Abstract
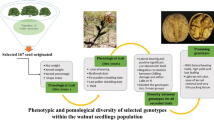
Cornelian cherry (Cornus mas) appears in a list of fruit and nut species growing in Europe considered neglected and underused economically. Although C. mas has a long-standing traditional medicinal use, only in recent years interest in products and food made from Cornelian cherries, said to have health-promoting effects, increased. This in turn raises the demand for improved planting material. In the Pielach Valley Region, Lower Austria, hundreds of centenary specimens of Cornus mas, but even a few millennial plants can still be encountered. The occurrence of these plants requested an active intervention to genetically characterize and preserve this valuable biodiversity, particularly in the light of changing environmental conditions. Efforts for the establishment of an in vitro collection of this valuable germplasm of centenary cornelian cherries yielded 193 mericlones initiated from single node explants from 41 selected plants. The selected donor plants were grouped by estimated age ranging from 10 years, > 50 years, > 100 years, > 200 years, > 400 years and 1000 years. The final goal of our efforts is to preserve these genetic resources, also checked for genetic and phytosanitary quality, for future generations and to use the superior clones for further breeding programs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cornus mas appears in a list of 27 fruit and nut species growing in Europe considered more or less neglected by researchers or which have been underused economically. Although cultivars and ecotypes are mentioned, there exist no orchestrated efforts for germplasm conservation (Maggioni 2000). For the purpose of ex situ conservation of valuable plant germplasm both in vivo and in vitro genebanks are generally recognized measures (IBPGR 1988).
Cornelian cherry (Cornus mas) of the dogwood family Cornaceae, is a shrub or small tree distributed over Southern and Central Europe, the Black Sea basin and the Caucasus (Da Ronch et al. 2016). It bears large elliptical or roundish fruits each containing a single stone enclosed by some sweet pulp. The ripe fruits are collected from the wild still today and used for the preparation of sherberts and jellies, and for the fermentation of alcoholic beverages. Cornels were also held to have medicinal virtues in antiquity and the Middle Ages (Gismondi et al. 2012). Even today, products and food made from Cornelian cherry fruit are said to have health-promoting effects. As a consequence of the traditional uses as medicinal plant, researchers got attentive and the identification and quantification of possibly health-related substances became an important research topic (Borroto Fernandez et al. 2021a).
While Turkey is the leader in production and processing of Cornelian cherry (Ercisli 2004), the highest genetic variability has been reported from the Caucasus region, Georgia and Ukraine (Pirc 2015). In Lower Austria, in the Pielach Valley Region, 200–300-year-old specimen of C. mas from the time of Empress Maria Theresia (1717–1780), but even millennial plants can still be encountered (Pirc 2015). The occurrence of these centenary Cornus mas plants challenged us to establish a protocol for in vitro conservation of these valuable genetic resources.
The most common way of propagation of wild Cornelian cherry is done via seedlings, where seeds need to be stratified after harvest and germinate in a 3-year period. First inflorescences will appear only after 6–8 years (Pirc 2015). Alternatively, vegetative propagation of selected cultivars by budding onto 2-year old seedling rootstock bears the advantage of the offsprings already bearing fruits in the fourth year (Pirc 2015). However, this method encounters several limitations such as seasonal dependence, low coefficient of propagation, seed stratification requirements, etc. The propagation by layering is even more difficult, with uncertain results and non-applicable for a commercial scale (Ďurkovič 2008).
First efforts for tissue culture of Cornus spp. were reported from North American and Asian species (Lattier et al. 2014), mainly the highly valued ornamental species C. nuttalii Audubon (Edson et al. 1994), C. florida (De Klerk and Korban 1994; Kaveriappa et al. 1997; Trigiano et al. 1992), C. canadensis L. (Feng et al. 2009) and Cornus alba (Ilczuk and Jacygrad 2016). On the other side, also the medicinal and edible plants C. officinalis Torr. ex Dur. (Lu 1985; Park et al. 1993; Xue et al. 2003), C. kousa (Ishimaru et al. 1998; Hadziabdic 2005) and C. mas Schönbrunner Gourmet (Swanson 2018) and selected high-yielding cultivars of the biofuel plant C. wilsoniana Wangerin in China (Li et al. 2015) were cultured in vitro.
Starting material in all cases involved either juvenile (seeds or seedlings) from breeding programs or 20–30-year-old specimen from plant collections. Ďurkovič (2008) describes the establishment of tissue culture from a mature 27-year-old tree of Cornus mas ‘Macrocarpa’ and the challenges encountered in establishing a stable in vitro culture.
Here we describe the efforts for the establishment of an in vitro collection of valuable germplasm of centenary cornelian cherries. The final goal of our efforts is to preserve these genetic resources for future generations and to use the superior clones for further breeding programs in the light of changing environmental conditions.
Materials and methods
Plant material
The in vitro conservation of valuable genetic resources of many centenary Cornus mas plants requested a protocol for in vitro establishment and maintenance. To achieve this goal, we addressed our questions subsequently to two populations.
The control population of 4 to 8 years old grafted individuals of 5 cultivars of Cornus mas, very popular in Austria, ‘Bulgarico’, ‘Flava’, ‘Kanzanlak, ‘Schönbrunner Gourmet’ and ‘Schumener’ was maintained in the greenhouse.
The main population consisted of centenary plants from different locations in Lower Austria (Table 1). The plants were grouped by estimated age ranging from 10 years (1 plant), > 50 (9 plants), > 100 (9 plants), > 200 (20 plants), > 400 (1 plant) and 1000 years (1 plant, Fig. 2a). The age of the plants was estimated from the information provided by the respective owners. The youngest plant and the plants in the category > 50 years have been planted by the owners. The plants > 100 were planted by the grand parents of the current owners. The plants in the categories > 200 and > 400 have achieved an impressive trunk size, and moreover have a historic link with the first mention of the farms in a given site. The oldest plant is even described as a national monument.
Genetic comparisons by microsatellite analyses revealed, that the 41 trees from 27 selected sites (at different elevations) represented unique genotypes, which could be explained by the pollination biology of the species (Borroto Fernandez et al. 2021b, Table 1). In brief, total genomic DNA was extracted from flower buds collected during ripening time. Seven different primer combinations (Wadl et al. 2014) were used for amplification of DNA from the different accessions.
To identify the population genetic structure of the selected genotypes of Cornus mas a model-based cluster analysis using STRUCTURE (Pritchard, Stephens and Donnelly, 2000) was implemented with a burn-in period and Markov chain Monte Carlo (MCMC) repetitions set to 5000 and 10,000, respectively. The admixture model with correlated allele frequencies was selected; K was set at 1–10 (K = 1–10) and K values were average across three iterations. The results from STRUCTURE were graphed on Microsoft Excel (2019) to obtain the plot (Fig. 1). A comparison with the entire population analysed (447 individuals) corroborated these results (Borroto Fernandez et al. 2021b).
A set of the following criteria were adopted for the selection of the individual genotpyes: fruit size, shape, and colour, yield and taste properties, and the fact, that the individuals belonged to the main different clusters as categorized by Borroto Fernandez et al. (2021b). Biochemical analyses were carried out on fruits from 11 plants (Borroto Fernandez et al. 2021a) and some plants exhibited particular traits, e.g. growing in a very humid or shady location, having an extremely small leaf size, or showing a different phyllotaxy (Table 1).
In vitro establishment
Culture initiation of selected cultivars
During vegetative shoot growth in springtime 2016 buds located at different positions on a twig were taken as explants from five greenhouse grown cultivars ‘Bulgarico’, ‘Flava’, ‘Kanzanlak, ‘Schönbrunner Gourmet’ and ‘Schumener’. According to Debergh and Maene (1981) the state of the donor plant essentially enhances tissue culture establishment.
Freshly collected twigs were cut into sections, rinsed under tap water and a few drops of liquid detergent for 20 min and incubated in 70% ethanol for 2–3 min. A fractionated treatment (5 min + 15 min) with 20% and 15% (v/v) commercial bleach (2.8% NaOCl) solutions with 2–3 droplets of Tween® 20 (Sigma-Aldrich Corporation, St. Louis, MO) was followed by 5 steps of washing with sterile water. Individual mericlones were initiated from single node explants from precisely marked sections to understand the impact of topophysis: A for apical buds, B for the first thin section of the twig, C for the central and D for the basal sections of the twigs. Special care was given to the nomenclature allowing to trace back the individual buds initiated from the same nodium on the branch.
Alternatively, a method for the in vitro establishment of Malus domestica from adult material (Laimer da Câmara Machado et al. 1991) was modified for Cornus mas, including important findings from Ďurkovič (2008). Twigs were immersed in an 0.1% 8-hydroxy-quinolinol-sulfate (8-HQS, Chinosol, Riedel de Haen) solution at 10 °C overnight, before buds were excised and processed as described before. Single buds were excised under a stereo microscope and placed in multiwell-plates (24 wells), covered with 0,1% 8-HQS overnight and subculture to fresh culture medium after 24 h, as originally described (Laimer da Câmara Machado et al. 1991). Initial culture media were based on MS medium (1962), supplemented with only 0.5 mg/l BA or in combination with 0.05 mg/l GA3 at pH 5.8.
Culture initiation of wildtypes from open field
Results obtained with the greenhouse grown material led us to elaborate a modified establishment procedure for the material from open field in spring 2016 (8 accessions), 2017 (12 accessions), 2018 (3 accessions) and 2019 (18 accessions). One of the techniques Durzan (1990) recommended for in vitro establishment of valuable individuals having reached the mature state, is the use of stump sprouts at the trunk of the tree. In fact, the first decision was already taken in the field, when harvesting the twigs with 40 vegetative buds, when available. Thin twigs were excluded, and only strong vital shoots were considered. Twigs were transported back to the lab, immersed and shaken in a 0.1% 8-HQS bath for 30 min, wrapped in tissue towels wetted in 0.1% 8-HQS solution and stored in the cold-room until further use. The next day twigs were rinsed under tap water and a few drops of liquid detergent for 20 min, incubated in 70% ethanol for 2–3 min and cut into 2–3 node sections to avoid tissue damage during surface sterilization. The fractionated surface sterilization involved a treatment (5 min + 15 min) with 20% and 15% (v/v) commercial bleach (2.8% NaOCl) with 2–3 droplets of Tween® 20.
Explants were cultured on a propagation medium consisting of WPM basal nutrients and vitamins, replacing the chelating agent for iron with EDDHA supplemented with 0.7 mg/l BA alone or in combination with 0.05 mg/l IBA or NAA, 100 mg l-1 myo-inositol, and 20 g l-1 sucrose. Medium was solidified with 7 g l-1 agar (Type A, Sigma-Aldrich) and a pH adjusted to 6.8–7.0.
Maintenance of the collection
Explants were cultured on propagation medium consisting of WPM basal nutrients and vitamins, replacing the chelating agent for iron with EDDHA, supplemented with 0.7 mg/l BA in combination with 0.05 mg/l IBA or NAA, 100 mg l-1 myo-inositol, and 20 g l-1 sucrose. The medium was solidified with 7 g/l-1 agar (Type A, Sigma-Aldrich) and the pH adjusted to 6.8–7.0. Regenerated axillary shoots were maintained by transferring to fresh regeneration medium every 4 to 6 weeks and incubated under standard culture conditions [24 ± 2 °C and a 16-h photoperiod of 100 µmol m-2 s-1 provided by cool-white fluorescent lamps].
Results and discussion
Culture initiation and long-term conservation of selected cultivars
On the single node explants after 28 days either one or both buds developed. To reduce the impact of apical dominance of one over the other, at the first subculture care was taken to split the original explant in two and to subculture the individual buds separately as x and y.
After 3 months from 124 established explants (Table 2), a total of 89 stably growing mericlones could be regenerated. Depending on the genotype, the number was variable from 29 of Schumener, 27 of Schönbrunner Gourmet, 16 of Kazanlak, 9 of Flava to 8 mericlones of Bulgarico. In total the most responsive buds were located at section D (31 mericlones), followed by the apical explants with 24 mericlones, and 17 mericlones for section B and C respectively, which corresponds to only 54% of section D. This could be most prominently observed in the cultivar Schumener, while it was not as pronounced, but still visible in the other cultivars. Regarding the cultivars Kazanlak, Flava and Bulgarico we observed less vitality and growth. Several buds turned brown and stopped growth, especially in the explants from the youngest section of the twigs. Interestingly from the apical explants the main meristem frequently developed into a small shoot and then died off, while the lateral buds formed on the first internode developed further and were therefore treated as individual mericlones x and y, which allowed to determine that 10/24 initially established nodes produced two vital shoots in the case of the cultivar Schumener, and 9/24 in the cultivar Schönbrunner Gourmet (data not shown).
Ďurkovič (2008) attempted to obtain axenic cultures by the application of commercial plant preservative mixtures and 0.1% mercuric chloride (HgCl2) solution; however, without mentioning the sterilisation rate. Given the toxicity of mercuric chloride, we searched for alternative solutions. The exposure of students and workers to HgCl2 solutions and the fact, that the washing water needs to be treated as contaminated waste, represent unnecessary occupational risks (Havermans et al. 2015). 8-HQ on the contrary has been known for its antimycotic and bactericidal activity, and apart from its use in human healthcare (Auterhoff et al. 1999) has been used for the treatment of budwood of fruit trees (Duhan, pers. comm). Read and Yang (1989) also showed that 8-hydroxy-quinoline citrate produced positive effects during the establishment of tissue cultures of woody species.
Therefore, even when starting from 24 original explants for every cultivar it was possible, after 28 days, to have enough material established to begin experiments for the optimization of the multiplication medium (Laimer da Câmara Machado et al. 1991). Both establishment methods yielded high numbers of vital shoots, which in dependence on the genotype varied from 33.3 to 100% (Table 2). A similar observation of the impact of the genotype, and even of the individual donor tree has been impressively documented already by Edson et al. (1994). Comparisons with data obtained with other Cornus species also indicated this high variability. Kaveriappa et al. (1997) reported that nodal sections from seedlings of C. florida grown in the greenhouse were easily established in culture after a treatment with 20% commercial bleach and a few drops of Triton X-100. Only 2–5% of the cultures were lost to contaminations, which agrees well with other studies that utilized seedling material grown under similar conditions (Declerck and Korban 1994). Edson et al. (1994) treated C. nuttallii bud material with NaOCl concentrations of 1.5%. Ilczuk and Jacygrad (2016) incubated C. alba buds in 70% EtOH for 2 min, followed by a treatment with 3% NaOCl for 15 min. In comparison, the NaOCl concentrations in all these experiments were substantially higher, opening further optimization perspectives by increasing the concentration without risking higher explant losses.
The cytokinin BA alone was reported to be sufficient for shoot culture establishment and multiplication of C. nuttallii (Edson et al. 1994), C. florida (De Klerk and Korban 1994; Kaveriappa et al. 1997), and C. kousa (Hadziabdic 2005). However, low concentrations of auxin were necessary for efficient in vitro growth in other dogwood species (Lattier et al. 2014). Since we did not observe any beneficial impact when applying GA3 (data not shown), we concluded to omit this variant from future trials.
Culture initiation and long-term conservation of wildtype accessions
Among several parameters considered of importance for a successful establishment, both genotype and environment were recognized as highly influential. Here we discuss the approach under two perspectives: (a) the choice of the explant and (b) the role of the culture medium to allow vigorous growth.
Mericlones developing from actively growing buds from different donor plants varied from a single bud (4,1%) up to 15 buds (62.5%) from the originally established 24 explants (Table 1). From the youngest individual (planted 8 years ago) 4 buds (16.6%) gave rise to actively growing mericlones. Similar ratios were obtained from plant material from the oldest plants, the 400 and the 1000-year-old specimen, namely 4 and 6 (16.6 and 25%), respectively (Table 1). Among the plants in the age class of > 50, only one particularly early ripening individual developed only from a single explant, while the other plants yielded 3–8 (12.5–33.3%) actively growing mericlones. Interestingly, among the 9 plants in the age class of > 100 2 plants developed a single mericlone, while 1 plant yielded 14 (58.3%) mericlones. Out of the 20 plants in the age class of > 200 4 plants yielded only a single viable mericlone, while others yielded up to 11, 14 or even 15 actively growing mericlones (45.8, 58.3 and 62.5%), respectively. In conclusion, the success rate does not correlate with the age of the donor plants, but must be clearly attributed to the physiological stage of the explant material at the time of excision.
The major problem encountered were fungal contaminations, with infection rates ranging from 68.6 to 72.4%, depending on the sterilization method. However, these results are comparable to those reported by Edson et al. (1994). The methods applied were quite efficient in controlling bacterial contaminations, since they only accounted for the loss of 2.9% and 5.5% of explants. Contrary to the observation of Edson et al. (1994), that most of the bacterial contaminations disappeared after a few subcultures in C. nuttallii, here infected cultures of C. mas were discarded.
Sterility of explants is probably the most difficult challenge with field grown explants due to their high contamination levels (Lazo-Javalera et al. 2016, Laimer da Câmara Machado et al. 1991). The establishment of in vitro cultures from woody plant material collected from Cornus species growing in the open field since centuries is reported to be a difficult task, owing to explant losses by fungal contaminations (Edson et al. 1994) and to detrimental conditions such as vitrification or chlorosis (Ilczuk and Jacygrad 2016). In fact, Edson (1994) stated that more explants from the forest trees of Cornus nuttallii Audubon were contaminated with fungi than from the greenhouse seedlings (75% versus 18%). Trigiano et al. (1992) reported high contamination rates with explants from forest trees of C. florida L. Most of these cultures became necrotic after 3 months and only 14/400 original explants survived.
Orthotropic shoots originating from the base of the trunk or shoots that arise from epicormic buds in several hardwoods are more juvenile than branches from other parts of the plant (Bonga 1987). These shoots arise from arrested of from adventitious buds. Epicormic buds are stimulated to grow and produce shoots as a result of stress i.e. sudden environmental change, thinning, crown dieback, heavy pruning, root death, cold, change in the water table. In temperate regions this phenomenon has been reported in oak (Quercus), elm (Ulmus), linden (Tilia), poplar (Populus), rowan (Sorbus) and willow (Salix). Examples of angiosperm trees micropropagated from stemp sprouts are Quercus robur, Tilia cordata (Chalupa 1984; Youn et al. 1988) and from buds of almost 100-year-old Morus nigra, Sorbus domestica and Liquidambar orientalis (Durkovic et al. 2012; Durkovic and Misalova 2009; Bayraktar et al. 2015).
Although most known tissue culture media were tested for Cornus species (Lattier et al. 2014), basal nutrients of the woody plant medium (WPM, Lloyd and McCown 1980) have preferentially been used for the micropropagation of a diverse range of dogwoods, including C. nuttallii (Edson et al. 1994), C. officinalis (Xue et al. 2003), C. mas (Ďurkovič 2008) and C. florida (Kaveriappa et al. 1997). Ishimaru et al. (1998) established cultures of C. kousa on Broadleaf Tree (BW) medium, Hadziabdic (2005) on WPM or half-strength BW medium (Chalupa 1984).
A strong dependency of explant vitality, lack of it and rate of proliferation on the cultivation conditions has reported by multiple authors (Ilczuk and Jacygrad 2016; Edson et al. 1994), whereas no mention in the literature was found on direct influences of the establishment method on the later vitality of the explants. As described by Ďurkovič (2008) cultures of Cornus mas Macrocarpa are prone to decline either by re-appearing infections and culture media conditions. Growth of cultures, initially established on WPM medium with a pH adjusted to 5.8 ceased, shoot tip necrosis appeared, and cultures gradually died. For this reason, the living shoots were transferred to a modified WPM with the pH adjusted to 6.8–7.0. Similarly, explants of C. mas Schönbrunner Gourmet after the initial 6 weeks on the medium described by Ďurkovič (2008) showed leaves that were slender and exhibited vitrification. These phenotypes disappeared after a transfer of the cultures to Long and Preece medium (Long et al. 1995; Swanson 2018). Shoots of C. florida exhibiting fasciated growth patterns, hyperhydricity and inhibited elongation where reported exposed to TDZ as plant growth regulator (Kaveriappa et al. 1997).
In our cultures of Cornus mas signs of vitrification disappeared after the transfer from MS (Murashige and Skoog 1962) to WPM medium. The most successful medium with a pH adjusted to 7.0 was supplemented with 0.7 mg/L BA and 0.05 mg/L NAA. After several subcultures on this medium all explants were growing actively, with long shoots and large vital green leaves (Fig. 2). Mericlones were regularly subcultured and maintained with 12–18 plantlets each on a medium supplemented with 0.36 mg/L BA and 0.02 mg/L NAA. This population size allows the safe maintenance of valuable genotypes and a rapid multiplication for experimental purposes or release. The documentation in an in house developed database allowed continuous monitoring of the status of cultures.
Similar media compositions including WPM as a basis as well as supplementation by BA and NAA at moderate pH values have been reported as optimal for various Cornus species (Ilczuk and Jacygrad 2016). In most in vitro shoot regeneration studies on dogwoods BA has been used as an effective cytokinin, and alone was sufficient for shoot proliferation of C. nuttallii (Edson et al. 1994), C. florida (De Klerk and Korban 1994; Kaveriappa et al. 1997), and C. kousa (Hadziabdic 2005) (see Lattier et al. 2014). However low concentrations of auxin were necessary for efficient in vitro growth in other dogwood species. Ďurkovič (2008) reported shoot regeneration using BA supplemented with NAA for C. mas ‘Macrocarpa’. Xue et al. (2003) reported proliferation of C. officinalis shoots on media containing BA, zeatin and NAA.
Chlorosis appeared in later subcultures and in some cases even led to explant death. Since we attributed it to the non-optimal cultivation conditions rather than to the establishment methods, we searched for alternative chelating agents. The beneficial influence of FeEDDHA on in vitro culture was noticed for several horticultural crops, e.g. increased shoot length and reduced chlorosis, enhanced regeneration from somatic embryos in rose, reduced chlorosis in raspberry and higher adventitious regeneration in blackberry (Van der Salm et al. 1994; Zawadzka and Orlikowska 2006; Tsao and Reed 2002). Development of chlorosis in shoot cultures of raspberry was caused by a shortage of iron when supplied as FeEDTA (Zawadzka and Orlikowska 2006), known to be less stable than FeEDDHA due to its susceptibility to light (Van der Salm et al. 1994), or its precipitation with other nutrient compounds (Dalton et al. 1983; Schenk et al. 1991). An obvious increase in the quality of the raspberry leaves (chlorophylls and iron content)—and thus reduction of chlorosis—was attributed by Zawadzka and Orlikowska (2006) to the higher availability of iron when supplied as FeEDDHA over FeEDTA, known to be less stable due to its susceptibility to light (Van der Salm et al. 1994). However, these authors also stressed, that the extent of this influence was cultivar dependent.
In vitro propagation offers also major contributions for any conservation programme and for the preservation of the genepool (Laimer and Maghuly 2008). In fact, Gepts (2006) correctly states that Plant Genetic Resources Conservation and Utilization are two tightly linked aspects important for the future generations. As far as efforts for germplasm conservation are concerned, in vivo and in vitro genebanks are generally recognized measures for ex situ conservation of valuable plant germplasm (IBPGR 1988; Yilmaz et al. 2009; Rop 2010). Thorough evaluation of the genetic resources of the native genotypes is essential for selecting most useful genotypes for future breeding programs designed to introduce traits such as hardiness and disease resistance from wild genotypes into cultivated varieties (Klimenko 2004; Ercisli et al. 2008; Borroto Fernandez et al. 2021b).
As climate is a key agro-ecosystem driving force, climate change could have a severe impact on cultivated as well as on wild growing plants. Many assessments have been carried out to date on the possible effects of climate change on plant physiology (Salinari 2006). In particular, plant pathogens may be specifically responsive to global change, given both short generation times and effective dispersal mechanisms (Coakley et al. 1999). Because of altered temperature and precipitation regimes, climate change may alter growth stages and/or rates of development in the life cycle and pathogenicity of pathogens, as well as modify the physiology and resistance of host plants (Chakraborty 2005). EU directives define the demands to the genetic and phytosanitary quality of intensively grown crop plants, but the perception of what this means for the native flora or extensively grown plants is largely missing, except when it acts as possible reservoir of inoculum. Changing climate conditions and reduced space availability will increasingly replace them with selected plant material. Healthy planting material can be made available, which provides the basis for a successful production of healthy food. With data presented in this paper, we are able to apply our long-lasting experience in the conservation of temperate fruit cultivars and landraces, as well as of small fruits, e.g. Rubus and Vaccinium to the conservation of Cornelian cherries.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Abbreviations
- BA:
-
6-Benzylaminopurine
- FeEDTA:
-
Ethylenediaminetetraacetate ferric sodium
- FeEDDHA:
-
Ethylenediamine di 2-hydroxyphenylacetate ferric
- 8-HQS:
-
8-Hydroxy-quinolinol-sulfate
- IBA:
-
Indole-3-butyric acid
- MS:
-
Murashige and Skoog
- NAA:
-
1-Naphthalenacetic acid
- WPM:
-
Woody plant medium
References
Auterhoff H, Knabe J, Höltje HD (1999) Lehrbuch der pharmazeutischen Chemie, 14th edn. VerlagsGmbH, Stuttgart, Wiss
Bayraktar M, Hayta S, Parlak S, Gurel A (2015) Micropropagation of centennial tertiary relict trees of Liquidambar orientalis Miller through meristematic nodules produced by cultures of primordial shoots. Trees 29:999–1009
Borroto Fernandez E et al (2021a) Phenotypic characterisation of selected Cornelian cherries (Cornus mas L.) from Austria. submitted.
Borroto Fernandez E et al (2021b) Evaluation of the genetic diversity of 400 accessions of a wildtype population of Cornus mas in Austria. submitted
Bonga JM (1987) Clonal propagation of mature trees: problems and possible solutions. In: Bonga JM, Durzan DJ (eds) Cell and tissue culture in forestry. General principles and biotechnology, vol 1. Martinus Nijhoff Publishers, Dordrecht, pp 249–271
Chakraborty S (2005) Potential impact of climate change on plant–pathogen interactions. Aust Plant Pathol 34:443–448
Chalupa V (1984) In vitro propagation of oak (Quercus robur L.) and linden (Tilia cordata Mill.). Biol Plant 26:374–377
Coakley SM, Scherm H, Chakraborty S (1999) Climate change and plant disease management. Annu Rev Phytopathol 37:399–426
Dalton CC, Iqbal K, Turner DA (1983) Iron phosphate precipitation in Murashige and Skoog media Physiol. Plant. 57:472–476
Da Ronch F et al (2016) Cornus mas in Europe: distribution, habitat, usage and threats. In: San-Miguel-Ayanz J, de Rigo D, Caudullo G, Houston Durrant T, Mauri A (eds) European Atlas of Forest Tree Species. Publ. Off. EU, Luxembourg, pp. e01ddab+
Debergh PC, Maene LJ (1981) A scheme for the commercial propagation of ornamental plants by tissue culture. Sci Hortic 14:335–345
Declerck V, Korban SS (1994) Effects of source of macronutrients and plant growth regulator concentrations on proliferation of Cornus florida. Plant Cell Tissue Organ Cult 38:57–60
Ďurkovič J (2008) Micropropagation of mature Cornus mas ‘Macrocarpa.’ Trees 22:597–602
Ďurkovič J, Misalova A (2009) Wood formation during ex vitro acclimatization in micropropagated true service tree (Sorbus domestica L.). Plant Cell Tissue Organ Cult 96:343–348
Ďurkovič J, Kanuchova A, Kacık F, Solar R, Lengyelova A (2012) Genotype- and age-dependent patterns of lignin and cellulose in regenerants derived from 80-year-old trees of black mulberry (Morus nigra L.). Plant Cell Tissue Organ Cult 108:359–370
Durzan DJ (1990) Adult vs. Juvenile explants: directed totipotency. In: Rodríguez R, Tamés RS, Durzan DJ (eds) Plant aging. NATO ASI series (series A: life sciences), vol 186. Springer, Boston, MA, pp 19–25
Edson JL, Wenny DL, Leege-Brusven A (1994) Micropropagation of pacific dogwood. HortScience 29:1355–1356
Ercisli S (2004) Cornelian cherry germplasm resources of Turkey. J Fruit Ornam Plant Res 12:87–92
Ercisli S et al (2008) Relationships among some cornelian cherry genotypes (Cornus mas L.) based on RAPD analysis. Genet Resour Crop Evol 55:613
Feng C-M et al (2009) Shoot regeneration of dwarf dogwood (Cornus canadensis L.) and morphological characterization of regenerated plants. Plant Cell Tissue Organ Cult 97:27–37
Gepts P (2006) Plant genetic resources conservation and utilization: the accomplishments and future of a societal insurance policy. Crop Sci 46:2278–2292
Gismondi A et al (2012) Identification of ancient Olea europaea L. and Cornus mas L. seeds by DNA barcoding. C R Biol 335(7):472–479
Havermans J et al (2015) The effect of mercuric chloride treatment as biocide for herbaria on the indoor air quality. Herit Sci 3:39
Hadziabdic D (2005) In vitro regeneration of Cladrastis kentukea (American yellowwood) and Cornus kousa (kousa dogwood). University of Tennesee, Knoxville, Dissertation
IBPGR (1988) Conservation and movement of vegetatively propagated germplasm: In vitro culture and disease aspects. Report of a subcommittee meeting. International Board for Plant Genetic Resources. Advisory Committee on In Vitro Storage
Ilczuk A, Jacygrad E (2016) In vitro propagation and assessment of genetic stability of acclimated plantlets of Cornus alba L. using RAPD and ISSR markers. Vitro Cell Dev Biol 52:379–390
Ishimaru K et al (1998) Cornus kousa (Dogwood): In vitro culture, and the production of tannins and other phenolic compounds. In: Bajaj YPS (ed) Biotechnology in agriculture and forestry 41. Medicinal and aromatic plants X. Springer, New York, pp 113–131
Kaveriappa KM, Phillips LM, Trigiano RN (1997) Micropropagation of flowering dogwood (Cornus florida) from seedlings. Plant Cell Rep 16:485–489
Klimenko S (2004) The cornelian cherry (Cornus mas L.): collection, preservation, and utilization of genetic resources. J Fruit Ornam Plant Res 12:93–98
Laimer da Câmara Machado M et al (1991) A new, efficient method for the initiation and establishment of tissue cultures of apple from adult material. Plant Cell Tissue Organ Cult 27:155–160
Laimer M, Maghuly F (2008) Contributions of biotechnological tools to the conservation of valuable germplasm. 2. World Scientific Congress: Challenges in Botanical research and climate change, Delft
Lattier J, Touchell DH, Ranney TG (2014) Micropropagation of an interspecific hybrid dogwood (Cornus ‘NCCH1’). Prop Ornam Plants 14:184–190
Lazo-Javalera MF et al (2016) Surface disinfection procedure and in vitro regeneration of grapevine (Vitis vinifera L.) axillary buds. Springerplus 5:453
Li Y, Wang X, Chen J, Cai N, Zeng H, Qiao Z, Wang X (2015) A method for micropropagation of Cornus wilsoniana: an important biofuel plant. Ind Crops Prod 76:49–54
Lloyd G, McCown BH (1980) Commercially-feasible micropropagation of mountain laurel, Kalmia latifolia, by use of shoot-tip culture. Combined Proc Int Plant Propag Soc 30:421–427
Long LM, Preece JE, Van Sambeek JW (1995) Adventitious regeneration of Juglans nigra L. (eastern black walnut). Plant Cell Rep 14:799–803
Lu WX (1985) Rapid propagation of plants from the immature embryos of Cornus officinalis. China J Chin Materia Med 10:9–10
Maggioni L (2000) Report of a Network Coordinating Group on Minor Crops. First Meeting. 16 June 1999, Turku, Finland. International Plant Genetic Resources Institute, Rome, Italy.
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Park CH et al (1993) Studies on multiplication of Cornus officinalis by in vitro culture. Korean J Med Crop Sci 1(1):63
Pirc H (2015) Enzyklopädie der Wildobst- und seltenen Obstarten. Stocker Vlg. 92- 112
Read PE, Yang G (1989) Response in vitro of explants chemically treated via forcing solutions. Proc Int Plant Prop Soc 38:406–408
Rop O et al (2010) Selected cultivars of cornelian cherry (Cornus mas L.) as a new food source of human nutrition. Afr J Biotechnol 9:1205–1210
Salinari F et al (2006) Downy mildew (Plasmopara viticola) epidemics on grapevine under climate change. Glob Change Biol 12:1299–1307
Schenk N, Hsiao K-C, Bornman CH (1991) Avoidance of precipitation and carbohydrate breakdown in autoclaved plant tissue culture media. Plant Cell Rep 10:115–119
Swanson MA (2018) Cornus mas l. cultivar selection based on hardiness and propagation. Master Thesis. Fargo, North Dakota
Trigiano RN, Beaty RM, Lowe KW (1992) Micropropagation of dogwoods (Cornus spp). In: Bajaj YPS (ed) Biotechnology in agriculture and forestry. High-tech and micropropagation IV, vol 20. Springer, Berlin, pp 81–90
Tsao CWV, Reed BM (2002) Gelling agent, silver nitrate, and sequestrene iron influence adventitious shoot and callus formation from Rubus leaves In Vitro Dev. Biol Plant 38:29–32
Van der Salm TPM et al (1994) Importance of the iron chelate formula for micropropagation of Rosa hybrida L. ‘Moneyway.’ Plant Cell Tissue Organ Cult 37(1):73–77
Wadl PA et al (2014) Development of microsatellites from Cornus mas L. (Cornaceae) and characterization of genetic diversity of cornelian cherries from China, central Europe, and the United States. Sci Hortic 179:314–320
Xue JP et al (2003) Study on plant tissue culture of Cornus officinalis. China J Chin Materia Med 28:118–121
Yilmaz KU et al (2009) Biodiversity, ex-situ conservation and characterization of Cornelian Cherry (Cornus mas L.) genotypes in Turkey. Biotechnol Eq 23(1):1143–1149
Youn Y, Ishii K, Saito A, Ohba K (1988) In vitro plantlet regeneration from axillary buds of mature trees of Tilia cordata. J Jpn Soc 70(7):315–317
Zawadzka M, Orlikowska T (2006) The influence of FeEDDHA in red raspberry cultures during shoot multiplication and adventitious regeneration from leaf explants. Plant Cell Tissue Organ Cult 85(2):145–149
Acknowledgements
The technical assistance of Sabine Mansky, Lena Achleitner, Andreas Albinus with the establishment and maintenance of the collection is acknowledged.
Funding
Open access funding provided by University of Natural Resources and Life Sciences Vienna (BOKU). Research Grant 101176 supported by the Federal Ministry of Agriculture, Regions and Tourism, and Land Niederösterreich, Austria.
Author information
Authors and Affiliations
Contributions
ML has designed the experiments and together with EB written the paper, VH has established the cultures, MZ propagated the cultures, and EB carried out the genetic analyses.
Corresponding author
Ethics declarations
Conflict of interest
Not applicable.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Laimer, M., Zeiser, M., Hanzer, V. et al. In vitro conservation of centennial Austrian Cornelian cherry accessions. Plant Biotechnol Rep 15, 289–298 (2021). https://doi.org/10.1007/s11816-021-00678-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11816-021-00678-1