (Ricci et al. 2020a, b), this antibiotic should be treated as toxic compound for adventitious shoot organogenesis starting from leaves of pear cultivars, at least when used at a concentration equal or higher than 630 mM. As referred by Schmitt et al., (1997), the lower efficiency in caulogenesis when cefotaxime (and vancomycin as well) was used at high concentrations, could be caused by an increasing of DNA methylation. Plants use the hypermethylation of DNA as protection system against fungal pathogens, which synthesize antibiotic compounds during their infection processes (Schmitt et al. 1997). Probably, depending on the susceptibility of plant tissue to this substance, the supplied antibiotic may simulate a fungal invasion, with consequent DNA hypermethylation and then reduction in morphogenesis ability until to plant cell death (Schmitt et al. 1997). Furthermore, the use of carbenicillin at 475 mM in the optimized regeneration medium seemed to have a negative effect on the adventitious regeneration only when leaves of Abate Fétel were used as starting material. As reviewed in our previous study (Ricci et al. 2020a, b), carbenicillin is considered as a potential promoter of plant organogenesis due to the chemical compounds derived from its breakdown, which play an auxin-like role able to improve the adventitious regeneration efficiency in several plant species. In the present study, an amount of carbenicillin higher than 475 mM may have caused an excess of auxin-like compounds in the leaves of both Abate Fétel and Farold®87, leading to a decrease of organogenetic competence (which is genotype-dependent) in this genotype. Therefore, if for problems of Agrobacterium overgrowth, it will be required an increased concentration of carbenicillin, it will be helpful to revise the composition of regeneration medium by reducing the amount of auxin. Furthermore, the key success in Agrobacterium-mediated genetic transformation also relies on the optimization of a suitable selection strategy, which is dependent on the genotype when kanamycin (the most common selection agent still used after Agrobacterium infection) is used. Our results showed that even when kanamycin was used at extremely low concentrations, the shoot regeneration starting from Abate Fétel and Farold®87 leaves decreased dramatically compared to the respective controls (Fig. 3 and Fig. 4), indicating that they were both extremely sensitive to kanamycin as selection agent using leaf tissue as starting plant material. The impact of kanamycin on the shoot organogenesis of Conference leaves was even worse in comparison with the other two pear genotypes, considering that no adventitious shoot regeneration was observed even when the lowest kanamycin concentration tested was used. In contrast, Mourgues et al., (1996), describing an A. tumefaciens-mediated gene transfer method for Conference leaves, optimized a selection medium containing kanamycin at 207 μM. Similarly, other authors obtained transgenic lines from leaf explants of many pear cultivars and a rootstock, such as Beurre Bosc, Bartlett, Silver Bell, La France and Old Home, using kanamycin concentrations from 52 up to 165 μM in the selection medium (Bell et al. 1999; Matsuda et al. 2005; Sun et al. 2011). These studies compared to our results demonstrated how large can be the range of kanamycin concentrations able to inhibit pear morphogenesis, which seems to be linked firstly to the plant species, genotype, and type of starting explant, other than the origin of the mother plant and composition of regeneration medium when the same genotype is under evaluation. Although 4 μM kanamycin should be appropriate for its use as selectable agent after co-culture of Abate Fétel and Farold®87 leaves with Agrobacterium, the discovery of this extreme sensitivity to kanamycin (even at very low concentrations) may require the use of delayed selection, that is the inclusion of the selective antibiotic into the regeneration medium few days/weeks after the infection with Agrobacterium. This strategy is used to allow the few transformed cells to proliferate after Agrobacterium infection, and consequently gaining more power to survive later to antibiotic selection. Considering its huge sensitivity to kanamycin, the influence of other antibiotics on Conference leaf organogenesis will be evaluated, for example choosing among those able to select nptII-expressing-transformed events, such as paromomycin, neomycin and geneticin.
Conclusions
In conclusion, efficient and new adventitious shoot regeneration protocols have been developed for Conference, Abate Fétel and Farold®87 leaves, reaching frequencies of regeneration up to 87.3%, 68%, and 53.3%, respectively. Although the protocol for Conference leaf regeneration has been already optimized by another research study (Leblay et al. 1991), to our knowledge, protocols for obtaining shoot organogenesis from Abate Fétel and Farold®87 leaves using TDZ have been reported here for the first time. Genetic variability, i.e. somaclonal variation, can be originated through plant tissue culture techniques, depending on the genotype, type of starting explant and medium composition. It is really limited when auxiliary buds are used in standard micropropagation protocols, while it can be induced with higher frequency when used in in vitro regeneration protocols (Caboni et al. 2002). Therefore, the risk of genetic variability determined by somaclonal variation is highly controlled for the development of protocols used for commercial scale multiplication of elite cultivars and rootstocks of woody species, which must maintain a high degree of genetic fidelity (Krishna et al. 2016). While, regeneration protocol can be used to create genetic variability, and if used for new breeding techniques applications, the selection of the homogenous and stable transformed lines will result as an important final step of the creation of new genetically modified plants.
To date, the pear plants of the two cultivars and the rootstock object of this study have a true-to-type phenotypic aspect (Fig. 5). However, the identification of somaclonal variation in the regenerated shoots determined by the optimized regeneration protocols could be the aim of an additional future study, through genotyping and phenotyping tools, as previously made by other authors (Bairu et al. 2011; Caboni et al. 2002; Palombi et al. 2007).
The present study was mainly aimed at optimizing an efficient in vitro regeneration protocol for the forthcoming insertion of useful genes in pear through genetic engineering approaches. For this reason, the effect of different type and concentration of decontamination antibiotics, usually used to control the overgrowth of Agrobacterium after explants co-culture, on the regeneration efficiency of the three pear genotypes has been evaluated; as well as the most suitable concentration of kanamycin to be applied for the future selection of pear transformed events has been identified for at least two of the three genotypes under study. The overall results of the present research will be considered for designing future genetic transformation trials for Conference, Abate Fétel and Farold®87 to apply the main NGTs for pear genetic improvement. For instance, these genotypes require further genetic improvement mainly due to their high susceptibility to brown spot disease, broadly spread in the Po valley (Italy) and caused by Stemphylium vesicarium, an anamorphic fungus that is becoming resistant to the most common fungicides (Alberoni et al. 2005; Cappai et al. 2018).
Availability of data and materials
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
References
Abdollahi H, Muleo R, Rugini E (2006) Optimisation of regeneration and maintenance of morphogenic callus in pear (Pyrus communis L.) by simple and double regeneration techniques. Sci Hortic (amsterdam) 108:352–358. https://doi.org/10.1016/j.scienta.2006.02.007
Abu-Qaoud H, Skirvin RM, Below FE (1991) Influence of nitrogen form and NH4+-N/:NO3–N ratios on adventitious shoot formation from pear (Pyrus communis) leaf explants in vitro. Plant Cell Tissue Organ Cult 27:315–319. https://doi.org/10.1007/BF00157597
Alberoni G, Collina M et al (2005) Resistance to dicarboximide fungicides in Stemphylium vesicarium of Italian pear orchards. Eur J Plant Path 113:211–219. https://doi.org/10.1007/s10658-005-2332-3
Bairu MW, Aremu AO, Van Staden J (2011) Somaclonal variation in plants: causes and detection methods. Plant Growth Regul 63:147–173. https://doi.org/10.1007/s10725-010-9554-x
Bell RL, Scorza R, Srinivasan C, Webb K (1999) Transformation of `Beurre Bosc’ Pear with the rolC Gene. J Am Soc Hortic Sci 124:570–574. https://doi.org/10.21273/JASHS.124.6.570
Bell RL, Scorza R, Lomberk D (2012) Adventitious shoot regeneration of pear (Pyrus spp.) genotypes. Plant Cell Tissue Organ Cult 108:229–236. https://doi.org/10.1007/S11240-011-0034-4
Bellini E, Nin S (2002) Breeding for new traits in pear. Acta Hort 596:217–224. https://doi.org/10.17660/ActaHortic.2002.596.31
Browning G, Ognjanov V, Passey AJ, James DJ (1987) Multiple shoot and root regeneration from pear embryo cotyledon explants in vitro. J Hortic Sci 62:305–311. https://doi.org/10.1080/14620316.1987.11515786
Caboni E, Tonelli MG, Lauri P et al (1999) In vitro shoot regeneration from leaves of wild pear. Plant Cell Tissue Organ Cult 59:1–7. https://doi.org/10.1023/A:1006351214343
Caboni E, Lauri P, Damiano C, D’Angeli S (2000) Somaclonal variation induced by adventitious shoot regeneration in pear and apple. Acta Hort 530:195–201. https://doi.org/10.17660/ACTAHORTIC.2000.530.22
Caboni E, D’Angeli S, Chiappetta A et al (2002) Adventitious shoot regeneration from vegetative shoot apices in pear and putative role of cytokinin accumulation in the morphogenetic process. Plant Cell Tiss Organ Cult 70:199–206. https://doi.org/10.1023/A:1016304106529
Cappai F, De Franceschi P, Ciriani A et al (2018) QTLs for susceptibility to Stemphylium vesicarium in pear. Mol Breed 38:1–11. https://doi.org/10.1007/S11032-018-0785-2
Chevreau E, Leblay C (1992) The effect of mother plant pretreatment and explant choice on regeneration from in vitro pear leaves. Acta Hort 336:263–268. https://doi.org/10.17660/ActaHortic.1993.336.34
Chevreau E, Skirvin RM, Abu-Qaoud HA et al (1989) Adventitious shoot regeneration from leaf tissue of three pear (Pyrus sp.) cultivars in vitro. Plant Cell Rep 7:688–691. https://doi.org/10.1007/BF00272062
Chevreau E, Thibault B, Arnaud Y (1992) Micropropagation of Pear (Pyrus communis L.). In: Bajaj YPS (ed) Biotechnology in agriculture and forestry. Springer, Berlin, pp 224–261
Chevreau E, Mourgues F, Neveu M, Chevalier M (1997) Effect of gelling agents and antibiotics on adventitious bud regeneration from in vitro leaves of pear. Vitr Cell Dev Biol Plant 33:173–179. https://doi.org/10.1007/s11627-997-0017-7
Dondini L, Sansavini S (2012) European pear. Fruit Breed. https://doi.org/10.1007/978-1-4419-0763-9_11
FAOSTAT (2020). https://www.fao.org/faostat/en/#home. Accessed on 15 July 2022
Javadi S, Kermani M, Irian S et al (2013) Indirect regeneration from in vitro grown leaves of three pear cultivars and determination of ploidy level in regenerated shoots by flow cytometry. Sci Hort. https://doi.org/10.1016/j.scienta.2013.09.056
Kadota M, Han D (2002) Plant regeneration from anther-derived embryos of apple and pear. HortScience 37:962–965. https://doi.org/10.21273/HORTSCI.37.6.962
Krishna H, Alizadeh M, Singh D, Singh U, Chauhan N, Eftekhari M, Sadh RK (2016) Somaclonal variations and their applications in horticultural crops improvement. 3 Biotech 6:1–18. https://doi.org/10.1007/s13205-016-0389-7
Lane WD (1979) Regeneration of pear plants from shoot meristem-tips. Plant Sci Lett 16:337–342. https://doi.org/10.1016/0304-4211790046-4
Leblay C, Chevreau E, Raboin LM (1991) Adventitious shoot regeneration from in vitro leaves of several pear cultivars (Pyrus communis L.). Plant Cell Tissue Organ Cult 25:99–105. https://doi.org/10.1007/BF00042180
Martinelli F, Busconi M, Fogher C et al (2014) Development of an efficient regeneration protocol for pear root-stock Pyrodwarf and assessment of SSR variability in regenerating shoots. Caryologia 62:62–68. https://doi.org/10.1080/00087114.2004.10589667
Matsuda N, Gao M, Isuzugawa K et al (2005) Development of an Agrobacterium-mediated transformation method for pear (Pyrus communis L.) with leaf-section and axillary shoot-meristem explants. Plant Cell Rep 24:45–51. https://doi.org/10.1007/S00299-005-0924-1
Mourgues F, Chevreau E, Lambert C, de Bondt A (1996) Efficient Agrobacterium-mediated transformation and recovery of transgenic plants from pear (Pyrus communis L.). Plant Cell Rep 16:245–249. https://doi.org/10.1007/BF01890877
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
Ochatt SJ, Caso OH (1986) Shoot regeneration from leaf mesophyll protoplasts of wild pear (Pyrus communis var. Pyraster L.). J Plant Physiol 122:243–249. https://doi.org/10.1016/S0176-1617(86)80123-7
Ochatt S, Osvaldo HC (1988) Plant regeneration from mesophyll protoplasts of Williams’ Bon Chretien (syn. Bartlett) pear (Pyrus communis L.). J Plant Phys 122:243–249. https://doi.org/10.1007/BF00272764
Palombi MA, Lombardo B, Caboni E (2007) In vitro regeneration of wild pear (Pyrus pyraster Burgsd) clones tolerant to Fe-chlorosis and somaclonal variation analysis by RAPD markers. Plant Cell Rep 26:489–496. https://doi.org/10.1007/s00299-006-0256-9
Pinet-Leblay C, Turpin FX, Chevreau E (1992) Effect of gamma and ultraviolet irradiation on adventitious regeneration from in vitro cultured pear leaves. Euphytica 62:225–233. https://doi.org/10.1007/BF00041757
Poudyal BK, Zhang Y, Du G (2008) Adventitious shoot regeneration from the leaves of some pear varieties (Pyrus spp.) grown in vitro. Front Agric China 2:82–92. https://doi.org/10.1007/S11703-008-0016-4
Predieri S, Fasolo Fabbri Malavasi F, Passey AJ et al (1989) Regeneration from in-vitro leaves of ‘Conference’ and other pear cultivars (Pyrus communis L.). J Hortic Sci 64:553–559. https://doi.org/10.1080/14620316.1989.11515990
Prohens J, Sestras AF, Plazas M, Lebedev V (2022) Stability of transgene inheritance in progeny of field-grown pear trees over a 7-year period. Plants 11:151. https://doi.org/10.3390/plants11020151
Quoirin M, Lepoivre P (1977) Improved media for in vitro culture of Prunus sp. Acta Hort. https://doi.org/10.17660/actahortic.1977.78.54
Ricci A, Sabbadini S, Prieto H et al (2020a) Genetic transformation in peach (Prunus persica L.): Challenges and ways forward. Plants 9:971. https://doi.org/10.3390/plants9080971
Ricci A, Capriotti L, Mezzetti B et al (2020b) Adventitious shoot regeneration from in vitro leaf explants of the peach rootstock Hansen 536. Plants 9:755. https://doi.org/10.3390/plants9060755
Rivalta L, Dradi M, Rosati C (2002) Thirty years of pear breeding activity at ISF Forlì, Italy. Acta Hort 596:233–238. https://doi.org/10.17660/ACTAHORTIC.2002.596.33
Sabbadini S, Capriotti L, Molesini B et al (2019) Comparison of regeneration capacity and Agrobacterium-mediated cell transformation efficiency of different cultivars and rootstocks of Vitis spp. via organogenesis. Sci Rep 9:1–10. https://doi.org/10.1038/s41598-018-37335-7
Sabbadini S, Capocasa F, Battino M et al (2021) Improved nutritional quality in fruit tree species through traditional and biotechnological approaches. Trend Fruit Sci Tech 117:125–138. https://doi.org/10.1016/j.tifs.2021.01.083
Schmitt F, Oakeley E, Jost JP (1997) Antibiotics induce genome-wide hypermethylation in cultured Nicotiana tabacum plants. J Biol Chem 272:1534–1540. https://doi.org/10.1074/jbc.272.3.1534
Sun Q, Zhao Y, Sun H et al (2011) High-efficiency and stable genetic transformation of pear (Pyrus communis L.) leaf segments and regeneration of transgenic plants. Acta Physiol Plant 33:383–390. https://doi.org/10.1007/S11738-010-0557-Z
Tang H, Luo Y, Liu C (2008) Plant regeneration from in vitro leaves of four commercial Pyrus species. Plant Soil Environ 54:140
Viseur J (1990) Evaluation of fire blight resistance of somaclonal variants obtained from the pear cultivar “Durondeau”. Acta Hort 273:275–284. https://doi.org/10.17660/ActaHortic.1990.273.41
Yousefiara M, Jafarkhani Kermani M, Bagheri A, Habashi A, Abdollahi H (2014a) Study of factors affecting direct shoot regeneration of pear (Pyrus communis L.). J Plant Mol Breed 2:21–28. https://doi.org/10.22058/JPMB.2014.8426
Yousefiara M, Kermani MJ, Bagheri A, Habashi AA, Abdollahi H (2014b) Induction of direct adventitious shoot regeneration in pear (Pyrus communis L). Plant Tissue Cult Biotechnol 24(1):87–92
Zhu LH, Welander M (2000) Adventitious shoot regeneration of two dwarfing pear rootstocks and the development of a transformation protocol. J Hortic Sci Biotechnol 75:745–752. https://doi.org/10.1080/14620316.2000.11511317
Acknowledgements
This study has been supported by Vitroplant Italia, Italy and provincial phytosanitary consortium of Modena and Reggio Emilia.
Funding
Open access funding provided by Università Politecnica delle Marche within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Contributions
SS and BM conceived and conceptualized the manuscript. AR wrote and prepared the original draft. AR conducted the experiments. ON provided the plant material and contributed to the experiments. AR analysed data. SS, BM, AR, ON contributed to review and critically revise the manuscript. BM and SS visualized and carefully supervised the work and the manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ricci, A., Mezzetti, B., Navacchi, O. et al. In vitro shoot regeneration from leaves of Pyrus communis L. rootstock and cultivars. Plant Biotechnol Rep 17, 341–352 (2023). https://doi.org/10.1007/s11816-023-00823-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11816-023-00823-y