Abstract
The hook of Uncaria rhynchophylla is an important external phenotype representing the quality of Gou-Teng. The hook formation pattern of U. rhynchophylla displays a unique feature that solitary hook and opposite hooks alternately occur in the leaf axils forming a liner series. The solitary hook only grows on the lower side of the leaf axil, resulting from the decline in the total yield of hooks. However, the reasons for solitary hook formation in U. rhynchophylla are not clear. Therefore, a survey on the molecular mechanisms of solitary hook formation needs to be performed urgently. We obtained 250.67 million high-quality clean reads from the lower side of axillary stem segments (HS) and the upper side of axillary stem segments (HSCK) libraries. We detected 42 DEGs (differentially expressed genes) between HS and HSCK libraries; 11 were down-regulated, and 31 were up-regulated. Gene ontology functional classification of the DEGs indicated that they included two genes that encoded LAZY1 related to the regulation of polar auxin transport, two genes that encoded AUX/IAA and AP2/ERF related to plant hormone signaling transduction, and two genes that encoded F3H related to the flavone synthase activity. These six genes were associated with lateral polar auxin transport (PAT) regulation, and their expression level had a high correlation with the pattern of IAA distribution upon gravity stimulation. And the inhibition of solitary hook development was observed after treatment with N-1-naphthylphthalamic acid (NPA) or quercetin, which was known as the polar auxin inhibitor. The above findings might provide new information about the molecular mechanisms of regulation of lateral PAT in the axillary bud development process under gravity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Uncaria rhynchophylla, belonging to the genus Uncaria of the family Rubiaceae, is one of five botanical sources for traditional medicine Gou-Teng (in Chinese), which has been used for a long time in China (Kuramochi et al. 1994; Ling and Zhang 2020). The characteristic active constituents of U. rhynchophylla mainly are indole alkaloids: rhynchophylline, isorhynchophylline, corynoxeine, and isocorynoxeine, which are responsible for treating convulsion, hypertension, fever, epilepsy and eclampsia and so on (Ndagijimana et al. 2013; Lim and Lee 2022). However, only hooks and hook-bearing stems of U. rhynchophylla are officially documented in Chinese pharmacopoeia as crude medicinal materials of Gou-Teng (Liang et al. 2020). In the Traditional Chinese Medicines system, hooks and hook-bearing stems are considered to have better pharmacological effects than stems without hooks (Geng et al. 2019). The comparison results of active constituents between thinner hooks and stems also show that thinner hooks have higher alkaloid contents, which is consistent with conventional wisdom (Hou et al. 2018). In the herbal market, the price of Gou-Teng is determined by the ratio of the hook to stem. The higher the ratio of the hook to stem, the higher the price of Gou-Teng (Hou et al. 2018). It follows then that the hook of U. rhynchophylla is an important external phenotype that represents the quality of Gou-Teng. However, numerous studies have only concentrated on the chemical constituents and pharmacology of U. rhynchophylla. Conversely, researches on the growth and development of U. rhynchophylla, such as hook development, which is one of the key regulators of Gou-Teng yield, remain to be revealed.
The hook formation pattern of U. rhynchophylla displays a unique feature that solitary hook and opposite hooks alternately occur in the leaf axils forming a liner series. The solitary hook only grows on the lower side of the leaf axil, resulting in a decline in the total yield of hooks. As a climbing vine, hooks are the essential organs of U. rhynchophylla, which provide stable structural supports for climbing, allowing the plant to occupy more niches and obtain higher-quality light and air (Isnard and Silk 2009). However, climbing organs originate from a variety of morphological structures for different plants (Sousa‐Baena et al. 2018). For instance, tendrils of grapes are identified as modified reproductive structures (Boss and Thomas 2002), tendrils of peas represent modified leaflets (Hofer et al. 2009), whereas they are considered to be modified lateral shoots in Cucurbitaceae (Mizuno et al. 2015; Wang et al. 2015). As for the hooks of Uncaria (Rubiaceae) may be modified inflorescences developed from specific primordia on the flanks of the axillary bud (Sperotto et al. 2020). Axillary bud development is regulated by a network of endogenous phytohormone signals, where auxin plays a key role (Shinohara et al. 2013).
Auxin is one of the most important phytohormones responsible for all aspects of plant growth and development (Weijers et al. 2018). Especially, the formation of an appropriate auxin gradient plays a virtual and fundamental role in regulating the axillary bud dormancy and active transduction in many plant species (Min et al. 2017), as the fate of the axillary bud is determined by the auxin sensitivity of the growing cells (Teale et al. 2006). However, auxin biosynthesis only occurs in particular tissues (Petersson et al. 2009). Therefore, it is widely accepted that the directional auxin transport, also known as polar auxin transport (PAT), has an essential role in establishing and maintaining the auxin gradient (Lv et al. 2019).
We observed that all solitary hooks of U. rhynchophylla only occurred on the lower side of the leaf axils. Whether the development of solitary hooks is mediated by regulating lateral PAT is not clear. Therefore, a survey on the molecular mechanisms of solitary hook formation needs to be performed urgently. In recent years, with the rapid development of next-generation sequencing technologies, RNA-seq has become an effective means to study transcription profiles (Wang et al. 2010). The completion of the sequencing of the U. rhynchophylla genome from our research group provides a new opportunity to research molecular mechanisms of solitary hook formation of U. rhynchophylla. In this study, solitary hooks of U. rhynchophylla provide ideal materials with the same genetic background subject to little interference from the environment for the study of complex regulatory networks of axillary bud development because solitary hooks only occur on the lower side of the leaf axils. Therefore, hook-bearing stem segments were collected from leaf axils only differentiated to solitary hooks of U. rhynchophylla and cut lengthwise into two halves, the lower and the upper side of axillary stem segments, and finally investigated for gene expression changes between the lower and the upper side of axillary stem segments by deep RNA sequencing. This research provides data for studying the molecular mechanism of axillary bud development of U. rhynchophylla and lays a solid foundation for ideal plant-type breeding in the studied species.
Materials and methods
Plant materials
The two samples were collected in Uncaria rhynchophylla, was obtained in spring from the Guangxi botanical garden of medicinal plants in Nanning, Guangxi Province, China. At the elongation stage of stem growth, during axillary bud differentiation and when the solitary hook first appeared, 150 solitary hook-bearing stem segments were collected. Then each axillary stem segment was cut lengthwise into two halves, the lower side of axillary stem segments (HS) and the upper side of axillary stem segments (HSCK) (see Fig. S1). 150 plant materials of each sample were randomly divided into six equal groups, three groups used for IAA and ACC measurement and the remaining three groups used for RNA sequencing. All plant materials wrapped with aluminium foil, immediately immersed in liquid nitrogen for 3–5 min, then put in the icebox with the regular ice packs and transported back to the laboratory. The frozen tissues were stored in a −80 ℃ freezer.
RNA isolation and sequencing
Total RNA was extracted from frozen samples using the RNAprep Pure Plant Kit (TIANGEN, Beijing, China). According to the manufacturer's protocols, RNA purity was checked using the kaiao K5500® Spectrophotometer (Kaiao, Beijing, China). RNA integrity and concentration were assessed using the RNA Nano 6000 Assay Kit of the Bioanalyzer 2100 system (Agilent Technologies, CA, USA). RNA degradation was monitored on agarose gels. Six groups of two samples were all deemed high quality and used to construct transcriptome libraries.
The construction of the cDNA library refers to the following methods: mRNA was enriched from total RNA using oligo (dT) magnetic beads. Using divalent cations under elevated temperature, Fragmentation was carried out in NEBNext First Strand Synthesis Reaction Buffer (5X). First-strand cDNA was synthesized using random hexamer primer and RNase H. Second-strand cDNA synthesis was performed using buffer, dNTPs, DNA polymerase I and RNase H. The library fragments were purified with QiaQuick PCR kits, elution with EB buffer, then a terminal repair, A-tailing, and adapter added were implemented. The aimed products were retrieved, PCR was performed, and the library was completed. The Agilent Bioanalyzer 2100 system and StepOnePlus™ Real-Time PCR System evaluated the library quality. Six libraries were sequenced on an Illumina HiSeq 2500 by Wuhan Benagen Tech Solutions Company Limited.
Mapping and sequence annotation
Raw reads were cleaned by removing sequencing adapters and trailing low-quality bases. Eventually, the clean reads were mapped to the whole genome of Uncaria rhynchophylla. The expression levels of the genes were normalized by the FPKM (fragments per kilobase per million fragments) method to compare the expression levels of different genes and samples. Mapping genes further performed functional annotation to the following six public databases: KEGG, Uniprot, GO, Pfam, NR and Interproscan.
Identification and analysis of DEGs
Significant DEGs were screened using read count data of the gene expression in each sample obtained by expression quantification with DESeq2 software. Genes with a Q value < 0.05 and |log2(fold change)|> 1 were identified as DEGs (differentially expressed genes). Fold changes in the expression levels between samples were used as the criteria in the screening process. After merging the DEGs from HS vs HSCK, clustering analyses were performed using Cluster (2.1.0).
Gene expression Analysis through real-time quantitative PCR (RT-qPCR)
Six DEGs involved in regulating lateral PAT and organ identification were selected for real-time quantitative PCR analysis. cDNA was synthesized according to the method recommended by the SuperScript® III Reverse Transcriptase kit manufacturer. And the product size range was 150–300 bp. The primer pairs were designed using Primer5 software with the following parameters: The primer sequence is 20 nucleotides in length, the GC content is 45–55%, Tm is about 60 °C and the product size range is 120–250 base pairs (Table S1). Real-time quantitative PCR (RT-qPCR) was performed using SYBR® Premix Ex Taq™ kit (Takara, Dalian, China) in the Bio-Rad CFX96 RT-qPCR platform (Bio-Rad Laboratories, Hercules, CA, USA) according to the manufacturer’s protocol. 20 µL reaction volumes contained 2 µL of tenfold diluted cDNA, 10 µL 2 × SYBR® Premix Ex Taq™, 1 µL 10 μmol/L forward primers, 1 µL 10 μmol/L reverse primers, and 6 µL sterile distilled water. The cycling conditions were as follows: 30 s at 95 °C, 40 cycles at 95 °C for 5 s, and 60 °C for 30 s. The specificity of the amplicons was verified by melting curve analysis (60–95 °C) after 40 PCR cycles. Each sample was tested three times. The relative expression of the target genes was calculated using the 2−ΔΔCT method with normalization against the internal control gene.
Measurement of endogenous plant hormones
The IAA and ACC were extracted and purified according to methods from previous research (Dobrev and Kamınek 2002). In summary, approximately 1 g of fresh weight of the sample was ground to powder in liquid nitrogen. 10 mL of 95% methanol precooled at 4℃ was added and extracted for 2 h at 4 °C. Extracts were centrifuged at 20,000 g for 15 min at 4 °C, and the supernatant was taken out and precipitated for another extraction. The supernatant was combined and passed through an MCX column and eluted with 3 mL methanol, and the methanol was dried with nitrogen and then dissolved in 200 μL methanol. The dissolved samples were filtered through 13 mm-diameter nylon membrane Millex filters (Ø 0.22 μm) (Millipore, Bedford, MA, USA) and placed into tubes for further analysis. The supernatant was separated using an Agilent1290 HPLC system fitted with a poroshell 120 sb-c18 (2.1 mm × 150 mm, 2.7 μm). The SCIEX-6500 Qtrap system (Applied Biosystems, California, USA) was used to perform the quantified analysis with the following settings: Spray voltage: 4500 V; Air curtain gas: 15 psi; Atomizing gas pressure: 65 psi; Auxiliary gas pressure: 70 psi; Atomization temperature: 400 °C.
Treatment with NPA or quercetin
To verify the role of regulation of PAT in solitary hook development, the polar auxin inhibitor N-1-naphthylphthalamic acid (NPA) or quercetin was supplied to apical axillary stem of U. rhynchophylla, where the hook outgrowth. A sprayer was filled with the NPA solution or quercetin at 25 μmol/L, and then 5 mL of polar auxin inhibitor was sprayed on the apical axillary stem before 8 AM and after 6 PM daily for one week. 5 mL of distilled water was sprayed on the apical axillary stem as a control. After one week of treatments, the development status of the hook was observed.
Results
Transcriptome sequencing
In this study, to investigate gene expression changes between the lower and the upper side of leaf axils at the transcriptional level, we utilized RNA-Seq to analyse DEGs in the stem segments of HS and HSCK. After filtering data and eliminating low-quality reads and adaptor sequences, 250.67 million high-quality clean reads were generated from the six samples, constituting 37.60 Gb of cDNA sequences. The average amount of clean reads per sample was more than 40 million. The Q20 values (sequence error rate was 1%) ranged from 97.92 to 98.04%, and the Q30 values (sequence error rate was 1‰) ranged from 93.75 to 93.97%, and the GC content was 43.46%, 43.50%, 43.39%, 43.39%, 43.03%and 43.22% for the HS1, HS2, HS3, HSCK1, HSCK2 and HSCK3 libraries, respectively. The reads in each library were aligned to the U. rhynchophylla reference genome. Finally, more than 91% of the clean reads mapped to a unique location on the genome (Table 1).
Defining differentially expressed genes and functional annotation
One of our primary goals of the transcriptomic analysis was to identify variation between HS and HSCK libraries. Differences in gene expression in two libraries were examined, and pairwise comparisons identified the DEGs. In the RNA-Seq data, we found 42 DEGs, including 31 up-regulated and 11 down-regulated DEGs in the HS/HSCK comparisons (Fig. 1; File S1).
To assign biological functions and identify DEGs related to axillary bud formation, the Upset Venn diagram illustrates that the number of DEGs was annotated by all the six databases (KEGG, Uniprot, GO, Pfam, NR and Interproscan) (Fig. 2). 40 genes (95.24%) mapped to a known gene among the DEGs. The unmatched DEGs may represent novel genes specifically expressed in axillary bud formation. A total of 8 DEGs (19.05%) were successfully annotated to at least one database. 35 DEGs (83.33%) had significant matches in the NR and Pfam databases, followed by 30 DEGs (71.43%) in the GO and Uniprot databases, respectively.
GO enrichment and KEGG pathway analysis
The DEGs were annotated with GO enrichment analysis to predict their functions and were enriched within three main ontologies, namely, biological process (BP), cellular component (CC) and molecular function (MF). Finally, the 37 subcategories and the analysis of 30 DEGs among two compare groups are shown in Fig. 3. The BP category contained 19 subcategories, of which the primary terms were: response to stimulus, metabolic process, cellular process, single-organism process, biological regulation and developmental process. The CC category contained 10 subcategories, including the primary terms cell, cell part, organelle and membrane. The MF category contained 8 subcategories, mainly binding, catalytic activity, and nucleic acid binding transcription factor activity functions.
Some key GO subcategories were summarized according to a corrected P value correction of less than 0.05 (Fig. 4). These results showed that the major biological processes involved in the development, gravity response and hormone regulation, such as shoot system development (GO:0048367, 10 DEGs), floral whorl development (GO:0048438, 4 DEGs), gravitropism (GO:0009630, 3 DEGs), regulation of hormone levels (GO:0010817, 5 DEGs) and regulation of polar auxin transport (GO:2000012, 2 DEGs). In the molecular function, flavone synthase activity (GO:0033759, 2 DEGs) was prominently represented.
The DEGs were also annotated to the reference pathways in the KEGG database (Fig. 5). Here, only 5 DEGs were assigned to 10 KEGG pathways. The top 4 pathways “Lysine biosynthesis (ko00300, 1 DEGs)”, “Fatty acid degradation (ko00071, 1 DEGs)”, “Tyrosine metabolism (ko00350, 1 DEGs)”, “Terpenoid backbone biosynthesis (ko00900, 1 DEGs)” that were related to substance biosynthesis and metabolism, such as amino acids and lipids. The pathways related to hormone regulation were “plant hormone signal transduction (ko04075, 2 DEGs)”.
The results of GO and KEGG enrichment analyses suggested that the DEGs between HS and HSCK are mainly related to genes involved in the regulation and signal transduction of hormone, indicating that hormone may play an important role in solitary hook formation. Interestingly, four genes are involved in floral whorl development, which is the signal for floral organ determination.
Differentially expressed genes related to the regulation of polar auxin transport and floral development
The establishment and maintenance of auxin gradient by regulating PAT play a key role in axillary bud dormancy and active transduction. Many genes related to regulating PAT were identified in our datasets, such as LAZY1 (g2096, g15745). LAZY1 gene is well known as a negative regulator of PAT influencing gravitropism in rice, Arabidopsis and maize (Yoshihara and Iino 2007; Taniguchi et al. 2017; Dong et al. 2013). For example, mutation of the Lazy1 gene in rice alters the endogenous auxin distribution in shoot by enhancing PAT and thus shows an increase in tiller numbers and angles (Chen et al. 2012). We found that two DEGs (g25750, g25751) annotated as F3H, which are involved in the flavonoid biosynthesis pathway. It is widely reported that flavonoids have been implicated in the blocking of auxin transport (Peer and Murphy 2007). In Arabidopsis, PAT is increased in transparent testa4, a flavonoid-deficient mutant (Buer and Muday 2004). In addition, one AUX/IAA (g38641) and one AP2/ERF (g2096) were annotated as involved in plant hormone signal transduction, which they are also potentially taking part in the regulation of PAT (Liu et al. 2021).
Interestingly, we found some DEGs were annotated as involved in floral whorl development, including AGAMOUS (g26340), SRS (g22750), GRP2 (g26185), EMS1 (g38544), suggesting that the hooks and the inflorescences of U. rhynchophylla are homologous organs.
As shown in Fig. 6, we analyzed the expression profiles of four classes of DEGs. Two LAZY1, one AUX/IAA and one AP2/ERF were down-regulated in HS compared with HSCK. Two F3H and some genes related to inflorescences development, including AGAMOUS, SRS, and GRP2 except for EMS1 were up-regulated in HS compared with HSCK.
Differential gene expression validation by qRT-PCR analysis
To further verify the reliability of transcriptome analysis, we designed primers. We performed qRT-PCR assays in a biologically independent experiment for six selected DEGs (three up-regulated genes and three down-regulated genes), including two genes related to plant hormone signaling, one gene related to polar auxin transport, one gene related to flavone synthase activity, one gene related to floral whorl development. The results showed that these DEGs exhibited consistent expression patterns in the qRT-PCR data and the RNA-Seq results (Fig. 7), thus supporting that our transcriptomic data and analyses were valid.
Hormone concentration in HS and HSCK
To determine the concentration differences of key hormones between HS and HSKCK, HPLC of the extracts from the stem segments of HS and HSKCK detected IAA and ACC (Fig. 8). The concentrations of IAA and ACC were different. In the HSCK sample, the average IAA concentration (21.95 ng/g) was more than twice the average IAA concentration in the HS sample (11.63 ng/g). The average ACC concentration in the HSCK sample (716.86 ng/g) was more than three times the average in the HS sample (198.43 ng/g).
Effect of treatment with NPA or quercetin on hook development
The growth and development of the apical axillary stem of U. rhynchophylla, treated by the polar auxin inhibitor NPA or quercetin, was observed (Fig. S2). Treatment with NPA significantly not only inhibited the elongation of the apical stem and the numbers of stem nodes but also abolished the hook formation in axillary meristems compared with the control treatment. Treatment with quercetin also showed a similar developmental characteristics to treatment with NPA.
Discussion
Auxin (IAA) is one of the most important phytohormones responsible for axillary bud development in many plant species (Chao et al. 2016). Thus, we determined the IAA concentration of axillary stem segments in this study by HPLC. The formation of an appropriate IAA gradient between the lower side (HS) and the upper side (HSCK) of leaf axils was observed, resulting in a higher concentration of IAA in the upper side of leaf axils without hook, while a lower concentration of auxin in the lower side of leaf axils with a hook (Fig. 2), suggesting that high IAA concentration might inhibit the forming of hook in the upper side of leaf axils. Previous studies have also shown that the biological functions of auxin affecting the development of axillary bud depend on the establishment and maintenance of proper auxin gradient by regulating PAT (Kramer and Bennett 2006; Robert and Friml 2009). In this study, to further investigate the molecular mechanism of regulation of PAT mediating the establishment and maintenance of IAA gradient involved in the formation and distribution of solitary hooks in U. rhynchophylla, a transcriptome analysis was performed in HS and HSCK. A total of 42 DEGs were identified, and the GO and KEGG enrichment analyses mainly showed several categories, including gravitropism, regulation of polar auxin transport, plant hormone signal transduction, and flavone synthase activity (Figs. 4 and 5).
Gravitropism is a morphological and developmental process that plants perceive and respond to gravity (Li et al. 2013). Following the gravity perception, the Cholodny-Went hypothesis, originally proposed in 1937, is proposed to explain that the lateral PAT across gravity-stimulated plant tissues drives differential growth (Trewavas 1992; Muday 2001), and which has been confirmed by auxin gradient identification in plant-specific-tissues (Muday and DeLong 2001; Blancaflor and Masson 2003; Morita and Tasaka 2004). However, most of the knowledge about gravity-induced regulation of PAT comes from studies of herbaceous plants, while less from studies of vine plants (Ajala and Hasenstein 2022; Dong et al. 2013; Li et al. 2007; Moseyko et al. 2002).
Our transcriptome datasets found that three DEGs were involved in gravitropism, two of which were both annotated with the LAZY1 protein-coding genes (g2096, g15745). Previous studies have demonstrated that LAZY1 performs a signalling role in mediating plant architecture in response to gravitropic stimulation by redirecting IAA transport in plants (Dong et al. 2013; Yoshihara et al. 2013; Zhang et al. 2018). For instance, in rice, LAZY1 plays a negative role in lateral PAT upon gravity stimulation, resulting from a higher endogenous IAA distribution in the upper than that in the middle or lower side of coleoptiles (Li et al. 2007); which is consistent with our results about the pattern of IAA distribution; Maize LAZY1 mediates shoot gravitropism and inflorescence development through regulating transport and signaling of IAA (Dong et al. 2013); The Arabidopsis LAZY1 family also regulates the direction of PAT in response to gravity, leading to the generation of asymmetric IAA distribution in roots and shoots (Taniguchi et al. 2017).
Another DEG involved in gravitropism was detected to be annotated by the AUX/IAA (g38641). It is reported that numerous gravitropism-specific AUX/IAA proteins have also been identified (Tatematsu et al. 2004; Vandenbussche et al. 2013). Mutations of AUX/IAA genes result in gravitropism defects. For example, auxin-resistant Arabidopsis mutants axr1 and axr2 have a low expression of AUX/IAA genes resulting in defects of auxin-related phenotypes, including gravitropism (Abel and Theologis 1995). Another Arabidopsis mutation IAA17/AXR3, an AUX/IAA family member, also performs gravitropism defects in roots (Rouse et al. 1998). And we observed that the hook formation was abolished by treatment with NPA known as the polar auxin inhibitor (Fig. S2). Therefore, the above results about IAA determination and DEGs involved in gravitropism indicated that when axillary stems of U. rhynchophylla were stimulated by gravity, lateral PAT was regulated by LAZY1 and AUX/IAA genes, resulting in the formation of auxin gradient between the lower and the upper side.
Meantime, the formation of an auxin gradient was also brought to the hormone signal transduction. In our study, AUX/IAA (g38641) also performed another function in the response to auxin signal transduction according to KEGG enrichment analyses. It’s reported that AUX/IAA genes were first isolated as members of a family of genes whose transcripts were rapidly induced by auxin (Reed 2001). And AUX/IAA proteins are key factors to participate in the regulation of axillary bud development by interacting with auxin response factors (ARFs) (Luo et al. 2018). Multiple mutants of AUX/IAA have altered numbers of lateral roots and/or inflorescence branches, suggesting that AUX/IAAs regulate cell division in both lateral shoot meristems and lateral root initials in the pericycle (Liscum and Reed 2002).
Another DEG was detected to be annotated by the AP2/ERF protein-coding genes (g2096), AP2/ERF was also identified as potentially taking part in the regulation of PAT upon gravity stimulation (Liu et al. 2021). For example, in Salix matsudana, gravity may affect the alternative splicing and expression levels of AP2/ERFs, finally inducing an asymmetric distribution of auxin and negative gravitropism of shoots (Liu et al. 2021). In the popular study, the high expression levels of ERFs in endodormancy bud are found, suggesting that ERFs may be related to bud dormancy initiation (Hao et al. 2017). AP2/ERFs are also known to be the transcription factors responding to ethylene signalling and regulating the expression of downstream genes (Feng et al. 2020). It has been reported that ethylene is another potential modulator of gravitropism (Buer et al. 2006). Thus, we also determined the concentration of ACC (a precursor of ethylene) in axillary stem segments by HPLC. The ACC concentration showed significant differences between HS and HSCK. Notably, ACC showed a higher concentration in HSCK compared with that in HS. It suggests that ethylene may be involved in axillary bud development response to gravity. It has been found that ethylene reduced PAT in shoot tissues of several species (Morgan and Gausman 1966; Suttle 1988). Additionally, ethylene reduced the lateral redistribution of auxin across gravity-stimulated corn roots (Lee et al. 1990). The gravitropic mutant eir1 is insensitive to ethylene inhibition of root growth (Luschnig et al. 1998). Intriguingly, the large-scale dataset demonstrates the activation effect of ACC treatment on AUX/IAA expression (Harkey et al. 2018). For example, the reduced ethylene signalling causes an impaired transference of AUX/IAA proteins to auxin in the root epidermis (Vaseva et al. 2018). Together, these results about ACC determination and two DEGs (AP2/ERF and AUX/IAA) involved in plant hormone signal transduction indicated that gravitropic signaling could also induce the asymmetrical distribution of ACC or ethylene between the lower and the upper side of axillary stems of U. rhynchophylla, and then converted to ethylene-mediated AP2/ERF signaling transduction, which affected the lateral PAT by regulating the expression of AUX/IAA gene.
In addition, one mechanism by which ACC can alter PAT is by modulating the synthesis of the endogenous auxin transport regulators, flavonoids (Brown et al. 2001; Peer et al. 2004). It has been found that flavanone-3-hydroxylase (F3H) plays important role in flavonoid biosynthesis, which can hydroxylate flavanones to form 3-hydroxy flavonol, a common precursor of flavanols (Owens et al. 2008; Flachowsky et al. 2012). For example, the high expression of CtF3H in quinochalcone-type safflower line is associated with the accumulation of flavonols (Tu et al. 2016). Naturally occurring flavonols have been established as PAT's most effective negative regulators (Murphy et al. 2000; Lazar and Goodman 2006). Changing flavonol levels by applying exogenous flavonols to activate or inactivate the flavonoid pathway has been shown to impair auxin transport capacity and thus affect many auxin-related developmental processes (Santelia et al. 2008; Buer and Djordjevic 2009). Consistently, we observed that two DEGs were annotated with the F3H gene (g25750 and g25751), up-regulated in HS compared with their expression in HSCK, and the hook formation was abolished by treatment with quercetin known as the polar auxin inhibitor (Fig. S2). This result indicated that the high expression of F3H in HS might promote the accumulation of flavonols, which inhibited lateral PAT and enhanced consequent auxin accumulation resulting from the axillary bud inhibition of HSCK.
Interestingly, we observed that the leaf axils generally gave rise to hooks, but upon flowering induction, they differentiated inflorescences in place of hooks. Based on their common origin, hooks and inflorescences (peduncles) have long been considered homologous organs (Sperotto et al. 2020), but their genetic and molecular bases are yet unknown. In our transcriptome datasets, the GO enrichment analyses showed that four DEGs were involved in floral whorl development and stamen development, including genes encoding AGAMOUS, SRS, GRP2, EMS1. AGAMOUS, a transcription factor of the MADS-box family, has been shown to play central roles in regulating the identity of reproductive organs (stamens and carpels) and ovules and controlling floral meristem determinacy during flower development (Dreni and Kater 2014; Pelayo et al. 2021). In Arabidopsis, activation of SRS gene contributes to anther development by regulating tapetum degeneration and another dehiscence (Kim et al. 2010). OsGRP2 functions in the establishment of the cell wall network in early tapetum development and plays important role in the differentiation and function of the tapetum and their cell fate and function in rice (Takebe et al. 2020). EMS1 gene encodes a putative leucine-rich repeat receptor protein kinase (LRR-RPK) that was identified to play important role in signalling an0ther cell differentiation (Canales et al. 2002; Zhao et al. 2002), suggesting that EMS1 mediates signals to control the fate of reproductive cells and their contiguous somatic cells (Zhao 2009). Therefore, our results provided some insights into the origin of hooks of Uncaria rhynchophylla from molecular genetics.
Conclusions
To conclude, we investigated the differential expression of genes between the lower side and the upper side of stem segments from leaf axils only differentiated to solitary hooks of U. rhynchophylla utilizing transcriptome sequencing technology. In this study, the analysis results showed a complex network that regulates solitary hooks development of U. rhynchophylla involving phytohormones, gravitropism, regulation of polar auxin transport, plant hormone signal transduction, flavone synthase activity. More importantly, the expression of genes related to PAT regulation had a high correlation with the pattern of IAA distribution. Based on our results and previous studies (Chen et al. 2012; Hao et al. 2017; Liscum and Reed 2002; Santelia et al. 2008; Vaseva et al. 2018), we deduced that interaction effects of genes LAZY1, AUX/IAA, AP2/ERF, F3H and phytohormones ACC might regulate lateral PAT of leaf axils upon gravity stimulation, which subsequently established and maintained auxin gradients, the auxin concentration in the upper side was higher than that in the lower side of leaf axils. In brief, the establishment and maintenance of auxin gradient by regulating PAT between the lower and the upper side of axillary stems could be the one of key roles that induced hook formation of leaf axils in U. rhynchophylla. In addition, our results provided genetic and molecular support for the homologous organs of the hooks and inflorescences of U. rhynchophylla. Certainly, like in many transcriptome profiling studies, more experiments must be performed to validate these results in the future. Nevertheless, the above findings provided new information about the molecular mechanisms of regulation of lateral PAT in the axillary bud development process under gravity.
Data availability statement
Data are available upon request.
References
Abel S, Theologis A (1995) A polymorphic bipartite motif signals nuclear targeting of early auxin-inducible proteins related to PS-IAA4 from pea (Pisum sativum). Plant J 8(1):87–96
Ajala C, Hasenstein KH (2022) Transcription profile of auxin related genes during positively gravitropic hypocotyl curvature of Brassica rapa. Plants 11(9):1191
Blancaflor EB, Masson PH (2003) Plant gravitropism Unraveling the ups and downs of a complex process. Plant Physiol 133(4):1677–1690
Boss PK, Thomas MR (2002) Association of dwarfism and floral induction with a grape ‘green revolution’ mutation. Nature 416(6883):847–850
Brown DE, Rashotte AM, Murphy AS, Normanly J, Tague BW, Peer WA, Taiz L, Muday GK (2001) Flavonoids act as negative regulators of auxin transport in vivo in Arabidopsis. Plant Physiol 126(2):524–535
Buer CS, Djordjevic MA (2009) Architectural phenotypes in the transparent testa mutants of Arabidopsis thaliana. J Exp Bot 60(3):751–763
Buer CS, Muday GK (2004) The transparent testa4 mutation prevents flavonoid synthesis and alters auxin transport and the response of Arabidopsis roots to gravity and light. Plant Cell 16(5):1191–1205
Buer CS, Sukumar P, Muday GK (2006) Ethylene modulates flavonoid accumulation and gravitropic responses in roots of Arabidopsis. Plant Physiol 140(4):1384–1396
Canales C, Bhatt AM, Scott R, Dickinson H (2002) EXS, a putative LRR receptor kinase, regulates male germline cell number and tapetal identity and promotes seed development in Arabidopsis. Curr Biol 12(20):1718–1727
Chao WS, Doğramaci M, Horvath DP, Anderson JV, Foley ME (2016) Phytohormone balance and stress-related cellular responses are involved in the transition from bud to shoot growth in leafy spurge. BMC Plant Biol 16(1):1–21
Chen YN, Fan XR, Song WJ, Zhang YL, Xu GH (2012) Over-expression of OsPIN2 leads to increased tiller numbers, angle and shorter plant height through suppression of OsLAZY1. Plant Biotechnol J 10(2):139–149
Dobrev PI, Kamınek M (2002) Fast and efficient separation of cytokinins from auxin and abscisic acid and their purification using mixed-mode solid-phase extraction. J Chromatogr A 950(1–2):21–29
Dong ZB, Jiang C, Chen XY, Zhang T, Ding L, Song WB, Luo HB, Lai JS, Chen HB, Liu RY (2013) Maize LAZY1 mediates shoot gravitropism and inflorescence development through regulating auxin transport, auxin signaling, and light response. Plant Physiol 163(3):1306–1322
Dreni L, Kater MM (2014) MADS reloaded: evolution of the AGAMOUS subfamily genes. New Phytol 201(3):717–732
Feng K, Hou XL, Xing GM, Liu J-X, Duan AQ, Xu ZS, Li MY, Zhuang J, Xiong AS (2020) Advances in AP2/ERF super-family transcription factors in plant. Crit Rev Biotechnol 40(6):750–776
Flachowsky H, Halbwirth H, Treutter D, Richter K, Hanke MV, Szankowski I, Gosch C, Stich K, Fischer TC (2012) Silencing of flavanone-3-hydroxylase in apple (Malus× domestica Borkh.) leads to accumulation of flavanones, but not to reduced fire blight susceptibility. Plant Physiol Biochem 51:18–25
Geng CA, Yang TH, Huang XY, Ma YB, Zhang XM, Chen JJ (2019) Antidepressant potential of Uncaria rhynchophylla and its active flavanol, catechin, targeting melatonin receptors. J Ethnopharmacol 232:39–46
Hao XY, Yang YJ, Yue C, Wang L, Horvath DP, Wang XC (2017) Comprehensive transcriptome analyses reveal differential gene expression profiles of Camellia sinensis axillary buds at para-, endo-, ecodormancy, and bud flush stages. Front Plant Sci 8:553
Harkey AF, Watkins JM, Olex AL, DiNapoli KT, Lewis DR, Fetrow JS, Binder BM, Muday GK (2018) Identification of transcriptional and receptor networks that control root responses to ethylene. Plant Physiol 176(3):2095–2118
Hofer J, Turner L, Moreau C, Ambrose M, Isaac P, Butcher S, Weller J, Dupin A, Dalmais M, Le Signor C (2009) Tendril-less regulates tendril formation in pea leaves. Plant Cell 21(2):420–428
Hou JJ, Feng RH, Zhang YB, Pan HQ, Yao S, Han SM, Feng ZJ, Cai LY, Wu WY, Guo D (2018) Characteristic chromatogram: a method of discriminate and quantitative analysis for quality evaluation of Uncaria stem with hooks. Planta Med 84(06/07):449–456
Isnard S, Silk WK (2009) Moving with climbing plants from Charles Darwin’s time into the 21st century. Am J Bot 96(7):1205–1221
Kim SG, Lee S, Kim YS, Yun DJ, Woo JC, Park CM (2010) Activation tagging of an Arabidopsis shi-related sequence gene produces abnormal anther dehiscence and floral development. Plant Mol Biol 74(4):337–351
Kramer EM, Bennett MJ (2006) Auxin transport: a field in flux. Trends Plant Sci 11(8):382–386
Kuramochi T, Chu J, Suga T (1994) Gou-teng (from Uncaria rhynchophylla Miquel)-induced endothelium-dependent and-independent relaxations in the isolated rat aorta. Life Sci 54(26):2061–2069
Lazar G, Goodman HM (2006) MAX1, a regulator of the flavonoid pathway, controls vegetative axillary bud outgrowth in Arabidopsis. Proc Natl Acad Sci 103(2):472–476
Lee JS, Chang WK, Evans ML (1990) Effects of ethylene on the kinetics of curvature and auxin redistribution in gravistimulated roots of Zea mays. Plant Physiol 94(4):1770–1775
Li P, Wang YH, Qian Q, Fu Z, Wang M, Zeng DL, Li BH, Wang XJ, Li JY (2007) LAZY1 controls rice shoot gravitropism through regulating polar auxin transport. Cell Res 17(5):402–410
Li HF, Chen XP, Zhu FH, Liu HY, Hong YB, Liang XQ (2013) Transcriptome profiling of peanut (Arachis hypogaea) gynophores in gravitropic response. Funct Plant Biol 40(12):1249–1260
Liang JH, Wang C, Huo XK, Tian XG, Zhao WY, Wang X, Sun CP, Ma XC (2020) The genus Uncaria: A review on phytochemical metabolites and biological aspects. Fitoterapia 147:104772
Lim H, Lee HR (2022) Safety and biological activity evaluation of Uncaria rhynchophylla ethanolic extract. Drugs Chem Toxicol 45(2):907–918
Ling LZ, Zhang SD (2020) The complete chloroplast genome of the traditional Chinese herb, Uncaria rhynchophylla (Rubiaceae). Mitochondrial DNA Part B 5(1):424–425
Liscum E, Reed JW (2002) Genetics of Aux/IAA and ARF action in plant growth and development. Plant Mol Biol 49(3):387–400
Liu GY, Li YX, Gao JF, Feng Z, Guo HY, Zou H, Chu XT, Li YQ, Chen YH, Yu CM (2021) Detecting the different responses of roots and shoots to gravity in Salix matsudana (Koidz). Forests 12(12):1715
Luo J, Zhou JJ, Zhang JZ (2018) Aux/IAA gene family in plants: molecular structure, regulation, and function. Int J Mol Sci 19(1):1–17
Luschnig C, Gaxiola RA, Grisafi P, Fink GR (1998) EIR1, a root-specific protein involved in auxin transport, is required for gravitropism in Arabidopsis thaliana. Gene Dev 12(14):2175–2187
Lv BS, Yan ZW, Tian HY, Zhang XS, Ding ZJ (2019) Local auxin biosynthesis mediates plant growth and development. Trends Plant Sci 24(1):6–9
Min Z, Zhao XF, Li RY, Yang BH, Liu M, Fang YL (2017) Comparative transcriptome analysis provides insight into differentially expressed genes related to bud dormancy in grapevine (Vitis vinifera). Sci Hortic 225:213–220
Mizuno S, Sonoda M, Tamura Y, Nishino E, Suzuki H, Sato T, Oizumi T (2015) Chiba Tendril-Less locus determines tendril organ identity in melon (Cucumis melo L.) and potentially encodes a tendril-specific TCP homolog. J Plant Res 128(6):941–951
Morgan PW, Gausman HW (1966) Effects of ethylene on auxin transport. Plant Physiol 41(1):45–52
Morita MT, Tasaka M (2004) Gravity sensing and signaling. Curr Opin Plant Biol 7(6):712–718
Moseyko N, Zhu T, Chang HS, Wang X, Feldman LJ (2002) Transcription profiling of the early gravitropic response in Arabidopsis using high-density oligonucleotide probe microarrays. Plant Physiol 130(2):720–728
Muday GK (2001) Auxins and tropisms. J Plant Growth Regul 20(3):226
Muday GK, DeLong A (2001) Polar auxin transport: controlling where and how much. Trends Plant Sci 6(11):535–542
Murphy A, Peer WA, Taiz L (2000) Regulation of auxin transport by aminopeptidases and endogenous flavonoids. Planta 211(3):315–324
Ndagijimana A, Wang XM, Pan GX, Zhang F, Feng H, Olaleye O (2013) A review on indole alkaloids isolated from Uncaria rhynchophylla and their pharmacological studies. Fitoterapia 86:35–47
Owens DK, Crosby KC, Runac J, Howard BA, Winkel BS (2008) Biochemical and genetic characterization of Arabidopsis flavanone 3β-hydroxylase. Plant Physiol Biochem 46(10):833–843
Peer WA, Murphy AS (2007) Flavonoids and auxin transport: modulators or regulators? Trends Plant Sci 12(12):556–563
Peer WA, Bandyopadhyay A, Blakeslee JJ, Makam SN, Chen RJ, Masson PH, Murphy AS (2004) Variation in expression and protein localization of the PIN family of auxin efflux facilitator proteins in flavonoid mutants with altered auxin transport in Arabidopsis thaliana. Plant Cell 16(7):1898–1911
Pelayo MA, Yamaguchi N, Ito T (2021) One factor, many systems: the floral homeotic protein Agamous and its epigenetic regulatory mechanisms. Curr Opin Plant Biol 61:102009
Petersson SV, Johansson AI, Kowalczyk M, Makoveychuk A, Wang JY, Moritz T, Grebe M, Benfey PN, Sandberg G, Ljung K (2009) An auxin gradient and maximum in the Arabidopsis root apex shown by high-resolution cell-specific analysis of IAA distribution and synthesis. Plant Cell 21(6):1659–1668
Reed JW (2001) Roles and activities of Aux/IAA proteins in Arabidopsis. Trends Plant Sci 6(9):420–425
Robert HS, Friml J (2009) Auxin and other signals on the move in plants. Nat Chem Biol 5(5):325–332
Rouse D, Mackay P, Stirnberg P, Estelle M, Leyser O (1998) Changes in auxin response from mutations in an AUX/IAA gene. Science 279(5355):1371–1373
Santelia D, Henrichs S, Vincenzetti V, Sauer M, Bigler L, Klein M, Bailly A, Lee Y, Friml J, Geisler M (2008) Flavonoids redirect PIN-mediated polar auxin fluxes during root gravitropic responses. J Bio Chem 283(45):31218–31226
Shinohara N, Taylor C, Leyser O (2013) Strigolactone can promote or inhibit shoot branching by triggering rapid depletion of the auxin efflux protein PIN1 from the plasma membrane. PLoS Biol 11(1):e1001474
Sousa-Baena MS, Lohmann LG, Hernandes-Lopes J, Sinha NR (2018) The molecular control of tendril development in angiosperms. New Phytol 218(3):944–958
Sperotto P, Acevedo-Rodríguez P, Vasconcelos TN, Roque N (2020) Towards a standardization of terminology of the climbing habit in plants. Bot Rev 86(3):180–210
Suttle JC (1988) Effect of ethylene treatment on polar IAA transport, net IAA uptake and specific binding of N-1-naphthylphthalamic acid in tissues and microsomes isolated from etiolated pea epicotyls. Plant Physiol 88(3):795–799
Takebe N, Nakamura A, Watanabe T, Miyashita A, Satoh S, Iwai H (2020) Cell wall glycine-rich protein2 is involved in tapetal differentiation and pollen maturation. J Plant Res 133(6):883–895
Taniguchi M, Furutani M, Nishimura T, Nakamura M, Fushita T, Iijima K, Baba K, Tanaka H, Toyota M, Tasaka M (2017) The Arabidopsis LAZY1 family plays a key role in gravity signaling within statocytes and in branch angle control of roots and shoots. Plant Cell 29(8):1984–1999
Tatematsu K, Kumagai S, Muto H, Sato A, Watahiki MK, Harper RM, Liscum E, Yamamoto KT (2004) Massugu2 encodes Aux/IAA19, an auxin-regulated protein that functions together with the transcriptional activator NPH4/ARF7 to regulate differential growth responses of hypocotyl and formation of lateral roots in Arabidopsis thaliana. Plant Cell 16(2):379–393
Teale WD, Paponov IA, Palme K (2006) Auxin in action: signaling, transport and the control of plant growth and development. Nat Rev Mol Cell Biol 7(11):847–859
Trewavas AJ (1992) What remains of the Cholodny-Went theory? A Summing up. Plant Cell Environ 15(7):793–794
Tu YH, Liu F, Guo DD, Fan LJ, Zhu Z, Xue YR, Gao Y, Guo ML (2016) Molecular characterization of flavanone 3-hydroxylase gene and flavonoid accumulation in two chemotyped safflower lines in response to methyl jasmonate stimulation. BMC Plant Biol 16(1):1–12
Vandenbussche F, Callebert P, Zadnikova P, Benkova E, Van Der Straeten D (2013) Brassinosteroid control of shoot gravitropism interacts with ethylene and depends on auxin signaling components. Am J Bot 100(1):215–225
Vaseva II, Qudeimat E, Potuschak T, Du Y, Genschik P, Vandenbussche F, Van Der Straeten D (2018) The plant hormone ethylene restricts Arabidopsis growth via the epidermis. Proc Natl Acad Sci 115(17):E4130–E4139
Wang L, Li PH, Brutnell TP (2010) Exploring plant transcriptomes using ultra high-throughput sequencing. Brief Funct Genom 9(2):118–128
Wang SH, Yang XY, Xu MN, Lin XZ, Lin T, Qi JJ, Shao GJ, Tian NN, Yang Q, Zhang ZH (2015) A rare SNP identified a TCP transcription factor essential for tendril development in cucumber. Mol Plant 8(12):1795–1808
Weijers D, Nemhauser J, Yang Z (2018) Auxin: small molecule, big impact. J Exp Bot 69(2):133–136
Yoshihara T, Iino M (2007) Identification of the gravitropism-related rice gene LAZY1 and elucidation of LAZY1-dependent and-independent gravity signaling pathways. Plant Cell Physiol 48(5):678–688
Yoshihara T, Spalding EP, Iino M (2013) AtLAZY1 is a signaling component required for gravitropism of the Arabidopsis thaliana inflorescence. Plant J 74(2):267–279
Zhang N, Yu H, Yu H, Cai YY, Huang L, Xu C, Xiong GS, Meng XB, Wang JY, Chen HF (2018) A core regulatory pathway controlling rice tiller angle mediated by the LAZY1-dependent asymmetric distribution of auxin. Plant Cell 30(7):1461–1475
Zhao D, zhong, (2009) Control of anther cell differentiation: a teamwork of receptor-like kinases. Sex Plant Reprod 22(4):221–228
Zhao DZ, Wang GF, Speal B, Ma H (2002) The excess microsporocytes1 gene encodes a putative leucine-rich repeat receptor protein kinase that controls somatic and reproductive cell fates in the Arabidopsis anther. Gene Dev 16(15):2021–2031
Funding
This work is supported by the National Natural Science Foundation of China (Grant No. 81903752), the Natural Science Foundation of Guangxi Province (Grant No. 2019GXNSFBA185026), the Guangxi Science and Technology Major Project of China (Grant No. AA22362), the Scientific Research Funding Project of Guangxi Botanical Garden of Medicinal Plants (Grant No. GYJ202013), the Research and Innovation Team Building Project of Guangxi Botanical Garden of Medicinal Plants (Grant No. GYCH2019008), the Key Laboratory Construction Program of Guangxi Health commission (Grant No. ZJC2020003).
Author information
Authors and Affiliations
Contributions
LYW, SGW, ZJZ conceived and designed the experiments. LYW, LMP, LSS performed the experiments and analyzed the data. LYW, SGW, ZJZ wrote the manuscript. QLH wrote parts of the manuscript and helped revise the language. JEF, XWJ performed parts of the data analysis.
Corresponding authors
Ethics declarations
Conflict of interest
No potential conflict of interest was reported by the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
11816_2022_808_MOESM2_ESM.jpg
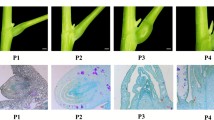
Supplementary file2 Morphological (A) and histological (B) structure of solitary hook. The original magnification of histological structure is 40 ×. (JPG 338 KB)
11816_2022_808_MOESM3_ESM.jpg
Supplementary file3 Growth and development of apical axillary stem treatment with NPA (A), quercetin (B) or distilled water (C) (JPG 253 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wan, L., Pan, L., Song, L. et al. Transcriptional analysis reveals formation of axillary solitary hook in vine plant Uncaria rhynchophylla. Plant Biotechnol Rep 17, 701–713 (2023). https://doi.org/10.1007/s11816-022-00808-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11816-022-00808-3