Abstract
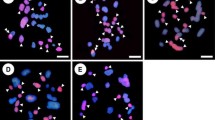
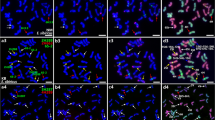
The analysis of chromosome pairing during meiosis is important for understanding the relationships between different genomes. To evaluate the diversity of chromosome pairing behavior in the wild species of Roegneria sinica var. media Keng with St and H genomes in Triticeae (Poaceae), differences and similarities in the meiotic chromosome pairing behaviors of the two genomes in two populations of R. sinica var. media, were analyzed using genomic in situ hybridization. Chromosome pairing at meiotic metaphase I in the two populations of R. sinica var. media mainly formed bivalents, although several univalents, trivalents and quadrivalents also occurred. Chromosome pairings occurred mainly between homologous chromosomes. However, some non-homologous pairings were observed under natural conditions. No significant differences in karyotype were found between the St and H genomes. Chromosome pairing behaviors differed between and within the two populations. Genetic variation occurred mainly within populations (94.04 %), and variation was more abundant in one population than the other. The genomes St and H differed, but there was some relationship between the two genomes. These findings suggest that homoeologous pairing of chromosomes or exchanges occurred between different genomes of the wild species in Triticeae during evolution. The findings also provide conclusive cytological evidence for genetic variation within the wild species, which forms the basis of their genetic diversity.


Similar content being viewed by others
References
Adamowski EV, Pagliarini MS, Batista LAK (2000) Chromosome number and microsporogenesis in Paspalum maritimum. Braz Arch Biol Technol 3:301–305
Baum BR, Yen C, Yang JL (1991) Roegneria: its genetic limits and justification for its recognition. Can J Bot 69:282–294
Benavente E, Orellana J, Fernandez-Calvin B (1998) Comparative analysis of the meiotic effects of wheat ph1b and ph2b mutations in wheat × rye hybrids. Theor Appl Genet 96:1200–1204
Bento M, Pereira H, Rocheta M, Gustafson P, Viegas W, Silva M (2008) Polyploidization as a retraction force in plant genome evolution: sequence rearrangements in Triticale. PLoS ONE 3(1):e1402
Bisht MS, Mukai Y (2001) Genomic in situ hybridization identifies genome donor of finger millet (Eleusine coracana). Theor Appl Genet 102:825–832
Bor NL (1960) Grasses of Burrna Ceylon, India and Pakistan. Pergamon Press, London, pp 652–680
Bowden WM (1965) Cytotaxonomy of the species and interspecific hybrids of the genus Agropyron in Canada and neighbouring areas. Can J Bot 43:1421–1448
Cai LB (1997) A taxonomical study on the genus Roegneria C. Koch from China. Acta Phytotaxon Sin 35:148–177
Cai LB (2002) Geographical distribution of Roegneria C Koch in China. Acta Bot Boreal Occident Sin 22:913–923
Cai X, Jones S (1997) Direct evidence for high level of autosyndetic pairing in hybrids of Thinopyrum intermedium and Th. ponticum with Triticum aestivum. Theor Appl Genet 95:568–572
Cao M, Sleper DA, Dong F, Jiang J (2000) Genomic in situ hybridization (GISH) reveals high chromosome pairing affinity between Lolium perenne and Festuca mairei. Genome 43:398–403
Crombach A, Hogeweg P (2007) Chromosome rearrangements and the evolution of genome structuring and adaptability. Mol Biol Evol 24:1130–1139
Devos KM, Millan T, Gale MD (1993) Comparative RFLP maps of the homoeologous group-2 chromosomes of wheat, rye and barley. Theor Appl Genet 85:784–792
Dong YC, Zheng DS (2000) Wheat genetic resources in China. Chinese Agricultrual Press, Beijing, pp 149–206
Dvorak J (1980) Homoeology between Agropyron elongatum chromosomes and Triticum aestivum chromosomes. Can J Genet Cytol 22:237–256
Ellneskog-Staam P, Salomon B, von Bothmer R, Anamthawat-Jonsson K (2001) Trigenomic origin of the hexaploid Psammopyrum athericum (Triticeae: Poaceae) revealed by in situ hybridization. Chromosome Res 9:243–249
Falistocco E, Torricelli R, Falcinelli M (2002) Genomic relationships between Medicago murex Willd. and Medicago lesinsii E. Small. investigated by in situ hybridization. Theor Appl Genet 105:829–833
Friebe B, Jiang J, Gill BS, Dyck PL (1993) Radiation-induced nonhomoeologous wheat-Agropyron intermedium chromosomal translocations conferring resistance to leaf rust. Theor Appl Genet 86:141–149
Gaeta R, Pires J (2010) Homoeologous recombination in allopolyploids: the polyploid ratchet. New Phytol 186:18–28
Guo PC, Yang H-L (1987) Flora Reipublicae Popularis Sinicae, Gramineae (3), Delectis Florae Reipublicae Popularis Sinicae Agendae. Academiae Sinicae Edita 9(3):5–104 (Beijing, Science Press)
Guo JY, Chen JF, Qian CT, Cao QH (2004) Meiotic chromosome pairing research and genome analysis in plants. Chin Bull Bot 21:513–520
Han F, Fedak G, Benabdelmouna A, Armstrong K, Ouellet T (2003) Characterization of six wheat × Thinopyrum intermedium derivatives by GISH, RFLP, and multicolor GISH. Genome 46:490–495
Han FP, Liu B, Fedak G, Liu ZH (2004) Genomic constitution and variation in five partial amphiploids of wheat-Thinopyrum intermedium as revealed by GISH, multicolor GISH and seed storage protein analysis. Theor Appl Genet 109:1070–1076
Hitchcock AS (1951) Manual of the grasses of the United States, 2nd edn. Revised by Chase A. Government Press, Washington, pp 230–280
Hou A, Peffley EB (2000) Recombinant chromosomes of advanced backcross plants between Allium cepa L. and A. fistulosum L. revealed by in situ hybridization. Theor Appl Genet 100:1190–1196
Jakob SS, Blattner FR (2009) Two extinct diploid progenitors were involved in allopolyploid formation in the Hordeum murinum (Poaceae: Triticeae) taxon complex. Mol Phylogenet Evol
Ji Y, Chetelat RT (2003) Homoeologous pairing and recombination in Solanum lycopersicoides monosomic addition and substitution lines of tomato. Theor Appl Genet 106:979–989
Keng YL, Chen SL (1963) A revision of the genus Roegneria C. Koch of China. J Nanjing Univ 3:1–29
Leitch A, Leitch I (2008) Genomic plasticity and the diversity of polyploid plants. Science 320:481
Liu WX, Liu WH, Wu J, Gao AN, Li LH (2010) Analysis of genetic diversity in natural populations of Psathyrostachys huashanica Keng using microsatellite (SSR) markers. Agric Sci China 9:463–471
Lu BR (1995) Diversity and conservation of Triticeae genetic resources. Chin Biodivers 3(63–68):3
Lu BR, Salomon B (1992) Differentiation of SY genomes in Asiatic Elymus. Hereditas 116:121–126
Lu BR, Salomon B, Bothmer RV (1991) Meiotic studies of the hybrids among Psetwloroegneria cognate, Elymus semicostatus and E. pendulinus (Poaeeae). Hereditas 114:117–124
Mukai Y (1993) Simultaneous discrimination of the three genomes in hexaploid wheat by multicolor fluorescence in situ hybridization using total genomic and highly repeated DNA probes. Genome 36:489–494
Nevski SA (1933) Uber das system der trib Hordeae Benth. Flora et systematica Plantae vasculares, Leningrad (2):9–32
Ørgaard M, Heslop-Harrison JS (1994) Investigations of genome relationships between Leymus, Psathyrostachys and Hordeum inferred by genome DNA: DNA in situ hybridization. Ann Bot 73:195–203
Poggio L, Confalonieri V, Comas C, Cuadrado A, Jouve N, Naranjo CA (1999) Genomic in situ hybridization (GISH) of Tripsacum dactyloides and Zea mays ssp. mays with B chromosomes. Genome 42:687–691
Reader SM, Abbo S, Purdie KA, King IP, Miller TE (1994) Direct labeling of plant chromosomes by rapid in situ hybridization. Trend Genet 10:265–266
Shannon CE, Weaver W (1949) The mathematical theory of communication. University of Illinois Press, Urbana, pp 3–14
Sharp PJ, Kreis M, Shewry PR, Gale MD (1988) Location of β-amylase sequences in wheat and its relatives. Theor Appl Genet 75:286–290
Shi JW, Gao AN, Liu JG, Li LH, Yang XM, Li XQ (2009) Morphological analysis on the diversity of Roegneria sinica var. media (Triticeae) populations. J Plant Genet Resour 10:547–552
Snowdon RJ, Kohler W, Kohler A (1997) Genomic in situ hybridization in Brassica amphidiploids and interspecific hybrids. Theor Appl Genet 95:1320–1324
Soltis D, Soltis P, Tate J (2003) Advances in the study of polyploidy since plant speciation. New Phytol 161:173–191
Stebbins G (1971) Chromosomal evolution in higher plants. Cambridge, UK: CUP 216 pp. Cytology evolution, genetics (PMBD, 185508067)
Sybenga J (1996) Chromosome pairing affinity and quadrivalent formation in polyploids: do segmental allopolyploids exist? Genome 39:1176–1184
Tian J, Zheng DS (1994) China crop genetic resources. China Agriculture Press, Beijing, pp 312–315
Tsvelev NN (1976) Grasses of the Soviet Union. Russian Translation Series 8, Rotterdam, pp 146–299
Wang QX, Xiang JS, Gao AN, Yang XM, Liu WH (2010) Analysis of chromosomal structural polymorphisms in the St, P, and Y genomes of Triticeae (Poaceae). Genome 53:241–249
Wang QX, Liu HT, Gao AN, Yang XM, Liu WH (2012) Intergenomic rearrangements after polyploidization of Kengyilia thoroldiana (Poaceae: Triticeae) affected by environmental factors. PLoS ONE 7:e31033
Wang QX, Han HM, Gao AN, Yang XM, Li LH (2014) P chromosomes involved in intergenomic rearrangements of Kengyilia thoroldiana affected by the environment. J Genet 93
Wendel J (2000) Genome evolution in polyploids. Plant Mol Biol 42:225–249
Wu RL, Gallo MM, Litteu RC, Zeng ZB (2001) A general polyploid model for analyzing gene segregation in qutcrossing tetraploid species. Genetics 59:869–882
Zheng Q, Li B, Mu SM, Zhou HP, Li ZS (2006) Physical mapping of the blue-grained gene(s) from Thinopyrum ponticum by GISH and FISH in a set of translocation lines with different seed colors in wheat. Genome 49:1109–1114
Acknowledgments
We appreciate the financial support provided by the National Natural Science Foundation of China (Project No: 30871520) and the Agricultural Science and Technology Innovation Program (ASTIP) of Chinese Academy of Agricultural Sciences (CAAS).
Author information
Authors and Affiliations
Corresponding author
Additional information
A. Gao and J. Shi contributed equally to this work.
Rights and permissions
About this article
Cite this article
Gao, A., Shi, J. & Liang, X. Research on meiotic chromosome pairing in Roegneria sinica var. media evaluated using genomic in situ hybridization. Plant Biotechnol Rep 10, 129–139 (2016). https://doi.org/10.1007/s11816-016-0393-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11816-016-0393-8